Abstract
Introduction
The Joint Commission Surgical Care Improvement Project (SCIP) includes performance measures aimed at reducing surgical site infections (SSI). One measure defines approved perioperative antibiotics for general operative procedures. However, there may be a subset of procedures not adequately covered with the use of approved antibiotics. We hypothesized that piperacillin-tazobactam is a more appropriate perioperative antibiotic for pancreaticoduodenectomy (PD).
Methods
In collaboration with hospital epidemiology and the Division of Infectious Diseases, we retrospectively reviewed records of 34 patients undergoing PD between March and May 2008 who received SCIP-approved perioperative antibiotics and calculated the SSI rate. After changing our perioperative antibiotic to piperacillin-tazobactam, we prospectively reviewed PDs performed between June 2008 and March 2009 and compared the SSI rates before and after the change.
Results
For 34 patients from March through May 2008, the SSI rate for PD was 32.4 per 100 cases. Common organisms from wound cultures were Enterobacter and Enterococcus (50.0% and 41.7%, respectively), and these were cefoxitin resistant. From June 2008 through March 2009, 106 PDs were performed. During this period, the SSI rate was 6.6 per 100 surgeries, 80% lower than during March through May 2008 (relative risk, 0.204; 95% confidence interval [CI], 0.086–0.485; P = .0004).
Conclusion
Use of piperacillin-tazobactam as a perioperative antibiotic in PD may reduce SSI compared with the use of SCIP-approved antibiotics. Continued evaluation of SCIP performance measures in relationship to patient outcomes is integral to sustained quality improvement.
Surgical site infection (SSI) is the second most common hospital-acquired infection after urinary tract infection, and the most common infection in surgical patients, with an incidence of about 1 of 24 patients undergoing an operation.1,2 The cost of SSIs is enormous, and nearly triples the dollar amount of postoperative care.3 SSI after pancreaticoduodenectomy (PD) has been reported to occur in between 6 and 17% of cases.4–15 A selection of these studies is summarized in Table I. Risk factors for SSI are numerous and include the general health of the patient (diabetes, obesity, smoking, nutritional status, American Society of Anesthesiology [ASA] score), operating time, blood loss, operative technique, and appropriate administration of antimicrobial prophylaxis, as well as the nature of the operation (clean, clean-contaminated, contaminated, gross spillage).16 An additional risk factor for SSI in the case of PD is the preoperative placement of a biliary stent or drain, attributable to the colonization of a foreign object.17
Table I.
Case series of pancreaticoduodenectomy (PD) and surgical site infection (SSI)
| Authors | Study year | N | Procedure | SSI % | Median age (range) | Antibiotic |
|---|---|---|---|---|---|---|
| Pisters et al5 (2001) | 1990–1999 | 300 | PD evaluating the effect of preoperative biliary decompression | 10.0 | 62 (8–92) | Second-generation cephalosporin with or without metronidazole; penicillin-allergic patients: Ciprofloxacin and metronidazole |
| Conlon et al2 (2001) | NR | 197 | PD and distal pancreatectomy evaluating the effect of intraperitoneal drainage | 10.2 | 68 (23–87) | Second-generation cephalosporin was the most commonly administered antibiotic |
| Kimura et al (2005) | 2001–2003 | 60 | PD and evaluating postoperative plasma level of IL-6 and IL-8 | 17 | 68 (36–84) | All patients received prophylactic antibiotics (cefmetazole sodium 2,000 mg/d) and gabexate mesilate at a dosage of 1,200 mg/d for 3 days after surgery. |
| Jethwa et al17 (2007) | 2000–2005 | 331 | HBP | 10.1 | NR | Antibiotic prophylaxis in gastrointestinal endoscopy. Clinical practice guidelines. British Society of Gastroenterology, 2007. |
| Kazanjian et al9 (2005) | 1988–2004 | 437 | PD | 8.2 | NR | NR |
| Ueno et al15 (2005) | 2002–2003 | 2,331 | PD (32.6%), PPPD (55.0%), and SSPPD (12.4%) | 6.8 | NR | 78% of institutions chose first- or second-generation cephalosporins; 22% of the institutions chose third-generation cephalosporins. |
| Yeo et al6 (2002) | 1996–2001 | 294 | PD and radical PD evaluating distal gastrectomy and extended retroperitoneal lymphadenectomy | 7.8 | 67 (NR) | NR |
| Kent et al12 (2012) | 2001–2009 | 550 | Elective pancreatic resection (PD + distal + central + other pancreatectomy) | 14 | NR | Cefazolin (2 g) for distal pancreatectomy and enucleations, and cefazolin (2 g)/metronidazole (500 mg) for PD. Penicillin-allergic patients: Clindamycin (600 mg) and gentamicin (2 mg/kg). |
| Cortes et al14 (2006) | 2002–2003 | 79 | PD evaluating the effect of bile contamination on immediate outcome | 11.4 | NR | Cefazolin |
| Rayes et al13 (2007) | NR | 80 | PPPD evaluating the effect of enteral nutrition and synbiotics on bacterial infection rates | 12.5 | NR | Cefuroxime (1.5 g) and metronidazole (500 mg) |
HBP, Hepatobiliary and pancreatic; Ig, immunoglobulin; PPPD, pylorus-preserving pancreaticoduodenectomy; NR, not recorded; SSPPD, subtotal stomach-preserving pancreaticoduodenectomy.
