Abstract
Proteomic analysis of the mouse photoreceptor sensory cilium identified a set of cilia proteins, including Poc1 centriolar protein b (Poc1b). Previous functional studies in human cells and zebrafish embryos implicated that Poc1b plays important roles in centriole duplication and length control, as well as ciliogenesis. To study the function of Poc1b in photoreceptor sensory cilia and other primary cilia, we expressed a tagged recombinant Poc1b protein in cultured renal epithelial cells and rat retina. Poc1b was localized to the centrioles and spindle bundles during cell cycle progression, and to the basal body of photoreceptor sensory cilia. A morpholino knockdown and complementation assay of poc1b in zebrafish showed that loss of poc1b led to a range of morphological anomalies of cilia commonly associated with human ciliopathies. In the retina, the development of retinal laminae was significantly delayed and the length of photoreceptor outer segments was shortened. Visual behavior studies revealed impaired visual function in the poc1b morphants. In addition, ciliopathy-associated developmental defects, such as small eyes, curved body axis, heart defects, and shortened cilia in Kupffer's vesicle, were observed as well. These data suggest that poc1b is required for normal development and ciliogenesis of retinal photoreceptor sensory cilia and other cilia. Furthermore, this conclusion is supported by recent findings that mutations in POC1B gene have been identified in patients with inherited retinal dystrophy and syndromic retinal ciliopathy.
Keywords: poc1b, Photoreceptor sensory cilia (PSC), Basal body, Ciliopathy, Zebrafish, Inherited retinal degenerations (IRDs)
1. Introduction
Inherited retinal degenerations (IRDs), such as retinitis pigmentosa (RP), cone or cone-rod dystrophy (CRD), and Leber congenital amaurosis (LCA), are important causes of blindness that are characterized by progressive cell death of rod and cone photoreceptor cells in the retina. IRDs are among the most genetically heterogeneous groups of disorders known, involving over 230 disease genes (https://sph.uth.edu/RetNet). Many of the identified IRD genes encode proteins that are essential for formation, maintenance and function of primary cilia [1]. As primary cilia are present on almost all cell types of human body [2], mutations in cilia genes can lead to a range of multisystem disorders, known as ciliopathies, that include retinal degeneration, cystic renal disease, polydactyly, mental retardation, obesity and diabetes, gonadal malformations, and situs inversus [1].
The primary cilium is a microtubule-based organelle that projects from the surface of the cell into the extracellular space. It is composed of a basal body, transition zone, axoneme, ciliary membrane and cilia tip [3–5]. Primary cilia have been described as mechanosensors and chemosensors that are involved in many aspects of sensation, including flow force, light, smell and hearing. In addition, primary cilia are essential for several signaling pathways such as Hedgehog and Wnt signaling [6,7]. The outer segments elaborated by rod and cone photoreceptor cells of the retina are specialized primary cilia, known as photoreceptor sensory cilia (PSC) [8,9]. Similar to other cilia, the photoreceptor sensory cilium contains a microtubule-based axonemal backbone, which anchors to the basal bodies in the inner segment, passes through a transition zone (also called the connecting cilium) and extends into the outer segment [10]. A number of proteins have been identified to be components of the cytoskeletons of PSC, primarily through studies of proteins produced by IRD and ciliopathic genes [11,12]. These proteins are often involved in transport activities between the cell body and cilia [11,12]. A comprehensive proteomic analysis of the PSC complex has revealed many known and novel cilia proteins not previously identified in photoreceptor cells, including almost all BBSome proteins, IFT proteins, and centrosome proteins [10]. Since at least one third of the proteins produced by IRD genes are part of PSC [9], we hypothesize that genes encoding proteins localized to the PSC are good candidate disease genes for retinal degeneration and/or related ciliopathies. Support for this concept is provided by the observation that a majority of the newly identified IRD and/or ciliopathy disease genes encode proteins that are detected in the PSC proteome (https://sph.uth.edu/RetNet).
First found in the alga Chlamydomonas in centriole proteomics study [13], poc1b is one of the most abundant proteins in centriole proteomics, and has a highly conserved WD40 domain. In 2009, Pearson et al. found that Poc1b was required for the structural maintenance of centrioles in both invertebrate and vertebrate primary cilia [14]. In this study, we selected Poc1b, one of the cilia proteins in the PSC proteome, to investigate its role in photoreceptor and other cilia biology. While we were completing the experiments described here, three studies reported that POC1B gene is indeed a disease gene for recessive retinal degeneration and severe syndromic ciliopathy [15–17]. Thus, our data support POC1B function in primary cilia and photoreceptor cells.
