Abstract
Past research involving cocaine and resting-state functional connectivity (RSFC) has shown altered functional connectivity within the frontal and between the frontal and other cortical and subcortical brain regions in chronic users of cocaine. However, there have been discrepancies in literature regarding the relationship between RSFC between brain regions and cocaine use behavior. This study explored the RSFC between brain regions in cocaine smokers abstinent from cocaine use for 72 h and healthy controls. Also, the relationship between RSFC between brain regions and various cocaine use measures (cocaine use duration; frequency, and money spent on cocaine/week) was examined. Twenty chronic cocaine users and 17 controls completed a resting-state scan and an anatomical MPRAGE scan. Group independent component analysis performed on functional magnetic resonance imaging data identified 13 ICs pertaining to distinct resting-state networks, and group-level differences were examined. To examine inter-network functional connectivity between brain regions, these 13 ICs were divided into 61 distinct regions of interest (ROIs). Correlations were calculated between 61 ROI time series. For the ROI pairs that significantly differed from controls in connectivity strength, correlations were computed between connectivity strength and cocaine use measures. Results showed an enhanced RSFC within the sensory motor cortex and the left frontal–parietal network in cocaine users than controls. An increased inter-network RSFC between frontal–temporal and frontal–parietal brain regions, and a decreased RSFC between parietal–parietal, occipital–limbic, occipital–occipital, and occipital–parietal brain regions was found in cocaine users. This study demonstrated that intra-network connectivity strength of sensory motor cortex was negatively correlated with years of cocaine use. Inter-network connectivity strength between occipital–limbic brain regions was positively correlated with years of cocaine use, while connectivity strength within occipital brain regions was negatively related to cocaine use frequency and money spent on cocaine per week in abstinent cocaine users.
Key words: : brain, cocaine, functional connectivity, resting, use
Introduction
Human drug addiction has been characterized as a complicated process of the brain (Goldstein and Volkow, 2002). Addicted individuals are unable to control their behavior in the presence of the strong motivation to consume a drug. It has been hypothesized that executive dysfunction plays a critical role in drug addiction. The chronic use of cocaine affects the structures of the brain that are responsible for the cognitive control of behavior that contributes directly to the addiction process. Chronic cocaine users have been shown to perform poorly on neuropsychological tests of executive function (Ardila et al., 1991; DiSclafani et al., 2002). Repeated cocaine use has been associated with structural (Franklin et al., 2002; Matochik et al., 2003) and metabolic (Ernst et al., 2000) abnormalities in the brain, particularly in the prefrontal and midline structures that are crucial for executive control (Miller and Cohen, 2001). In addition to the proposed structural changes in the frontal brain areas associated with chronic cocaine use, functional magnetic resonance imaging (fMRI) studies suggest that chronic cocaine use is associated with changes in frontal lobe functioning. For example, compared to healthy controls, cocaine users show enhanced activation in the prefrontal brain regions during cue-induced cocaine craving (Grant et al., 1996; Wang et al., 1999; Wilcox et al., 2011). Resting-state fMRI approaches have revealed altered functional connectivity (RSFC) in frontal brain areas of cocaine users (Camchong et al., 2011; Kelly et al., 2011). RSFC, assessed by the correlation of spontaneous fluctuations of blood oxygen level-dependent (BOLD) signals in different regions of the resting brain, is believed to provide a measure of the brain's functional organization (Fox and Raichle, 2007). RSFC has been related to self-monitoring and introspective processes (Eryilmaz et al., 2011).
Previous results have shown enhanced connectivity within the anterior cingulate cortex network in cocaine-dependent individuals compared to controls (Camchong et al., 2011) as well as reduced prefrontal interhemispheric RSFC in cocaine users relative to control participants (Kelly et al., 2011). A handful of resting state cocaine studies have also examined functional connectivity between frontal and limbic brain areas (Cisler et al., 2013; Gu et al., 2010; Verdejo-Garcia et al., 2012; Wilcox et al., 2011) as cocaine addiction is characterized by persistent decision-making deficits, which are linked to functional abnormalities within the frontolimbic systems. Results demonstrate enhanced frontal–striatal (Wilcox et al., 2011) and prefrontal–insula (Cisler et al., 2013) connectivity as well as reduced connectivity within the mesocorticolimbic circuits [e.g., between ventral tegmental area and nucleus accumbens, between amygdala and medial prefrontal cortex (Gu et al., 2010)], and between anterior cingulate cortex, thalamus, insula, and brain stem areas in cocaine users compared to healthy controls (Verdejo-Garcia et al., 2012).
Although the resting state cocaine studies have shed light on how the functional connectivity within the frontal as well as between frontal and other cortical and subcortical brain areas is altered due to chronic cocaine use, very little is known regarding how the connectivity between brain regions is related to an individual's cocaine use behavior. Only a handful of existing resting state studies involving cocaine users have addressed this important issue (Camchong et al., 2011; Gu et al., 2010; Konova et al., 2013; McHugh et al., 2014). Using treatment-seeking cocaine users, McHugh et al. (2014) showed that early relapse risk was associated with reduced RSFC between the amygdala and the prefrontal cortex compared to cocaine users who remained abstinent. Lower RSFC of the striatum was correlated with less cocaine use severity in a study by Konova et al. (2013). Camchong et al. (2011) did not find any significant correlation between strength of functional connectivity within the dorsolateral prefrontal cortex, medial temporal gyrus, and cocaine use duration, whereas Gu et al. (2010) demonstrated that functional connectivity between frontal–limbic brain areas was negatively correlated with duration of cocaine use. A careful examination of study participants' cocaine use history revealed that although they had abused cocaine for a relatively long duration [average 14.6 years in Camchong et al.'s (2011) study; 13.8 years in Gu et al.'s (2010) study], participants varied from very light users to daily users [Camchong et al. (2011) study: average frequency of cocaine use in the past month 3.2 (SD=1.63); Gu et al. (2010) study: once a week to daily users]. Additionally, over 50% of participants in Gu et al's (2010) study tested positive for cocaine immediately before participating in the study. It is conceivable that most of the participants in Camchong et al.'s (2011) study also had cocaine in their system, as on the day of the study, they reported an average number of days since the last cocaine use was 1.6 (SD=.82). It is estimated that 72 h are required to allow for elimination of the active cocaine metabolites from one's system (Van Gorp et al., 1999; Wilcox et al., 2011). Thus, the studies that examined a relationship between RSFC strength and cocaine use behavior were confounded as previous research shows that the presence of cocaine in urine alters RSFC in cocaine users (Gu et al., 2010).