In 2003, the Centers for Medicare and Medicaid Services initiated a program to reduce preventable surgical complications including surgical site infections. This resulted in the creation of the Surgical Care Improvement Project (SCIP) measures.18 The 3 SCIP measures with regard to antibiotics and SSI prevention include (1) administration of antibiotics within 1 hour of incision time, (2) selection of appropriate antibiotic therapy, and (3) discontinuation of antibiotic within 24 hours after surgery end time (or 48 hours for cardiac procedures). The antibiotic recommendations classified by case-type are summarized in Table II. For non–penicillin-allergic patients, these include any of the following for colonic/abdominal surgery: Cefotetan, cefoxitin, cefazolin with metronidazole, or ampicillinsulbactam. These antibiotics have pharmacologic activity against microbes encountered in colonic surgery, namely gram-negative bacilli and anaerobes.
Table II.
Joint Commission Surgical Care Improvement Project-approved antibiotics based on surgery type
| Operative procedure | Approved antibiotic | Beta-lactam allergy |
|---|---|---|
| CABG, other cardiac or vascular | Cefazolin, cefuroxime, or vancomycin | Vancomycin or clindamycin |
| Hip/knee arthroplasty | Cefazolin, cefuroxime, or vancomycin | Vancomycin or clindamycin |
| Colon | Cefotetan, cefoxitin, ampicillin/sulbactam or ertapenem or cefazolin or cefuroxime + metronidazole | Clindamycin + aminoglycoside or clindamycin + quinolone or clindamcyin + aztreonam or metronidazole with aminoglycoside or metronidazole + quinolone |
| Hysterectomy | Cefotetan, cefazolin, cefoxtin, cefuroxime, or ampicillin/sulbactam | Clindamycin + aminoglycoside or clindamycin + quinolone or clindamcyin + aztreonam or metronidazole with aminoglycoside or metronidazole + quinolone |
CABG, Coronary artery bypass grafting.
Despite adherence to SCIP-guidelines at our own institution, the observation was made that there was a cluster of SSI cases after PD, for which antibiotics from the colon/abdominal category are used because there are no more specific guidelines. Thus, an investigation into the nature of the operations and bacterial cultures was initiated.
METHODS
Data collection
In collaboration with hospital epidemiology and the Division of Infectious Diseases, we retrospectively reviewed the records of consecutive patients undergoing PD between March and May 2008 and calculated the SSI rate. The wound culture results from patients with SSIs were reviewed, and based on organism identification and sensitivities the perioperative antibiotic for PD to was changed to piperacillin-tazobactam 4.5 g intravenously (or tigecycline 70 mg intravenously for those allergic to penicillin). After this change in June 2008, we prospectively reviewed consecutive PD cases performed between June 2008 and March 2009 and compared the procedure-specific SSI rates before and after the change. All operations were performed by the same 2 surgeons at the same large, academic hospital.
Demographic data and patient comorbidities were was recorded including the presence of obesity, body mass index, diabetes, smoking history, and immunosuppression, as well as preoperative albumin level, ASA score, operative time, and intraoperative blood loss. Use of preoperative biliary decompression with a stent or percutaneous transhepatic drain was also recorded.
SSI classification
We defined SSI according to CDC guidelines.2 Specifically, an SSI is defined as a wound infection that occurs within 30 days of the date of the operation and is characterized by either purulent exudate draining from the surgical site, a positive fluid culture from a surgical site that was closed primarily, a surgical site that requires opening, or a surgeon’s diagnosis of wound infection. SSIs were classified as superficial (skin or subcutaneous tissues), deep (deep tissues of an incision), or organ space (intra-abdominal). Wound culture data were recorded.
Statistical analysis
Descriptive statistics were calculated for all variables by treatment group (before and after antibiotic prophylaxis change). Mean values and standard deviations were calculated for continuous variables and counts and percentages for categorical variables. Box plots and histograms were used to graphically check for normality of data sets. Natural logarithm transformation was applied to estimated blood loss (EBL) to improve normality owing to a large standard deviation. Independent 2-sample t tests were used to compare continuous variables between the 2 treatment groups, and Pearson’s Chi-square tests were used for categorical variables. Effects of treatment (post piperacillin-tazobactam vs pre piperacillin-tazobactam) and other potential risk factors were assessed using univariate and multivariate logistic regression models, with SSI as the outcome variable. Relative risk or odds ratio and 95% confidence intervals were calculated. The Hosmer-Lemeshow test was used to examine goodness-of-fit of the models. All statistical analyses were performed using SAS software (Version 9.2; SAS Institute Inc, Cary, NC).