2. Materials and methods
2.1. Plasmid construct
Human POC1B ORF Gateway™ pENTR(tm)221 vector was obtained from the Ultimate ™ ORF Clones collection (Invitrogen). Mouse Poc1b cDNA was RT-PCR-amplified from mouse retina and cloned into pENTR/D-TOPO entry vector. The coding sequences of human POC1B and mouse Poc1b in the entry vector was moved into the Gateway destination expression vector pCAG-ORF-IRES-EGFP containing V5 or Flag epitope tag in-frame using recombination mediated by LR clonase II (Life Technologies) to generate V5 or Flag tagged hPOC1B or mPoc1b expression plasmids. Plasmid DNAs were purified using the EndoFree plasmid maxi kit (Qiagen) and verified by direct DNA sequencing. Three mouse Poc1b shRNA sequences (shRNA-1, shRNA-2, shRNA-3) were selected using the RNAi Central website (Cold Spring Harbor, http://hannonlab.cshl.edu). The shRNA oligos were synthesized at IDT (Coralville, IA) and then PCR-amplified and cloned into a pCAG-miR30-IRES-EGFP vector [18,19].
2.2. RT- PCR
The poc1b RNA expression level in zebrafish was evaluated using RT-PCR. Zebrafish embryos (both standard control and morphant group) at different stages during 120 h post fertilization were used for total RNA extraction. Isolated total RNA was reverse-transcribed using the superscript first-strand synthesis system and PCR amplified according to standard protocol from Invitrogen.
2.3. Cell culture and immunofluorescence microscopy
A mIMCD3 stable cell line expressing SSTR3-EGFP, a ciliary membrane marker (gift of Gregory J. Pazour, University of Massachusetts Medical School) was used for Poc1b subcellular localization. A wild-type mIMCD3 cell line from ATCC was used for phenotypic analysis of Poc1b shRNA knockdown assay. The mIMCD3 cells were maintained in DMEM:F12 media supplemented with 10% fetal bovine serum (FBS) and 0.5 mM sodium pyruvate (Invitrogen). Expression plasmids (Flag-Poc1b or V5-Poc1b) and shRNA knockdown plasmids (Poc1b shRNA-1, shRNA-2, shRNA-3) were transfected using Lipofectamine 2000 or Lipofectamine LTX reagent. The cells were processed for immunocytochemistry analysis at 72 h after transfection as described previously [19]. Rabbit anti-mouse Poc1b (Novus), mouse anti-V5 (Invitrogen), mouse anti-Flag, rabbit anti-ɤ-tubulin and mouse anti-acetylated α-tubulin (Sigma) antibodies were used in this study. Fluorescence signals were visualized using a Nikon Ti-E fluorescence microscope and images were analyzed using NIS-Elements software (Nikon).
2.4. Western blot
To verify the knockdown efficiency of the mouse Poc1b shRNAs, 2 μg of V5-Poc1b plasmid with each of the three Poc1b shRNA or shRNA control constructs were co-transfected in CHO cells using Lipofectamine 2000 (Invitrogen). Two days after transfection, total protein was extracted using LDS sample buffer (Invitrogen). Proteins were separated on a NuPAGE Gel and the V5-Poc1b recombinant protein was detected using anti-v5 antibody. The expression levels of Poc1b were quantified using Image Studio Lite Software from LI-COR.
2.5. Animals
The animal work described in this study has been carried out in accordance with EU Directive 2010/63/EU for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm) and the Massachusetts Eye and Ear Infirmary Guidelines for Animal Care and Use, and was specifically approved by Institutional Animal Care and Use committees at the Massachusetts Eye and Ear Infirmary. Timed pregnant Sprague–Dawley rats were purchased from Charles River Laboratories. Wild-type adult zebrafish of the AB strain were obtained from Zebrafish International Resource Center (ZIRC, Eugene, OR) and were maintained at the Zebrafish facility at the Massachusetts Eye and Ear Infirmary as described in the Zebrafish Book [20].
2.6. Sub-retinal injection and in vivo electroporation
To localize POC1B in photoreceptor cells, pCAG-V5-POC1B-IRES-EGFP expression plasmid was injected into the sub-retinal space of the eyes at P0–P1. Injected plasmid was electroporated into retinal cells using tweezer-type electrodes as described previously [21]. Retinas were dissected 3 weeks following injection, and frozen sections of retinas were stained using anti-V5 antibody to localize the recombinant POC1B protein.
