Abstract
Osteoporosis and sarcopenia are common in older age and associated with significant morbidity and mortality. Consequently, they are both attended by a considerable socioeconomic burden. Osteoporosis was defined by the World Health Organisation (WHO) in 1994 as a bone mineral density of less than 2.5 standard deviations below the sex-specific young adult mean and this characterisation has been adopted globally. Subsequently, a further step forward was taken when bone mineral density was incorporated into fracture risk prediction algorithms, such as the Fracture Risk Assessment Tool (FRAX®) also developed by the WHO. In contrast, for sarcopenia there have been several diagnostic criteria suggested, initially relating to low muscle mass alone and more recently low muscle mass and muscle function. However, none of these have been universally accepted. This has led to difficulties in accurately delineating the burden of disease, exploring geographic differences, and recruiting appropriate subjects to clinical trials. There is also uncertainty about how improvement in sarcopenia should be measured in pharmaceutical trials. Reasons for these difficulties including the number of facets of muscle health available, e.g. mass, strength, function, and performance, and the various clinical outcomes to which sarcopenia can be related such as falls, fracture, disability and premature mortality. It is imperative that a universal definition of sarcopenia is reached soon to facilitate greater progress in research into this debilitating condition.
Keywords: Osteoporosis, Sarcopenia, Bone, Muscle, Epidemiology, Definition
Introduction
Osteoporosis and sarcopenia are common diseases that predominantly affect older individuals [1, 2]. They are both associated with significant morbidity and can therefore lead to considerable health and social costs [3, 4]. Specially, sarcopenia is associated with increased rates of disability, poor mobility, frailty, and hospitalisation [5, 6] and it has been estimated that, in the United States, sarcopenia resulted in additional healthcare costs of over $18 billion in 2001 [4]. Furthermore, in common with hip and vertebral fracture fractures, a decline in muscle health has also been shown to predict future mortality from middle-age into later life [7]. Given current secular trends in population demographics with greater longevity, the burden of both osteoporosis and sarcopenia may continue to increase.
In addition to the similar population in which they occur, there is also growing evidence of a link between the two conditions. Studies have shown associations between bone and muscle health by dual energy x-ray absorptiometry (DXA) and more recently using cross-sectional imaging techniques [8, 9]. DXA studies have focussed on relationships between facets of muscle health and either bone mass or density and have tended to show positive relationships [10-12]. The use of peripheral quantitative computed tomography (pQCT) has additionally shown bone size and strength to be associated with muscle size, and to a lesser extent, muscle strength. Relationships of muscle with cortical and trabecular volumetric bone mineral density (vBMD) have been less consistent [8, 9].
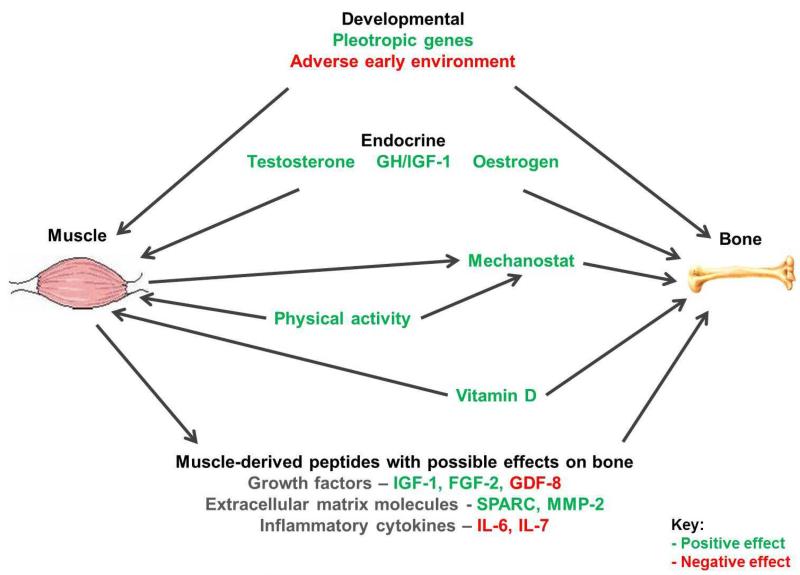
There are several potential explanations for these interrelationships (Figure 1). The mechanostat hypothesis describes the action of muscle contraction providing a direct mechanical stimulus to bone which promotes osteogenesis [13]. Hormones, such as growth hormone, can have positive effects on the growth of muscle and bone [14, 15]. Furthermore, exercise and levels of activity clearly augment both of these components of the musculoskeletal system. There are also likely to be common genetic and developmental components to muscle and bone health [16, 17].