To begin to address the discrepancies in the literature involving the relationship between RSFC between brain regions and cocaine use behavior, we first examined RSFC between frontal and other cortical and subcortical brain regions in nontreatment-seeking chronic cocaine smokers who smoked cocaine at least twice a week, and were abstinent from cocaine use for at least 72 h and healthy control participants. We then examined the relationship between functional connectivity of brain regions and various cocaine use measures: frequency of cocaine use per week, duration of cocaine use (in years), and the amount of money spent on cocaine per week. Based on the previous resting state cocaine studies we predicted that cocaine users would show an altered functional connectivity between frontal and other cortical and subcortical brain regions compared to controls. We also expected significant correlations between the functional connectivity of brain regions and various cocaine use measures.
Materials and Methods
Participants
Twenty (15M; 5F) nontreatment-seeking chronic cocaine smokers abstinent from cocaine use for 72 h, and 17 (13M; 4F) age-, education-, and ethnic background-matched healthy volunteers took part in the study (Table 1).1 The groups did not significantly differ in terms of age, education, alcohol use quantity, nicotine use frequency and quantity, and caffeine use frequency and quantity.
Table 1.
Demographic and Substance Use Information for Cocaine Users and Controls
| Cocaine (n=20)Mean, range (SD) | Control (n=17)Mean, range (SD) | t-Stats | p | |
|---|---|---|---|---|
| Age (years) | 46 (6.4) | 46 (7) | 0.10 | 0.92 |
| Education (years) | 13.4 (2.4) | 13.5 (2.1) | −0.17 | 0.86 |
| Race/ethnicity | ||||
| Caucasian | 7 | 5 | ||
| African American | 11 | 11 | ||
| Hispanic | 2 | 1 | ||
| Female (n) | 5 | 4 | ||
| Cocaine use by all users | ||||
| Frequency (days/week) | 3, 2–6 (1.2) | na | ||
| Duration of use (years) | 16, 3–34 (8) | na | ||
| Money spent ($/week) | $220, $70–550 (131) | na | ||
| Cocaine use by noncocaine-dependent/abusers | ||||
| Frequency (days/week) | 3, 2–6 (1.5) | |||
| Duration of use (years) | 9, 3–19 (6) | |||
| Money spent ($/week) | $172, $80–350 (93) | |||
| Alcohol use | ||||
| Frequency (days/month) | 1.9, 1–2.5 (0.55) | 4.0, 2.5–6.5 (1.4) | −4.89 | 0.00* |
| Quantity (drinks/occasion) | 2.1, 1–3.5 (0.92) | 1.7, 1–2 (0.42) | 0.92 | 0.37 |
| Drinkers (no.) | 13 | 6 | ||
| Nicotine use | ||||
| Frequency (days/week) | 5.1, 1–7 (2.3) | 5.7, 3–7 (2.3) | −0.40 | 0.70 |
| Quantity (cigarettes/day) | 6.3, 1.5–13 (3.0) | 2.8, 2.5–3 (.29) | 2.00 | 0.07 |
| Smokers (no.) | 13 | 6 | ||
| Caffeine use | ||||
| Frequency (days/week) | 4.4, 1–7 (2.5) | 3.6, 1–7 (2.4) | 0.78 | 0.44 |
| Quantity (cups/day) | 1.3, 1–2 (0.43) | 1.3, 1–4 (0.90) | 0.26 | 0.80 |
| Caffeine users (no.) | 13 | 11 | ||
| Clinical characteristics | ||||
| DSM-IV-R cocaine dependence | 10 | na | ||
| DSM-IV-R cocaine abuse | 3 | na | ||
| Cocaine nondependent/abusers | 7 | |||
denotes significant group difference.
The main inclusion criteria for the study participants included English as first language, right handedness, near 20/20 vision (or corrected), and no report of childhood learning disability or special education. The main exclusion criteria for the study participants included serious medical conditions, a history of psychiatric or neurological disorder or treatment, lifetime diagnosis of any substance use disorder on the part of the prospective participant's biological mother (to rule out prenatal exposure effects), alcohol abuse and dependence, including past dependence on alcohol, MRI contraindications, and for women, pregnancy.
Participants were included in the cocaine group if they had a history of smoking cocaine for at least two times per week for the past 6 months (assessed by self-report), and had a current spending of at least $70 per week on cocaine. Participants in the cocaine group were instructed to abstain from cocaine use for at least 72 h before their study appointment. The primary current drug of choice for the cocaine group was cocaine and they did not meet a DSM-IV-R diagnosis of abuse or dependence for any other drugs, as confirmed by SCID (First et al., 1997). The inclusion criteria for the control participants included no current or past drug use history and no alcohol abuse history on part of their first-degree family members.
On the day of the study, all participants gave written informed consent and were administered a urine screen to rule out pregnancy in women, and to ensure negative urine toxicology for cocaine, methamphetamine, tetrahydrocannabinol (THC), opiate, and benzodiazepines (One Step Multi-Drug Screen Test Panel). Their abstinence from alcohol was confirmed with a breathalyzer. At the end of the study, participants received a gift certificate worth $100 for their participation and were paid for their transportation expenses.