2.7. Morpholino injection of zebrafish embryo
The morpholino (MO) injection of zebrafish embryo protocol was based on our previous study [19]. Three morpholino oligos were designed (MO-TB, block poc1b RNA translation, 5’-TCCTCCATTACAGACGCCATGATTC-3’; MO-SP, block splicing, 5’-ACTAAATCATCTTACCATTACAGAC-3’; control, 5’-CCTCTTACCTCAGTTACAATTTATA-3’) and synthesized from Gene Tools (Philomath, OR). For rescue experiments, human POC1B cDNA was amplified by PCR, cloned into the pCS2P + vector and linearized with SnaBI. Capped human POC1B mRNA was synthesized in vitro using mMESSAGE mMACHINE® SP6 Transcription Kit (Ambion) and purified by the RNeasy Plus micro kit (Qiagen). 0.3–0.5 mM MO were injected alone or with rescue mRNA (100–300 ng/embryo) into embryos at 1–2 cell stages. Injected embryos were allowed to develop at 28.5 °C for 2 days and imaged with a Nikon SMZ-1500 stereomicroscope. The defect rates of each group were calculated at 2 days post fertilization (dpf). Zebrafish larvae at 3 dpf and 5 dpf were processed for histology. Semi-thin sections (1 μm) were cut and stained with alkaline toluidine blue for light microscopy.
2.8. Optokinetic response analysis (OKR)
Vision of zebrafish larvae in standard control and morphants was evaluated on 5 dpf. The larvae were embedded in 6% prewarmed (28 °C) methylcellulose to prevent body movements with dorsal side up, and placed within a drum lined with black and white stripes, 1 cm in width. The drum was illuminated with a white light source and rotated at 5 rpm. The OKR response was recorded at 4 frames/second for 1.5 min with direction changed at a 30-s interval [22]. The frequency and degree of the full smooth pursuit–saccade cycles in both the directions was recorded and analyzed using ImageJ software (Fig. 3D); and the data were processed and graphed with Metalab 2013.
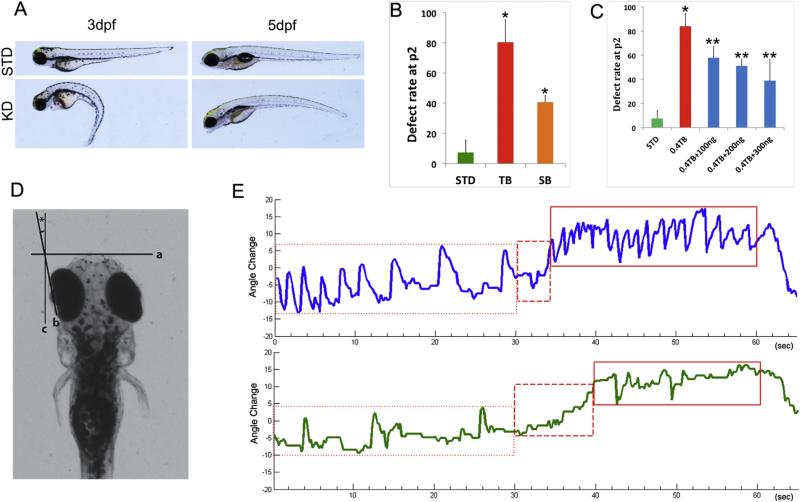
Fig. 3.
Knockdown of poc1b causes visual impairment in zebrafish. (A) Representative lateral view of 3 and 5 dpf larvae injected with poc1b morpholino. (B) The phenotypic defect rates of zebrafish injected with TB-MO (n = 153) and SB-MO (n = 40) were calculated at 2 dpf. The abnormality rate is more than 80% in the embryos injected with TB-MO, which is significantly higher than that in the controls (n = 253) (p < 0.01). Reproducibly phenotypic defects in morphants injected with SB-MO was also significantly increased (p < 0.05), but with less severity. (C) The defect rates were rescued when co-injected with indicated dose of wild-type mRNA (100, 200 or 300 ng/embryo), in poc1b TB morphants (n = 3) as marked (**p < 0.05). (D) Schematic diagram of eye movement measurement. Two points in the zebrafish larvae eye creates an axis line (b) that can decide the angle of eye movement. (E) An example of the OKR responses from poc1b morphants (green) and their STD control fish (blue) were displayed.
2.9. Statistical analysis
Statistical analyses were performed with SPSS19.0. For the cilia length data analyses, Student's t test with unequal variance was used. For analysis of the defect rate of zebrafish larvae, both Students' t test with unequal variance and chi-square test were used.