Figure 1.
Interrelationships between muscle and bone.
Despite their similarities and interrelationships, study into these diseases is at very different stages of evolution, with research into osteoporosis considerably ahead. This review describes the progress that has been made in defining these conditions and explores the reasons for the discrepancy in progress made.
The history of osteoporosis
Osteoporosis is a skeletal disease characterised by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture [18]. The term literally means “porous bone” and was first introduced in France and Germany when it described a histological diagnosis. We now know this to represent bone tissue which is normally mineralised but reduced in quantity. This abnormality is the mechanism through which bones become weaker, increasing the risk of fractures occurring.
A significant leap forward was made with the development of non-invasive techniques able to assess bone mineral density (BMD) in vivo. Up to that point, attempts had been made to quantify bone health purely using plain radiographs, such as assessments of cortical morphometry [19]. Single photon absorptiometry was introduced in the 1960s and was subsequently replaced by dual photon absorptiometry. Both relied on radionuclide sources [20] and took over 15 minutes to complete (per site). The radionuclide decayed and consequently had a finite lifespan, needing to be changed at regular intervals. Around 25 years ago, the radionuclide source was superseded by an X-ray source and DXA scanners were born with faster scanning times and greater spatial resolution. This technique allows measurement of areal bone mineral density (aBMD) primarily at the hip and lumbar spine.
In 1994, the next step change in the definition of osteoporosis occurred, when a working group of the World Health Organisation (WHO) used bone density measurements by DXA to provide a practical definition of osteoporosis as an aBMD of less than 2.5 standard deviations (SD) below the young normal mean [21]. As earlier definitions had incorporated fracture, in order to provide comparability, the subset of women with osteoporosis who had also suffered one or more fragility fractures were deemed to have severe (established) osteoporosis. Osteopenia was defined as an aBMD level between 1 and 2.5 SD below the young normal mean.
This definition has been adopted throughout the world and has allowed great strides forward within this disease area. Prevalence was compared between different geographical locations and this led to hypotheses regarding likely aetiology. Study participants could be more easily selected and beneficial effects on bone could be quantified facilitating research into pharmaceutical agents to treat osteoporosis. This has led to the licensing of several medications with good evidence for efficacy in fracture risk reduction.
Overall within a population, higher aBMD is associated with greater bone strength. Specifically, it has been shown that there is an almost doubling of fracture risk for every one standard deviation reduction in aBMD [22]. However, these measures alone do not explain all of the variance in fracture risk. This is partly related to the inability to measure cortical and trabecular bone separately, and to take into consideration the bone’s material quality or structural geometry [23, 24]. Recent studies have shown some additional fracture discrimination using cross-sectional imaging techniques but the incremental gains tend to be relatively small [25, 26]. These techniques may however allow better understanding of the specific pathogenesis of osteoporosis at the structural level. In contrast, a considerable improvement in fracture prediction has been achieved with the development of fracture risk prediction algorithms, such as the Fracture Risk Assessment Tool (FRAX®).
FRAX® uses clinically available risk factors, with or without aBMD, to determine an individual’s risk of major osteoporotic fracture and hip fracture in the next 10 years [27]. As this is more accurate than using aBMD alone, it allows better targeting of treatments to those at greatest risk with positive effects on the ratio of risk to benefit. Therefore, we are now at a point where we can evaluate whether or not to treat an individual and have several effective therapies to do so, with more in the pipeline.