Procedure
Each participant completed a resting-state scan and an anatomical MPRAGE scan. During the resting-state scan, participants were instructed to lie quietly without any movements and visually fixate on a cross for 6 min.
Image acquisition
Imaging data were obtained using a 3T Siemens Trio head-only fMRI scanner equipped with a standard Siemens head coil. While participants visually fixated on the cross, T2*-weighted echo planar images were acquired (35 axial slices, voxel size 3×3×3 mm, interslice gap 1 mm, matrix size 64×64 mm, FOV=192 mm, TR=2000 msec, TE 25 msec, flip angle=90°) covering the entire brain. A sagittal T1-weighted structural scan (TR=1900 msec, TE=2.52 msec, matrix=256×256, FOV=256 mm, voxel size 1×1×1 mm, 176 1-mm slices with 0.5 mm gap) was acquired to coregister it with the fMRI data.
Data processing
For each of the participants, a SPM8-based data processing scheme was implemented based on our earlier studies (Di et al., 2013). Data processing included motion correction with respect to the mean image using realign function. Following motion correction, each of the participant's functional images was coregistered to his/her anatomical image. Each anatomical image was also segmented into gray matter, white matter, and cerebral spinal fluid (CSF) images using SPM8 “new segment” tool and deformation field was calculated. This deformation field was applied to each of the functional images to transform them into MNI standard space. Following segmentation CSF/WM images were thresholded at p>0.95, and binary masks representing CSF/WM were created. Using the CSF/WM masks, time series were extracted from functional data and principal components were calculated. A total of 34 nuisance time series were regressed from the BOLD fMRI data. These included first five principal components of CSF time series, first five principal components of WM time series, 6 motion parameters, 6 motion parameters from one time point before and 12 corresponding square of motion parameters (Friston 24-parameter model) (Behzadi et al., 2007). Following time series regression, each participant's fMRI data were temporally band-pass filtered between 0.01 and 0.1 Hz and spatially smoothed with 9 mm FWHM Gaussian kernel.
For each participant, maximum motion in any of the six directions (roll, pitch, yaw, x-, y-, and z-) was calculated along with frame-wise displacement as defined by Jenkinson et al. (2002). Participants displaying higher than 3 mm maximum motion in any direction or higher than 1 mm mean frame-wise displacement were planned to be removed from further analysis.
Data analysis
Following data preprocessing, group independent component (IC) analysis was applied to filtered and smoothed BOLD fMRI data by combining fMRI data from both cocaine users and controls. Group IC analysis was performed on this BOLD fMRI data using the temporal concatenation approach in MELODIC software available in the FSL software package (Beckmann et al., 2005; Jenkinson et al., 2010). A total of 20 ICs were extracted. Visual inspection followed by spatial correlation with IC maps from the FCP-1000 project was performed to identify ICs representing known resting-state networks (Taylor et al., 2012). Of the 20 ICs derived, 13 ICs were found to represent known resting-state networks. These group IC maps were back projected in each participant's BOLD fMRI data to derive participant-level IC time series. These participant-level time series were used as temporal regressors in a second regression model to derive participant-level spatial IC maps. In the second level regression model, each participant was processed separately. A voxel-wise two-sample t-test was conducted using the “randomize” program in FSL to derive group-level differences between cocaine users and healthy controls. Family wise error correction was performed using threshold-free cluster enhancement (TFCE) available in FSL.
To study functional connectivity between subcomponents of the brain networks derived from the IC analysis, the 13 IC maps were segmented into 61 distinct regions of interest (ROIs). For each of the 61 ROIs, voxel coordinates that described the maximum Z-stat values were extracted. Table 2 lists peak voxels' coordinates and corresponding ROI numbers, as well as ROI names in MNI space. The ROI names were derived based on the MNI atlas in AFNI (Cox and Hyde et al., 1997). For each of these coordinates, a 6 mm sphere consisting of 33 voxels was created around the peak coordinate to create 61 distinct ROI masks. For each of these ROI masks, preprocessed fMRI time series were extracted and pair-wise correlation was calculated for each of the ROI pairs. These correlation values, which represent functional connectivity strength, were converted into Fisher's Z-values. Two-sample t-tests were performed to compare these connectivity strength values between cocaine users and controls. For each of the ROI pairs that significantly differed from the controls in connectivity strength, correlations were computed between connectivity strength and various cocaine use measures for the cocaine group.
Table 2.