3. Results
3.1. Poc1b is evolutionarily conserved, stably expressed and located at the basal body of primary cilia
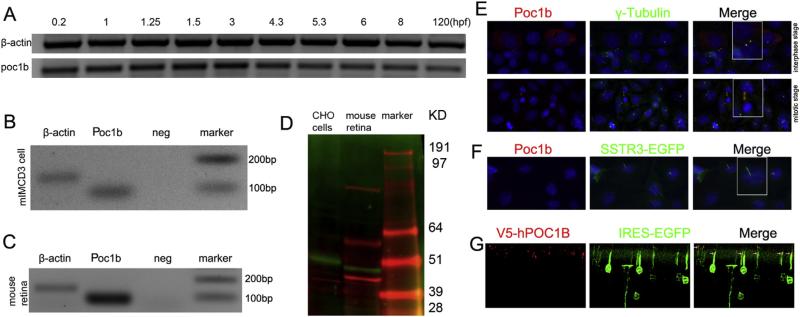
Alignment of amino acid sequences revealed a high degree of conservation among different species. For example, the similarities between human and zebrafish, mouse or rat Poc1b protein are 67%, 77% and 84%, respectively. Importantly, human, mouse and zebrafish poc1b proteins all share the conserved protein domains, including a WD40-repeat domain at the N-terminus and a coiled coil located at the C-terminus. poc1b developmental expression profile was first assessed at the transcript level in zebrafish. RT-PCR results of the zebrafish embryos demonstrated that poc1b was stably expressed during 0–120 h post fertilization (hpf) in zebrafish (Fig. 1A), implying the maternal transcript and functional importance in cell cleavage period. We confirmed the expression of Poc1b mRNA in mIMCD3 cells and mouse retina (Fig. 1B and C). Further, we determined by Western blot the expression of Poc1b protein in the mouse retina, using recombinant Poc1b protein (green band) expressed in Chinese Hamster Ovary (CHO) cells as a positive control (Fig. 1D). Two additional molecular sizes of proteins (the top 2 red bands) were detected, which may indicate non-specific proteins. As most of cilia proteins containing WD40 domain are localized in the centriole or basal body, the association of Poc1b with centriole or the basal body of cilium was determined by immunostaining. Interestingly, Poc1b localization was dependent upon cell-cycle stages. During the interphase, Poc1b localized in the centrioles (mother and daughter) and co-localized with γ–tubulin (Fig. 1E), whereas during the mitosis stage, Poc1b localized in the mitotic spindle instead. When cells exited cell cycle and cilia were formed, Poc1b is located at the daughter centriole and basal body of the cilium, which was highlighted by the green SSTR3-EGFP fluorescent protein (Fig. 1F). In the retina, recombinant POC1B was localized to the basal body at the tip of the inner segment of photoreceptor cells that were transfected with the V5-POC1B construct by sub-retinal injection and 1in vivo electroporation (Fig. 1G). Taken together, Poc1b is a centriole and basal body protein in the photoreceptor sensory cilium and primary cilium.
Fig. 1.
Poc1b is constitutively expressed and localized at the centrioles/basal body of primary cilia. (A) RT-PCR from zebrafish embryos demonstrated stable transcription of poc1b during 0–120 hpf. (B, C) Expression of Poc1b in mIMCD3 cell and mouse retina at transcripts level. (D) Expression of Poc1b protein was detected by anti-V5 (green) or anti-Poc1b (red) antibodies in total protein extraction from transfected CHO cell or mouse retina. (E) Localization of Poc1b in mIMCD3 cells. During interphase, Poc1b co-localized with the centrioles marker γ–tubulin (green) in both of two centrioles (red); when cells enter mitotic stage, Poc1b expressed in the spindle (red), and do not co-localized with the γ–tubulin (green). (F) Poc1b (red) was located at the basal body of cilium, next to the green cilium labeled by SSTR3-GFP. (G) Recombinant human POC1B localized in the basal body of the mouse photoreceptors (red). Green fluorescence indicated the express of GPF in the inner segments and outer nuclear layer of positively transfected cells with pCAG-V5-POC1B-IRES-EGFP.