The history of sarcopenia
The term sarcopenia was first coined in 1989 by Irwin Rosenberg who used it to pertain to the loss of muscle mass with age [28, 29]. It has since become apparent that muscle function, in addition to muscle mass, is necessary to describe sarcopenia and so the definition has undergone an evolution to reflect this. Although muscle mass would intuitively be thought to be the central factor, it is only weakly associated with function and disability. It does, however, relate to low muscle strength which is strongly associated with these clinical outcomes [30]. Furthermore, although both muscle mass and muscle function decline from the age of 35 years [31], muscle strength and power decrease more rapidly than muscle mass [32] implying that they may be more sensitive to the changes that are occurring.
Several consensus definitions have been proposed to define sarcopenia clinically [33-35], most recently from the Foundation of the National Institute of Health (FNIH) sarcopenia project [30]. Although they all differ to some extent, each includes a measurement of both muscle size and muscle function. The assessment of muscle size utilised in the European Working Group on Sarcopenia in Older People (EWGSOP) and International Working Group on Sarcopenia (IWGS) definitions is that of skeletal mass index (appendicular mass relative to height squared) [33, 34]. This mitigates against defining individuals as sarcopenic or not based solely on their size. In contrast, the FNIH definition recommends use of muscle mass relative to body mass index (BMI) focussing more on the importance of adequate muscle for a given level of adiposity [30]. This is in keeping with the concept of sarcopenic obesity which has been developed to describe those individuals with both low muscle mass/function and excess adiposity. This combination is important as there is evidence that it may adversely affect health to a greater extent than either condition alone [36].
Muscle mass cut points have been suggested for each of the definitions. In the IWGS definition it equates to an appendicular lean mass divided by height squared of <7.23kg/m2 in men and <5.67kg/m2 in women [34]. The EWGSOP offers several DXA cut points all of which are of a similar magnitude [33]. From their large observational dataset, the FNIH definition suggests cut offs of appendicular lean mass over BMI of <0.789 for men and <0.512 in women, both of which predicted impaired mobility as assessed by a gait speed of <0.8 m/s [30].
Usual gait speed is the measure of muscle function in both the IWGS and ESPEN SIG definitions and this can be assessed using a 4 metre walk test. Interestingly, the cut offs differ by study group being <1m/s for the IWGS definition but more stringent at <0.8m/s for the ESPEN SIG definition [34, 35]. The EWGSOP definition is similar in that it suggests sarcopenia is present with the occurrence of low muscle mass along with low gait speed (<0.8m/s) but differs in that the later could be replaced by low muscle strength (grip strength) [33]. In contrast, the FNIH definition uses low grip strength (<26kg for men and <16 kg for females) alone as the required measure of muscle function [30]. Cut offs were higher in the EWGSOP definition and rates of sarcopenia were correspondingly greater [37].
In addition to those measures of muscle function already explored, there are other related parameters, such as muscle endurance and levels of habitual activity. Although both may relate to functional ability, they will clearly also be strongly influenced by factors other than sarcopenia. Furthermore, there are few published data concerning these outcomes and a lack of consistency in the way they are defined. Consequently they have not been included in current consensus definitions.
Although there are certainly similarities between the four consensus definitions described above, the subtle differences result in different groups of individuals being identified as sarcopenic [37]. Currently, there is therefore no universally accepted way to determine which patients are sarcopenic. This leads to several difficulties in investigating the condition.
The importance of defining sarcopenia
As sarcopenia is associated with significant morbidity and is a predictor of premature mortality, it is clearly important for further research in this area to take place. A universal consensus definition of sarcopenia would allow an evaluation of prevalence across different geographical areas. This would allow a more accurate quantification of the burden of disease and, as in the case of osteoporosis, potentially provide further clues to the aetiology. It would also allow identification of individuals at risk of the disease in order to evaluate its natural history and to target treatment where appropriate. Interventions may include diet, exercise and, in the future, pharmaceutical agents.
Furthermore, a universal definition of sarcopenia would also simplify participant selection for studies, including those of therapeutic agents, and would likely facilitate regulatory approval. In addition, it is also imperative to produce an easily measureable and clinically important study outcome. Options would include change in muscle size, muscle strength, or physical performance. However, as will be discussed, there are benefits and limitations to each of these. Furthermore, in line with fracture risk reduction in osteoporosis, it would also be beneficial if a drug could be shown to ameliorate one or more of the adverse clinical outcomes of sarcopenia.