Regions of Interest Names, Numbers, and Peak Voxels' Coordinates
| IC number/network name | ROI numbers | ROI name | x | y | z |
|---|---|---|---|---|---|
| IC01 visual cortex | ROI-01 | L-calcarine gyrus | 0 | 79 | 10 |
| IC02 sensory motor cortex | ROI-02 | R-postcentral gyrus | −30 | 28 | 61 |
| ROI-03 | L-paracentral lobule | 0 | 22 | 58 | |
| ROI-04 | L-postcentral gyrus | 24 | 28 | 64 | |
| IC03 default mode network | ROI-05 | L-precuneus | 0 | 52 | 34 |
| ROI-06 | L-angular gyrus | 45 | 58 | 28 | |
| ROI-07 | R-angular gyrus | −48 | 58 | 37 | |
| ROI-08 | L-middle temporal gyrus | 60 | 28 | −8 | |
| ROI-09 | L-superior frontal gyrus | 12 | −32 | 52 | |
| ROI-10 | L-middle frontal gyrus | 39 | −14 | 46 | |
| ROI-11 | R-middle temporal gyrus | −57 | 31 | −5 | |
| ROI-12 | R-middle frontal gyrus | −36 | −44 | 31 | |
| ROI-13 | L-medial frontal gyrus | 6 | −53 | 19 | |
| IC04 higher visual cortex | ROI-14 | R-middle occipital gyrus | −33 | 88 | 13 |
| ROI-15 | L-middle occipital gyrus | 36 | 85 | 1 | |
| ROI-16 | R-inferior frontal gyrus | −48 | −8 | 31 | |
| ROI-17 | R-inferior parietal lobule | −27 | 52 | 49 | |
| ROI-18 | L-inferior parietal lobule | 27 | 55 | 55 | |
| IC05 frontal Cortex | ROI-19 | L-superior frontal gyrus | 24 | −44 | 34 |
| IC06 left frontal parietal network | ROI-20 | L-middle frontal gyrus | 48 | −11 | 34 |
| ROI-21 | L-inferior parietal lobule | 45 | 46 | 49 | |
| ROI-22 | L-middle temporal gyrus | 54 | 52 | −8 | |
| ROI-23 | R-inferior parietal lobule | −42 | 40 | 49 | |
| ROI-24 | L-medial frontal gyrus | 6 | −29 | 43 | |
| IC07 saliance network | ROI-25 | R-precuneus | −3 | 52 | 55 |
| ROI-26 | L-middle cingulate cortex | 3 | −11 | 40 | |
| ROI-27 | R-supra marginal gyrus | −60 | 34 | 31 | |
| ROI-28 | R-middle frontal gyrus | −33 | −47 | 25 | |
| ROI-29 | L-middle frontal gyrus | 33 | −47 | 19 | |
| ROI-30 | L-supra marginal gyrus | 57 | 37 | 31 | |
| IC08 motor network | ROI-31 | R-postcentral gyrus | −60 | 19 | 28 |
| ROI-32 | L-postcentral gyrus | 57 | 19 | 40 | |
| ROI-33 | L-SMA | 0 | 1 | 49 | |
| IC09 inferior frontal gyrus | ROI-34 | R-inferior frontal gyrus | −48 | −23 | −2 |
| ROI-35 | L-inferior frontal gyrus | 45 | −20 | −2 | |
| ROI-36 | L-superior frontal gyrus | 0 | −11 | 61 | |
| ROI-37 | R-supra marginal gyrus | −60 | 40 | 25 | |
| IC10 cerebellum | ROI-38 | L-declive | 21 | 67 | −23 |
| IC11 default mode network | ROI-39 | L-anterior cingulate cortex | 3 | −47 | −5 |
| ROI-40 | L-cingulate gyrus | 0 | 37 | 43 | |
| ROI-41 | L-superior frontal gyrus | 15 | −5 | 73 | |
| ROI-42 | R-cerebellar vermis | −9 | 58 | −35 | |
| ROI-43 | L-angular gyrus | 42 | 73 | 34 | |
| ROI-44 | R-angular gyrus | −45 | 70 | 34 | |
| ROI-45 | L-middle frontal gyrus | 21 | −35 | 40 | |
| ROI-46 | R-middle frontal gyrus | −24 | −38 | 34 | |
| ROI-47 | R-para hippocampal gyrus | −27 | 22 | −14 | |
| ROI-48 | L-para hippocampal gyrus | 24 | 37 | −11 | |
| IC12 right frontal parietal network | ROI-49 | R-middle frontal gyrus | −42 | −14 | 49 |
| ROI-50 | R-inferior parietal lobule | −51 | 46 | 46 | |
| ROI-51 | L-pyramis | 39 | 67 | −41 | |
| ROI-52 | R-middle temporal gyrus | −60 | 31 | −8 | |
| ROI-53 | R-medial frontal gyrus | −6 | −35 | 37 | |
| ROI-54 | R-cingulate gyrus | −6 | 46 | 40 | |
| ROI-55 | R-precuneus | −9 | 70 | 43 | |
| ROI-56 | L-cerebellum | 12 | 76 | −29 | |
| IC 18 temporal gyrus | ROI-57 | R-superior temporal gyrus | −54 | 49 | 13 |
| ROI-58 | L-superior temporal gyrus | 54 | 55 | 16 | |
| ROI-59 | R-medial temporal pole | −54 | −11 | −23 | |
| ROI-60 | L-cingulate gyrus | 0 | 34 | 34 | |
| ROI-61 | R-lingual gyrus | −15 | 55 | −5 |
IC, independent component; ROIs, regions of interest.
To derive whether there was any systematic difference in motion between the two groups of participants, two-sample t-tests were conducted on various subject-wise motion parameters. For each participant, frame-wise displacement was calculated using Jenkinson et al. (2002) model. Average and maximum frame-wise displacement was also calculated for each participant along with the maximum motion in any of the six directions. Two-sample t-tests were conducted for each of these motion parameters between two groups.
Results
Motion comparison
All participants met the motion threshold as set for the study. That is, for all participants, the mean frame-wise displacement was less than 1 mm and the maximum motion was less than 3 mm in any direction. A group-level unpaired t-test revealed that groups did not differ in the mean frame-wise displacement (p=0.8120) or maximum motion (p=0.8044). No significant group-level differences were observed in mean or maximum movement across participants.