3.2. Poc1b is critical for primary cilia formation in cultured kidney epithelial cells
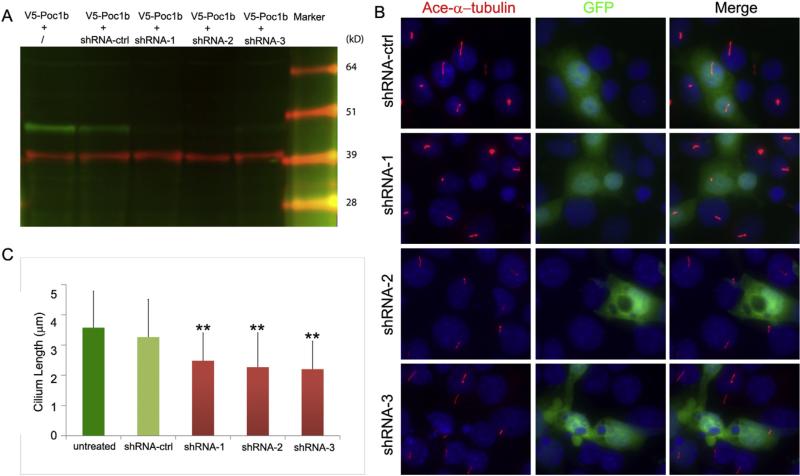
The identification of Poc1b as a centriole/basal body protein suggests that it may play an important role in cilia formation and function. To determine how depletion of Poc1b affects the ciliogenesis, we generated three short hairpin RNA (shRNA) plasmids (Poc1b-shRNA-1, -2 and -3) targeting mouse Poc1b and evaluated their knockdown efficiency on the Poc1b in CHO cells expressing a recombinant V5-Poc1b protein. As shown in Fig. 2A, the expression of V5-Poc1b (green band) was significantly suppressed when cells were co-transfected with Poc1b-shRNAs. In contrast, the expression of the internal control of β actin was not changed (red) (Fig. 2A). The quantitative data of the expression level was not shown. Further, we used serum starved mIMCD3 cells to test the effect of shRNA-mediated knockdown of Poc1b on ciliogenesis. As shown in Fig. 2B, the formation of the cilia was observed in mIMCD3 cells transfected with each of the three Poc1b-shRNAs and control shRNA (labeled by anti-acetylated α-tubulin antibody, red) (Fig. 2B). We then quantitatively analyzed the cilia lengths in approximately one hundred shRNA-transfected cells and four non-transfected surrounding cells. The mean cilia lengths showed no significant difference between non-transfected cells and control shRNA transfected cells (p > 0.05). In contrast, cilia lengths in all three Poc1b-shRNA transfected groups were significantly shorter than that in the negative control group (**p < 0.05).
Fig. 2.
shRNA knockdown of Poc1b in mIMCD3 cell causes shortened cilia. (A) Co-expression of a Poc1b-V5 plasmid with three Poc1b shRNA plasmids (shRNA-1, -2, and -3) in CHO cells. Poc1b protein (green bands) and internal control (red bands) were detected by anti-V5 and anti-b-actin. (B, C) Ciliogenesis was assessed by measuring the lengths of cilia marked with anti-acetylated a-tubulin antibody (red) in the transfected IMCD3 cells co-expressing an IRES-EGFP protein (green). The cilia lengths in shRNA-transfected cells were significantly shorter than that in negative control shRNA group and non-transfected group (**p < 0.05); while there was no significant difference of the cilia lengths between non-transfected group and negative control group (p > 0.05).
3.3. Knockdown of poc1b causes defects in visual behavior of zebrafish
To elucidate the function of poc1b in vision, both poc1b translation block morpholino (TB-MO) and splicing block morpholino (SB-MO) were injected into zebrafish embryos at 1–2 cell stages. Several gross defects could be observed in the poc1b-MO treated fish, including small eyes, small body, curved tail and heart defects (Fig. 3A). Survival rate and defect rate of zebrafish larvae in the first 2 days were calculated, and there is no significant difference in survival rate between the standard control and morpholino knockdown group; but the defect rate was much higher in the morpholino knockdown group than the standard control group (*p < 0.05) (Fig. 3B). The specificity of the phenotypic defects observed in the morphants was further confirmed by a complementation assay. The phenotypic defects in poc1b morphants were partially rescued in embryos co-injected with the synthetic zebrafish mRNA (100–300 ng/embryo) and TB-MO (**p < 0.05) (Fig. 3C). We further evaluated the visual behavior of larvae by testing the optokinetic responses (OKR) at 5 dpf larvae [22]. The OKR data were collected from approximately 10 individuals each at three different experiments. The poc1b morphants moved their eyes less robustly and less frequently than control MO fish in the OKR assay (Fig. 3D, E). An example of the OKR responses from poc1b morphants and their control fish were displayed in Fig. 3E. The maximum angle change (degree) of the eye movements in poc1b morphants was significantly reduced (data not shown) (Fig. 3D). The average frequency of the smooth pursuit–saccade cycles in poc1b morphants (6.8 ± 5.4 saccades/min) was significantly less than that in control larvae (29 ± 9.1 saccades/min). The finding of aberrant visual behavior in poc1b morphants is consistent with other studies [15], indicating that Poc1b protein has an important role in photoreceptor function and that its dysfunction can lead to visual impairment.