Difficulties in defining sarcopenia
When a definition of sarcopenia is developed in clinical practice, it is important that it is practical, affordable, and acceptable to patients. Although it may be possible to use a more complicated and expensive method within research, it would be favourable to have a definition that could be used in both settings allowing results of studies to be more easily translatable into clinical practice.
The principle reason that progress in sarcopenia research has not advanced as rapidly as osteoporosis research, is the difficulty in establishing a consensual definition of sarcopenia that achieves the objective of a diagnostic criterion, as well as serving as an appropriate outcome measure for clinical trials of treatment. It is clear that any definition could use various combinations of muscle mass, strength and physical performance. However, the extent to which these three measures may be combined in a universal definition remains controversial. Some approaches attempt to incorporate all three components (EWGSOP) while others focus on simply one measure, such as gait speed.
Another difficulty has been a decision on which measures of muscle mass, strength and physical performance are most appropriate. Which is chosen may depend on what clinical outcomes are felt to be most important. For example, gait speed would be most closely associated with mobility and both gait speed and grip strength have been associated with several other outcomes including premature death [7].
This question also arises when assessing for clinical improvement. In osteoporosis, aBMD has been universally adopted as a good proxy for bone strength. Whereas in sarcopenia the most appropriate measure is not as apparent. Each possible endpoint has its own advantages and disadvantages (table 1). Similarly, the important clinical event in osteoporosis is obviously fracture. However, sarcopenia is associated with several adverse outcomes including falls, fractures, disability and death. Choosing the primary clinical outcome to include in clinical trials is therefore more problematic.
Table 1.
Potential endpoints in trials of interventions for sarcopenia and osteoporosis. Adapted with permission from Cooper et al. [38].
| Advantages | Disadvantages | |
|---|---|---|
| Sarcopenia | ||
| Muscle mass |
|
|
| Muscle strength |
|
|
| Muscle power |
|
|
| Muscle fatigue |
|
|
| Osteoporosis | ||
| Bone mineral density |
|
|
| Fracture |
|
|
The move towards a consensus is urgently needed and it may be that the two objectives (classification of individuals as disease or non-disease, and an outcome measure for clinical trials of novel therapies) require distinct definitional approaches [38].
Conclusion
There are many similarities between osteoporosis and sarcopenia including patient demographics, high prevalence, and great socioeconomic cost. There is also evidence of a mechanistic interrelationship between muscle and bone with sarcopenic individuals at greater risk of osteoporosis and vice versa. Although both are well recognised, there is a considerable difference in the progress that has been made in managing the two conditions.
A universal definition was established for osteoporosis in 1994 and this has allowed thorough assessment of its epidemiology, aetiology and fracture risk assessment, and for treatments to be developed. In contrast, there is still ongoing debate regarding how best to define sarcopenia. Although it tends to be accepted that this should include a measure of muscle size and muscle function, the precise nature of these measures and the cut offs to be used have not be confirmed.
It is imperative that a definition is agreed on soon to drive forward progress in this field. A better understanding of the prevalence and burden of sarcopenia will potentially build the case for further funding and interest from industry. It will also facilitate appropriate recruitment to studies and allow optimal measurement of improvement in muscle health in therapeutic trials.
One way to aid in the determination of which definition of sarcopenia should be adopted may be to more thoroughly examine their relationships, and those of their individual facets, to adverse clinical outcomes such as falls, fracture, disability, and death. This may allow an approach based on a more comprehensive evidence base.