Connectivity analysis
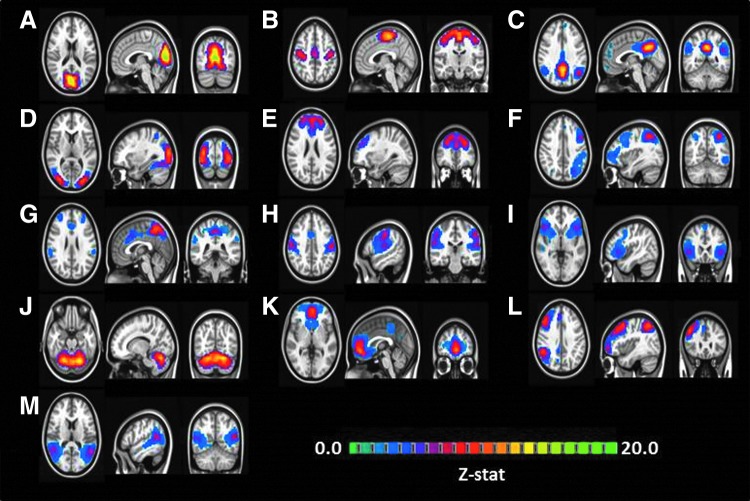
Group-level IC analysis resulted in 20 different ICs, out of which 13 ICs were found to be representing known resting-state networks. Figure 1 displays the group-level IC maps. The known resting-state networks were (A) visual cortex, (B) sensory motor cortex, (C) default mode network, (D) higher visual cortex, (E) frontal cortex, (F) left frontal–parietal network, (G) salience network, (H) motor network, (I) inferior frontal gyrus, (J) cerebellum, (K) default mode network, (L) right frontal–parietal network, and (M) temporal gyrus. Similar to splitting of the default-mode network into the frontal and the posterior section as observed in the earlier studies (Biswal et al., 2010), we also observed that the default-mode network was split into a frontal segment and a posterior segment.
FIG. 1.
Group independent component (IC) analysis on combined resting-state functional magnetic resonance imaging data from both cocaine users and healthy controls identified 13 ICs representing known resting-state networks: (A) visual cortex, (B) sensory motor cortex, (C) default mode network, (D) higher visual cortex, (E) frontal cortex, (F) left frontal–parietal network, (G) salience network, (H) motor network, (I) inferior frontal gyrus, (J) cerebellum, (K) default mode network, (L) right frontal–parietal network, and (M) temporal gyrus.
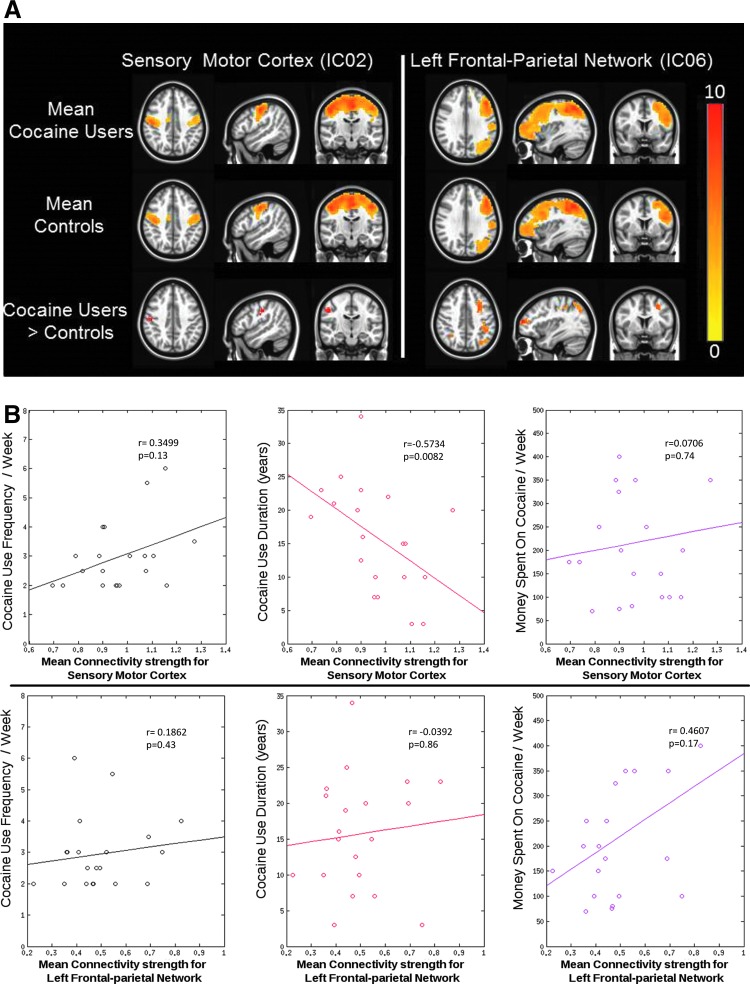
Significant group-level differences were observed in group IC maps representing the sensory motor cortex and the left frontal–parietal network. Connectivity in the right postcentral gyrus from the sensory motor cortex and between the left middle frontal gyrus and left inferior parietal lobule of the left frontal–parietal network was found to be increased in cocaine users compared to controls (p<0.05, FWE corrected) (Fig. 2). For both the networks that displayed significant differences in the connectivity strength between the groups, mean connectivity strength was extracted from cocaine users and correlated with cocaine use measures. A significant negative correlation was observed between the mean connectivity strength of sensory motor cortex and years of cocaine use (p<0.05, Bonferroni corrected) (Fig. 2B).
FIG. 2.
(A) Group level differences between cocaine users and controls for the sensory motor cortex and the left frontal–parietal network. Group-level activation maps for cocaine users are shown in row 1. Group-level activation maps for healthy controls are in row 2, and the last row displays group-level difference between cocaine users and controls (cocaine users>controls) (p<0.05, FWE corrected). (B) Scatter plots between various cocaine use measures and connectivity strength within the sensory motor cortex (top row) and the left frontal–parietal network (bottom row). A significant correlation was observed between resting-state functional connectivity of the sensory motor cortex and cocaine use duration.