3.4. Knockdown of poc1b disrupts the development of the retina
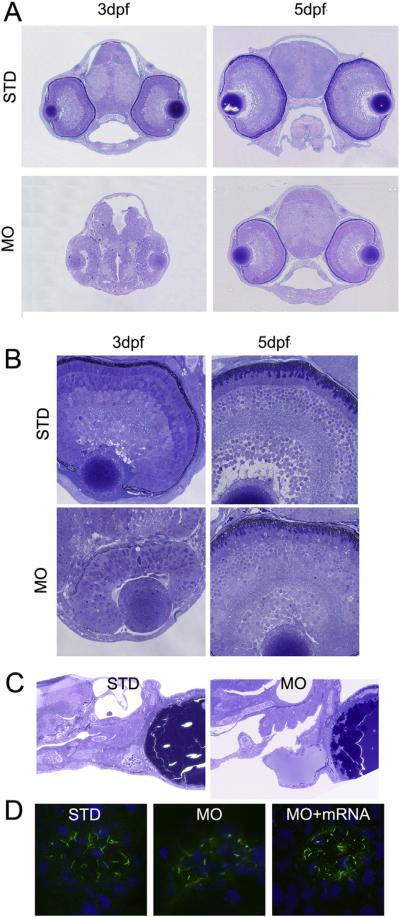
Given the vision behavioral defects discovered in the poc1b morphants, histological examination of poc1b-MO treated zebrafish was conducted to elucidate if poc1b is required for normal eye development. At both 3 and 5 dpf, whole head sections through coronal planes revealed a significantly smaller head, eye and brain in the poc1b morphants (Fig. 4A). Sections of the retinal histology demonstrated delayed development of the retina in the poc1b morphants. The retinas in poc1b morphants were not laminated and lacked any neuronal organization at 3 dpf. At 5 dpf, shortened and disorganized outer segments can be observed in the poc1b morphants (Fig. 4B). In the control larvae, the five principal laminaes of retina (three cellular and two plexiform layers) are differentiated and the photoreceptor outer segments start to appear at 3 dpf, which were later fully formed with well-defined outer segments and inner segments at 5 dpf (Fig. 4B). The delayed lamination at 3 dpf and almost normal lamination at 5 dpf in poc1b morphants was probably due to the loss of poc1b-MO potency on suppressing the differentiation of retinal cells after 3 days, as MO function usually lasts only 1–3 days. Thus, knockdown of poc1b in zebrafish leads to delayed body development in general and abnormal photoreceptor development in particular (Fig. 4B).
Fig. 4.
Knockdown of poc1b disrupts the morphogenesis of cilia in retina, heart and Kupffer's vesicle. (A) The whole head histology through coronal planes at 3 dpf and 5 dpf fish was illustrated. The head and brain size in poc1b morphants were approximately one-third of the size in control fish; and the size of eye was moderately smaller, in comparison to control. (B) The higher magnification views of the morphant retinas revealed no clear retina lamination at 3 dpf, but shortened and disorganized photoreceptor outer segments at 5 dpf in the retinas of poc1b morphants, with otherwise clear but thinner laminae. (C) In the control group, heart atrium and ventricle were developed normally with normal connection between them. However, in poc1b TB morphants, zebrafish larvae presented dilated ventricle, which have no proper connect with the atrium. (D) The Kupffer's vesicle cilia stained by acetylated alpha tubulin antibody in green were shortened and the morphology of the cilia was disrupted in poc1b TB morphants (middle panel). When co-inject poc1b MO with normal POC1B mRNA, the Kupffer's vesicle cilia disruption was partially rescued (right panel).
3.5. Knockdown of poc1b causes heart defects and abnormal Kupffer's vesicle
To determine the possible ciliopathy-related defects in the cilia of other organs, we examined the heart and Kupffer's vesicle morphology. Zebrafish larvae at 5 dpf presented with a pericardial effusion in poc1b MO treated group compared to the standard controls. In addition, heart rate was significantly decreased (69.41 ± 26.71/min) compared to control group (99.75 ± 3.45/min). The structure of the heart was also significantly disrupted with dilated ventricles, which have no proper connection with the atrium (Fig. 4C). Previous studies demonstrated that genes that have an impact on the ciliogenesis could disturb the Wnt signaling pathway, which is critical to the development of heart and left-right patterning [15]. Kupffer's vesicle morphogenesis appeared normal in MO injected embryos. However, immunostaining of the motile cilia in Kupffer's vesicle demonstrated shortened cilia length as compared with controls, and this abnormal cilia morphogenesis can be partially rescued by wild type POC1B mRNA (Fig. 4D).