References
- 1.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 2.Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC, Edwards M, Dennison E, Cooper C, Aihie Sayer A. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013;42:378–384. doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. Journal of the American Geriatrics Society. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 5.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, Waite LM, Seibel MJ, Sambrook PN. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J.Am.Geriatr.Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr.Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. Bmj. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards M, Gregson C, Patel H, Jameson K, Harvey N, Sayer AA, Dennison E, Cooper C. Muscle size, strength and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner.Res. 2013;28(11):2295–2304. doi: 10.1002/jbmr.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank AW, Lorbergs AL, Chilibeck PD, Farthing JP, Kontulainen SA. Muscle cross sectional area and grip torque contraction types are similarly related to pQCT derived bone strength indices in the radii of older healthy adults. J Musculoskelet.Neuronal.Interact. 2010;10:136–141. [PubMed] [Google Scholar]
- 10.Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C. Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology. 2001;47:207–212. doi: 10.1159/000052800. [DOI] [PubMed] [Google Scholar]
- 11.Segal NA, Torner JC, Yang M, Curtis JR, Felson DT, Nevitt MC. Muscle mass is more strongly related to hip bone mineral density than is quadriceps strength or lower activity level in adults over age 50 year. J Clin Densitom. 2008;11:503–510. doi: 10.1016/j.jocd.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirola J, Tuppurainen M, Honkanen R, Jurvelin JS, Kroger H. Associations between grip strength change and axial postmenopausal bone loss--a 10-year population-based follow-up study. Osteoporos.Int. 2005;16:1841–1848. doi: 10.1007/s00198-005-1944-y. [DOI] [PubMed] [Google Scholar]
- 13.Frost HM. Bone’s mechanostat: a 2003 update. Anat.Rec.A Discov.Mol.Cell Evol.Biol. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 14.Tritos NA, Biller BM. Growth hormone and bone. Curr.Opin.Endocrinol.Diabetes Obes. 2009;16:415–422. doi: 10.1097/MED.0b013e3283319e6d. [DOI] [PubMed] [Google Scholar]
- 15.Urban RJ. Growth hormone and testosterone: anabolic effects on muscle. Horm.Res.Paediatr. 2011;76(Suppl 1):81–83. doi: 10.1159/000329184. [DOI] [PubMed] [Google Scholar]
- 16.Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner.Res. 2008;23:788–802. doi: 10.1359/jbmr.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ.Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 19.Dequeker J. Quantitative radiology: radiogrammetry of cortical bone. Br.J Radiol. 1976;49:912–920. doi: 10.1259/0007-1285-49-587-912. [DOI] [PubMed] [Google Scholar]
- 20.Cameron J, Sorensson J. Measurement of bone mineral in vivo: an improved method. Science. 1963;142:230–232. doi: 10.1126/science.142.3589.230. [DOI] [PubMed] [Google Scholar]
- 21.Report of a WHO Study Group Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech.Rep.Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 22.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ott SM. When bone mass fails to predict bone failure. Calcif.Tissue Int. 1993;53(Suppl 1):S7–13. doi: 10.1007/BF01673395. [DOI] [PubMed] [Google Scholar]
- 24.Recker RR. Low bone mass may not be the only cause of skeletal fragility in osteoporosis. Proc.Soc.Exp.Biol Med. 1989;191:272–274. doi: 10.3181/00379727-191-42919. [DOI] [PubMed] [Google Scholar]
- 25.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner.Res. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 26.Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Yin PT, Cosman F, Nieves J, Guo XE, Shane E. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner.Res. 2010;25:2572–2581. doi: 10.1002/jbmr.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX((R)) with and without bone mineral density. Calcif.Tissue Int. 2012;90:1–13. doi: 10.1007/s00223-011-9544-7. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 29.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 30.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. Journal of applied physiology (Bethesda, Md. : 1985) 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Sarcopenia: alternative definitions and associations with lower extremity function. Journal of the American Geriatrics Society. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 33.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van KG, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De MC, Donini L, Harris T, Kannt A, Keime GF, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M, International working group on sarcopenia Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med.Dir.Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Current opinion in clinical nutrition and metabolic care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S. An evidence-based comparison of operational criteria for the presence of sarcopenia. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, Reid K, Boonen S, Dere W, Epstein S, Mitlak B, Tsouderos Y, Sayer AA, Rizzoli R, Reginster JY, Kanis JA. Tools in the assessment of sarcopenia. Calcified tissue international. 2013;93:201–210. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]