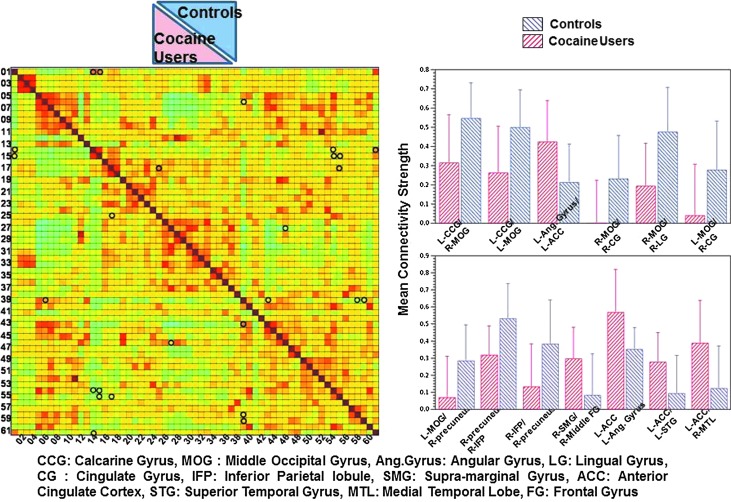
Figure 3 displays group-level differences for inter-network connectivity strength between different ROI pairs across controls and cocaine users (p<0.01, uncorrected). Significant differences were observed between cocaine smokers and controls in the interhemispheric and intrahemispheric connectivity of different ROI pairs. Cocaine smokers displayed significantly different connectivity compared to controls across 13 different ROI pairs. Connectivity between left anterior cingulate cortex and left superior temporal gyrus, right medial temporal lobe, and left angular gyrus was enhanced in cocaine users compared to controls. Connectivity between right middle frontal gyrus and right supramarginal gyrus was also enhanced in cocaine users compared to controls. On the contrary, controls showed an enhanced connectivity between right precuneus and right inferior parietal lobule and left middle occipital gyrus; between right middle occipital gyrus and right cingulate gyrus, right lingual gyrus, and left calcarine gyrus; and between left middle occipital gyrus and left calcarine gyrus and right cingulate gyrus.
FIG. 3.
Mean connectivity strength for different regions of interest (ROI) pairs for cocaine users and controls. Significant (p<0.01) connectivity values are marked by circles. Mean connectivity strength for each of the significantly different ROI pairs is displayed as barplot on the right side of the figure. The error bars represent standard deviation.
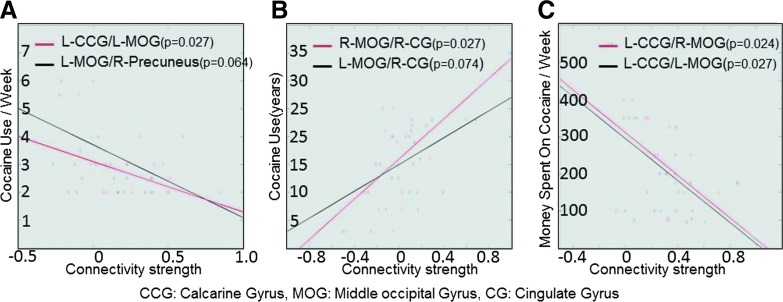
For each of the 13 ROI pairs which displayed significant difference in connectivity strength between cocaine users and controls, raw correlation values were extracted that represented the connectivity strength between these ROI pairs for cocaine users. Each of these connectivity strength values was correlated with three different cocaine use measures [cocaine use frequency per week, the amount of money spent on cocaine per week, and cocaine use duration (in years)]. Figure 4 displays the scatter plots between connectivity strength between ROI pairs and various cocaine use measures. Only the ROI pairs whose connectivity strength was highly correlated with various cocaine use measures are shown (r>0.4). Connectivity between right middle occipital gyrus and right cingulate gyrus was significantly (p<0.05) positively correlated with the cocaine use duration (in years). Connectivity between left middle occipital gyrus and left calcarine gyrus was significantly negatively correlated with cocaine use frequency per week, and connectivity between left calcarine gyrus and left and right middle occipital gyrus was significantly negatively correlated with the amount of money spent on cocaine per week. These analyses were not corrected for multiple comparisons due to small sample size.
FIG. 4.
Scatter plots between various cocaine use measures and connectivity strength between ROI pairs. Only the correlations higher than 0.4 (r>0.4) are displayed.
Discussion
In the present study, we examined RSFC differences between nontreatment-seeking chronic cocaine smokers who were abstinent from cocaine use for 72 h, and healthy controls. We then examined the relationship between intra-network and inter-network functional connectivity between brain regions and various cocaine use measures. Results showed enhanced connectivity within the sensory motor cortex and within the left frontal–parietal network in cocaine users compared to controls. RSFC within the sensory motor cortex was negatively correlated with years of cocaine use.
Furthermore, the analysis to study inter-network functional connectivity between brain regions revealed that cocaine smokers showed a significantly increased RSFC between interhemispheric frontal–temporal and intrahemispheric frontal–parietal brain areas, and decreased RSFC between intrahemispheric parietal–parietal, intra and interhemispheric occipital–limbic, occipital–occipital, and interhemispheric occipital–parietal brain regions compared to controls. This study demonstrated that inter-network connectivity strength between occipital–limbic brain regions was positively related to years of cocaine use, and connectivity strength within the occipital brain region was negatively related to the frequency of cocaine use and the amount of money spent on cocaine per week in abstinent cocaine users. Note that angular gyrus, supramarginal gyrus, and precuneus are parts of the parietal lobe, and lingual gyrus and calcarine gyrus are parts of the occipital lobe.