4. Discussion
The study reported here was based on the hypothesis that the WD40 repeat–containing protein Poc1b detected in the PSC proteome may play an important role in the biology and function of cilia, including photoreceptors. Expression of the Poc1b protein in cultured renal epithelial cells and rat photoreceptor cells resulted in its defined subcellular location at the basal body of cilia. Many proteins produced by inherited retinal and ciliopathy disease genes localize in these regions, suggesting that the Poc1b protein could have roles in ciliogenesis and/or maintenance [6,7]. Interestingly, the location of Poc1b is dynamically changed at different stages of cell cycle. It localizes at the mitotic spindles in proliferating cells and the centrioles in interphase cells. This indicates that Poc1b may have a distinct function in the cell cycle and proliferation that contributes to the body development in general and retinal lamination in the eye. Furthermore, the presence of Poc1b at both the basal body and daughter centriole in the post-mitotic photoreceptor cells and ciliated mIMCD3 cells suggests that Poc1b may function as a structural element in supporting ciliogenesis and maintenance of the PSC.
Further studies in zebrafish revealed that loss of poc1B led to a spectrum of cilia morphological anomalies commonly associated with human ciliopathies. In retina, the development of retinal lamination was significantly delayed when expression of poc1b was suppressed by MO, an indication of defect in retinal cell proliferation/differentiation. The length of photoreceptor outer segments in the larval retina is reduced, suggesting that poc1b is functional in both rod and cone photoreceptor cells. Consistent with the morphologic abnormalities, the visual function was impaired, as illustrated by the disrupted OKR response in the poc1b morphants (Fig. 3). Knockdown studies also showed that poc1b is necessary for ciliogenesis in other cilia. Typical ciliopathy-associated developmental defects, such as small eyes, curved body axis, heart defect, and shortened cilia in Kupffer's vesicle, were observed in poc1b morphant zebrafish (Fig. 4) as well. Rescue of these phenotypes associated with ciliogenesis was achieved with normal human POC1B mRNA. Taken together, our data indicate that poc1b plays an essential role in ciliogenesis and function in primary cilia and photoreceptor cells in zebrafish; and thus, disruption of POC1B in human may cause ciliopathy-related defects. Indeed, the latter has been evidenced by recent findings that mutations in POC1B gene cause autosomal recessive cone-rod dystrophy and syndromic retinal ciliopathy [15–17].
Cilia proteins located in mother centriole/basal body have been reported to play distinct roles during different stages of ciliogenesis. For example, an apical domain of the mother centriole named Cep164 is found to take part in the docking process to the membrane [23]; Cep135 specifically localizes at the basal body and is required for stabilization of the basal body [24]; FOR20 is required for the assembly of the transition zone in cilia [25]. Poc1b has been shown to act with Poc1a, one of the two isoforms of Poc1 protein, at the centriole/basal body to ensure centriole integrity in human cells [26]. In the retina, FAM161A was found to be a binary interaction partner of POC1B, and their interaction was disrupted when mutant POC1B was present [16]. As a RP disease gene, FAM161A has been found to interact with several other ciliopathic genes/proteins as well, including lebercilin, CEP290, OFD1 and SDCCAG8 [27,28]. These studies suggest that, through interacting with other basal body or centriole proteins, Poc1b may provide a molecular link between the assembly and stability maintaining of the basal body during ciliogenesis. Further studies are warranted to elucidate the biologic function of the POC1B in cilia and the mechanism by which mutations in POC1B lead to cilia dysfunction in the retina and other tissues.
Acknowledgments
We thank the morphology core facility in Massachusetts Eye and Ear Infirmary for assistance with zebrafish histology. This work was supported by the grants from the Foundation Fighting Blindness USA (BR-GE-0911-0539-MEEI, Q.L.); the Curing Kids Fund (Q.L.); the Research to Prevent Blindness (unrestricted funds to MEEI-Department of Ophthalmology, QL); the Department Fund of Massachusetts Eye and Ear Infirmary (Q.L.); and the National Institutes of Health [P30EY014104 (MEEI core support)].
Footnotes
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2015.06.083.
References
- 1.Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia. 2012;1:2046–2530. doi: 10.1186/2046-2530-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S, Dynlacht BD. Assembling a primary cilium. Curr. Opin. Cell Biol. 2013;25:506–511. doi: 10.1016/j.ceb.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuno N, Taschner M, Engel BD, Lorentzen E. Structural studies of ciliary components. J. Mol. Biol. 2012;422:163–180. doi: 10.1016/j.jmb.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 2009;19:220–229. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol. Cell. Biol. 2005;25:4129–4137. doi: 10.1128/MCB.25.10.4129-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrada-Cuzcano A, Roepman R, Cremers FP, den Hollander AI, Mans DA. Non-syndromic retinal ciliopathies: translating gene discovery into therapy. Hum. Mol. Genet. 2012;21:R111–R124. doi: 10.1093/hmg/dds298. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr., Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol. Cell. Proteomics MCP. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna, nature reviews. Mol. Cell. Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 12.Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J. The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell Biol. 2012;14:950–957. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller LC, Romijn EP, Zamora I, Yates JR, 3rd, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr. Biol. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Pearson CG, Osborn DP, Giddings TH, Jr., Beales PL, Winey M. Basal body stability and ciliogenesis requires the conserved component Poc1. J. Cell Biol. 2009;187:905–920. doi: 10.1083/jcb.200908019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roosing S, Lamers IJ, de Vrieze E, van den Born LI, Lambertus S, Arts HH, Peters TA, Hoyng CB, Kremer H, Hetterschijt L, Letteboer SJ, van Wijk E, Roepman R, den Hollander AI, Cremers FP. Disruption of the basal body protein POC1B results in autosomal-recessive cone-rod dystrophy. Am. J. Hum. Genet. 2014;95:131–142. doi: 10.1016/j.ajhg.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durlu YK, Koroglu C, Tolun A. Novel recessive cone-rod dystrophy caused by POC1B mutation. JAMA Ophthalmol. 2014;132:1185–1191. doi: 10.1001/jamaophthalmol.2014.1658. [DOI] [PubMed] [Google Scholar]
- 17.Beck BB, Phillips JB, Bartram MP, Wegner J, Thoenes M, Pannes A, Sampson J, Heller R, Gobel H, Koerber F, Neugebauer A, Hedergott A, Nurnberg G, Nurnberg P, Thiele H, Altmuller J, Toliat MR, Staubach S, Boycott KM, Valente EM, Janecke AR, Eisenberger T, Bergmann C, Tebbe L, Wang Y, Wu Y, Fry AM, Westerfield M, Wolfrum U, Bolz HJ. Mutation of POC1B in a severe syndromic retinal ciliopathy. Hum. Mutat. 2014;35:1153–1162. doi: 10.1002/humu.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paddison PJ, Cleary M, Silva JM, Chang K, Sheth N, Sachidanandam R, Hannon GJ. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat. Methods. 2004;1:163–167. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Liu Q, Austin C, Drummond I, Pierce EA. Knockdown of ttc26 disrupts ciliogenesis of the photoreceptor cells and the pronephros in zebrafish. Mol. Biol. Cell. 2012;23:3069–3078. doi: 10.1091/mbc.E12-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerfield M. The Zebrafish Book, a Guide for the Laboratory Use of Zebrafish (Danio rerio) fifth ed. University of Oregon Press; Eugene: 2007. [Google Scholar]
- 21.Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YY, Neuhauss SC. The optokinetic response in zebrafish and its applications. Front. Biosci. J. Virtual Libr. 2008;13:1899–1916. doi: 10.2741/2810. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J. Cell Biol. 2012;199:1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayless BA, Giddings TH, Jr., Winey M, Pearson CG. Bld10/Cep135 stabilizes basal bodies to resist cilia-generated forces. Mol. Biol. Cell. 2012;23:4820–4832. doi: 10.1091/mbc.E12-08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aubusson-Fleury A, Lemullois M, de Loubresse NG, Laligne C, Cohen J, Rosnet O, Jerka-Dziadosz M, Beisson J, Koll F. The conserved centrosomal protein FOR20 is required for assembly of the transition zone and basal body docking at the cell surface. J. Cell Sci. 2012;125:4395–4404. doi: 10.1242/jcs.108639. [DOI] [PubMed] [Google Scholar]
- 26.Venoux M TX, Hames RS, Straatman KR, Woodland HR, Fry AM. Poc1A and Poc1B act together in human cells to ensure centriole integrity. J. Cell Sci. 2013;126:163–175. doi: 10.1242/jcs.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Gioia SA, Letteboer SJ, Kostic C, Bandah-Rozenfeld D, Hetterschijt L, Sharon D, Arsenijevic Y, Roepman R, Rivolta C. FAM161A, associated with retinitis pigmentosa, is a component of the cilia-basal body complex and interacts with proteins involved in ciliopathies. Hum. Mol. Genet. 2012;21:5174–5184. doi: 10.1093/hmg/dds368. [DOI] [PubMed] [Google Scholar]
- 28.Zach F, Grassmann F, Langmann T, Sorusch N, Wolfrum U, Stohr H. The retinitis pigmentosa 28 protein FAM161A is a novel ciliary protein involved in intermolecular protein interaction and microtubule association. Hum. Mol. Genet. 2012;21:4573–4586. doi: 10.1093/hmg/dds268. [DOI] [PubMed] [Google Scholar]