The present study addresses the discrepancies in the literature involving the relationship between RSFC between brain regions and cocaine use behavior by examining functional connectivity between brain regions in cocaine users who abstained from cocaine for at least 72 h. This experimental manipulation allowed us to examine intra- and inter-network RSFC between brain regions in cocaine users wherein cocaine's primary active metabolite benzoylecgonine had left their system as evidenced by a negative urine drug test result before the scan. Thus, the altered functional connectivity as observed in cocaine users compared to controls cannot be attributed to recent cocaine intoxication. Previous cocaine RSFC study results that either did not show any significant correlation between the strength of functional connectivity within certain brain regions and years of cocaine use (Camchong et al., 2011) or found a negative correlation between frontal–limbic brain regions and duration of cocaine use (Gu et al., 2010) were confounded as the majority of study participants had cocaine in their system while they took part in the resting-state scan. Since the earlier research has shown that the presence of cocaine in urine can alter RSFC in cocaine users (Gu et al., 2010), it is conceivable that the findings in these studies were confounded as the majority of study participants had cocaine in their system while they took part in the resting-state scan.
The current results are consistent with previous cocaine RSFC studies that have shown an enhanced functional connectivity between frontal and other cortical brain regions (Cisler et al., 2013), and a reduced functional connectivity between cortical–cortical brain regions (Verdejo-Garcia et al., 2012) in cocaine users compared to healthy controls. This study extends the previous literature by demonstrating that the intra-network connectivity strength of the sensory motor cortex is negatively correlated, and the inter-network connectivity strength between cortical–cortical (middle occipital gyrus–cingulate gyrus) brain regions is positively correlated with the duration of cocaine use in cocaine users who were abstinent from cocaine for 72 h. Also, the connectivity strength within the occipital brain regions is negatively related to the frequency of cocaine use and the amount of money spent on cocaine per week. An enhanced RSFC between frontal–temporal and frontal–parietal brain regions suggests a chronic cocaine user's weakened strength of cognitive control (Baler and Volkow, 2006).
The existing cocaine literature has suggested that repeated cocaine use leads to the disruption of dopaminergic pathways and causes neuroadaptations in dopaminergic function as well as neuroadaptations in glutamatergic, GABAergic, and other catecholaminergic systems (Beveridge et al., 2005; Borgland et al., 2006; Cunningham et al., 1992; Volkow et al., 2007). It is further suggested that these neuroadaptations could alter the functional connectivity of brain regions regulated by dopamine (Aharonovich et al., 2003; Goldstein et al., 2004; Volkow et al., 1997). Dopamine receptors are in abundance in the frontal, temporal, occipital, cingulate gyrus, and parietal brain regions (Meador-Woodruff et al., 1996; Miller et al., 2009; Piggott et al., 2007; Sawaguchi and Goldman-Rakic, 1991; Valentini et al., 2006; and Volkow et al., 2002), and continued cocaine use may disrupt dopamine functions in these areas. Neuroadaptations as a result of impaired dopaminergic function may have contributed to the observed altered intra-network and inter-network functional connectivity between brain regions in nontreatment-seeking abstinent cocaine smokers compared to healthy controls during rest. A negative correlation between RSFC of the sensory motor cortex and years of cocaine use, and a positive correlation between RSFC between occipital–limbic brain regions and years of cocaine use may signify different neuroadaptation patterns within the sensory motor cortex and between occipital–limbic brain regions due to chronic use of cocaine.
Future research should include characterizing the directionality of the functional connectivity among brain regions as observed in chronic users of cocaine by using a graph theoretic approach such as IMaGES (IMaGES: Independent Multisample Greedy Equivalence Search); Ramsey et al., (2011), which is based on causal modeling. Another important future direction will include using both fMRI and diffusion tensor imaging techniques to look for a relationship between functional and anatomical connectivity (Skudlarski et al., 2008) between frontal–temporal, frontal–parietal, occipital–parietal, occipital–limbic, and within the parietal and occipital brain regions in cocaine users. Any evidence of preserved structural connectivity would encourage hope for recovery of function, which may either result from abstinence from use, or as a result of cognitive interventions. Studies involving cognitive training interventions posit that functional deficits can be reversible (Kelly and Garavan, 2005). Future research will further examine whether the altered intra-network as well as the inter-network functional connectivity between brain regions during rest as observed in chronic users of cocaine preceded the initiation of cocaine addiction, which may contribute to a vulnerability to the development of drug-related disorders. As potential brain level biomarkers of chronic cocaine use, these identified resting-state connectivity within a network and connectivity between brain regions from different networks may be usefully applied in prevention and treatment development. These results suggest that it is necessary to regulate behavior (i.e., increase self-control), and necessary to regulate functional connectivity between the frontal–temporal and the frontal–parietal brain regions.
Finally, we need to consider a few caveats while considering the results of the present study. First, although we made every effort to match the cocaine and control groups in terms of their age, educational and ethnic/racial background, it was not possible to match them exactly in terms of their alcohol use frequency (see Table 1). Second, our analysis to examine group-level differences for inter-network connectivity strength between different ROI pairs across cocaine users and controls were not corrected for multiple comparisons due to small sample size. Third, there were not enough female cocaine smokers (n=5) to examine the influence of sex on functional connectivity between brain regions in chronic users of cocaine during rest. In addition, in the current study, a high correlation was observed between the cocaine use measures (cocaine use frequency and cocaine use duration) and the age of the participants. Hence, the correlation between the cocaine use measures and the connectivity strength may also be explained by the age of the cocaine users. Although, the connectivity strength differences observed in the current study was derived based on comparison with age, education, and ethnicity-matched controls, further studies are warranted to examine the effect of cocaine use measures on connectivity strength while accounting for changes in participants' age. Future studies need to examine these important research questions. Finally, although during debriefing, participants confirmed that they followed the study instruction to keep their eyes open and visually fixate on the fixation cross, we did not use an eye tracker to ensure that they were actually awake during the resting-state scan. Despite these limitations, the results of the present study provide a model of the intra-network and inter-network functional connectivity between brain regions in nontreatment-seeking chronic smokers of cocaine who had no active cocaine metabolites in their system during the resting-state scan.
Conclusions
Cocaine smokers showed an enhanced intra-network RSFC within the sensory motor cortex and within the left frontal–parietal network compared to controls. Also, the inter-network RSFC between brain regions showed that cocaine smokers had a significantly increased RSFC between interhemispheric frontal–temporal and intrahemispheric frontal–parietal brain regions, and a decreased RSFC between intrahemispheric parietal–parietal, intra and interhemispheric occipital–limbic, occipital–occipital, and interhemispheric occipital–parietal brain regions compared to controls. The present study demonstrated that the intra-network connectivity strength of the sensory motor cortex was negatively correlated, and the inter-network connectivity strength between occipital–limbic brain regions was positively correlated with years of cocaine use in abstinent cocaine users. Also, the inter-network connectivity strength within occipital brain regions was negatively related to cocaine use frequency and money spent on cocaine per week in abstinent cocaine users. These findings indicate a chronic cocaine user's weakened strength of cognitive control (Baler and Volkow, 2006). Results have important implications for prevention and treatment development.
Supplementary Material
Acknowledgments
We would like to thank Aradhana Srinagesh, Ashley Aya, Brian Foster, and Leila Hazavei for assistance with subject recruitment, data collection, and behavioral data coding. This research was supported by a National Institute on Drug Abuse grant (K01DA029047) to Dr. Suchismita Ray. The NIH had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Author Disclosure Statement
No competing financial interests exist.
For histogram of cocaine use duration (in years) for study participants, see the Supplementary Fig. S1. (Supplementary Data are available online at www.liebertpub.com/brain)
References
- Aharonovich E, Nunes E, Hasin D. 2003. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend 71:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. 1991. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci 57:73–79 [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. 2006. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med 12:559–566 [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. 2005. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T, Smith H, Nader M, Porrino L. 2005. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology 180:781–788 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, et al. . 2010. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland S, Taha S, Sarti F, Fields H, Bonci A. 2006. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601 [DOI] [PubMed] [Google Scholar]
- Camchong J, MacDonald III AW, Nelson B, Bell C, Mueller BA. 2011. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry 69:1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, James JA. et al. . 2013. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res 213:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Hyde J. Software tools for analysis and visualization of FMRI Data. 1997. NMR Biomed 10:171–178 [DOI] [PubMed] [Google Scholar]
- Cunningham K, Paris J, Goeders N. 1992. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse 11:112–123 [DOI] [PubMed] [Google Scholar]
- DiSclafani V, Tolou-Shams M, Price LJ, Fein G. 2002. Neuropsychological performance of individuals dependent on crack-cocaine, or crackcocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend 66:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Gohel S, Kim E, Biswal B. 2013. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front Hum Neurosci 7:493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Oropilla G, Gustavson A, Speck O. 2000. Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Res 99:63–74 [DOI] [PubMed] [Google Scholar]
- Eryilmaz H, Van De Ville D, Schwartz S, Vuilleumier P. 2011. Impact of transient emotions on functional connectivity during subsequent resting state: a wavelet correlation approach. Neuroimage 54:2481–2491 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 1997. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0, 4/97 revision)
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA. et al. . 2002. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 51:134–142 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS. et al. . 2004. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia 42:1447–1458 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C. et al. . 1996. Activation of memory circuits during cue-elicited cocaine craving. Proc Natil Acad Sci U S A 93:12040–12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron JB, Ross JT, Geng X, Zhan W, Stein EA, Yang Y. 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann C, Behrens T, Woolrich M, Smith S. 2012. FSL. Neuroimage 62:782–790 [DOI] [PubMed] [Google Scholar]
- Kelly C, Garavan H. 2005. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15:1089–1102 [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo X, Gotimer K, Cox CL, Lynch L, Brock D. et al. . 2011. Reduced interhemispheric resting state functional connectivity in cocaine users. Biol Psychiatry 69:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. 2013. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry 70:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth D, Cadet JL, Bolla KI. 2003. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage 19:1095–1102 [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Salmeron BJ, Devous Sr MD, Stein EA, Adinoff B. 2014. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. FPSYT 5:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V. Davis KL. et al. . 1996. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 15:17–29 [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202 [DOI] [PubMed] [Google Scholar]
- Miller WM, Powrozek TA, Vogt BA. 2009. Dopamine systems in the cingulate gyrus: organization, development, and neurotoxic vulnerability. In: Vogt BA. (ed.) Cingulate Neurobiology and Disease, 1st ed. Oxford: Oxford University Press; p. 164 [Google Scholar]
- Piggott MA, Ballard CG, Rowan E, Holmes C, McKeith IG. et al. . 2007. Selective loss of dopamine D2 receptors in temporal cortex in dementia with Lewy bodies, association with cognitive decline. Synapse 61:903–911 [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Hanson SJ, Glymour C. 2011. Multi-subject search correctly identifies causal connections and most causal directions in the DCM models of the Smith et al. simulation study. Neuroimage 58:838–848 [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. 1991. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251:947–950 [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. 2008. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage 43:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Gohel S, Di X, Walter M, Biswal BB. 2012. Functional covariance networks: obtaining resting-state networks from intersubject variability. Brain Connect. 2:203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini V, Cacciapaglia F, Frau R, DiChiara G. 2006. Differential alpha-mediated inhibition of dopamine and noradrenaline release in the parietal and occipital cortex following noradrenaline transporter blockade. J Neurochem 98:113–121 [DOI] [PubMed] [Google Scholar]
- Van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD. et al. . 1999. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiatry 56:85–89 [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Contreras-Rodríguez O, Fonseca F, Cuenca A, Soriano-Mass C, Rodriguez J. et al. . 2014. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict Biol 19:272–81 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. 2002. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn Mem. 78:610–624 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson J, Telang F. 2007. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64:1575–1579 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN. 1997. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830 [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR. et al. . 1999. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64:775–784 [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. 2011. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 115:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.