RESULTS
Demographic data for all patients is summarized in Table III. Wound classification for all cases in both groups was class II (clean/contaminated). There were no differences between the 2 groups with regard to age, gender, body mass index or obesity, diabetes, smoking, or hypertension. Furthermore, there were no differences in operating times before or after the change in antibiotics, nor in EBL. The groups had comparable ASA scores and comparable rates of preoperative biliary stenting. No patients in either group took corticosteroids or other immunosuppressive agents. Albumin was lower in the group receiving SCIP-approved antibiotics (2.925 ± 0.403 vs 3.537 ± 0.786; P < .001). However, albumin was not significant in either the univariate or the multivariate logistic regression model to estimate its association with SSI. Stent placement, EBL, and ASA score were also analyzed in the logistic regression models, and none of these variables were significantly associated with an increased SSI risk in our series. These results are summarized in Table IV. Interaction terms of these variables with treatment were tested to determine whether they had different effects on SSI in different treatments. No significant interaction effect was detected (data not shown).
Table III.
Demographics of pancreaticoduodenectomy patients
| Variables | SCIP regimen (n = 34) | Piperacillin-tazobactam (n = 106) | P value |
|---|---|---|---|
| Age | 63.4 ± 13.4 | 63.3 ± 14.4 | .955 |
| Male gender (%) | 35.3 | 46.6 | .249 |
| BMI (kg/m2) | 27.0 ± 6.7 | 25.7 ± 6.2 | .387 |
| Diabetes | 18.2% | 29.4% | .205 |
| Obesity | 29.2% | 24.1% | .613 |
| Smoking | 12.9% | 8.8% | .503 |
| Hypertension | 47.1% | 50% | .766 |
| Corticosteroids/immunosuppression | 0% | 0% | — |
| Albumin | 2.925 ± 0.403 | 3.537 ± 0.786 | <.001 |
| Operative time (min) | 259.3 ± 109.9 | 235.7 ± 99.1 | .244 |
| EBL (mL) | 308.0 ± 360.6 | 195.6 ± 171.2 | .107 |
| ASA score ≥ 3 | 64.70% | 58.30% | .506 |
| Biliary stent | 35.30% | 37.70% | .798 |
Plus–minus values are mean values ± standard deviation.
ASA, American Society of Anesthesiologists; BMI, body mass index; EBL, estimated blood loss.
Table IV.
Odds ratio (95% confidence interval) of risk factors for surgical site infection (SSI)
| Variables | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| Treatment (piperacillin-tazobactam vs SCIP) | 0.148 (0.052–0.423) | .0004 | 0.068 (0.010–0.466) | .006 |
| Albumin | 1.466 (0.648–3.314) | .359 | 2.332 (0.621–8.763) | .210 |
| EBL (log-transformed) | 0.574 (0.326–1.010) | .054 | 1.863 (0.851–4.075) | .119 |
| ASA (≥3 vs 2) | 0.941 (0.341–2.600) | .907 | 0.641 (0.150–2.738) | .548 |
| Biliary stent | 0.918 (0.332–2.538) | .870 | 1.173 (0.271–5.068) | .831 |
ASA, American Society of Anesthesiology; EBL, estimated blood loss.
For the period of March through May 2008, 34 patients underwent PD with 11 SSI cases. Fifteen percent of patients in this group received piperacillin-tazobactam (or tigecycline if they had penicillin allergies), and the rest received SCIP-approved antibiotics. Regimens included cefoxitin (62%), cefazolin and metronidazole (15%), and clindamycin (8%). No patients receiving piperacillin-tazobactam had SSIs. Antibiotics were re-dosed per guidelines based on the half-life of the antibiotic. All SSIs had positive culture results, and revealed growth of Enterobacter species in 6 cases (55%), Enterococcus species in 5 (45%), coagulase-negative Staphylococcus in 5 (45%), yeast in 2 (18%), and Streptococcus pneumoniae in 1 (9%).
From June 2008 through March 2009, 106 PDs were performed. During this period, the SSI rate was 6.6 per 100 surgeries, 80% lower than during March through May 2008 (11/34 vs 7/106; relative risk, 0.204; 95% confidence interval, 0.086–0.485; P = .0004). Overall during that period, 86% of patients received either piperacillin-tazobactam or tigecycline. It should be noted that in the final 3 months studied after the antibiotic change (January to March 2009), 93% of patients received this regimen and the SSI rate was 3.3%. The other patients received cefoxitin (n = 3), imipenem (n = 2), or 1 of 6 other antibiotic regimens (cefotaxime and metronidazole, cefazolin, clindamycin and gentamicin, ciprofloxacin and metronidazole, clindamycin, or meropenem). The reduction in SSI occurring with the change in antibiotic usage is represented in the Figure. Five of the 7 SSI cases were culture positive, with 2 cases of Enterobacter species, and 1 case each (14%) of Enterococcus, coagulase-negative Staphylococcus, and yeast. All data, including culture results and nature of the SSI (superficial vs deep vs organ space) both before and after the antibiotic change, are summarized in Table V.
Figure.
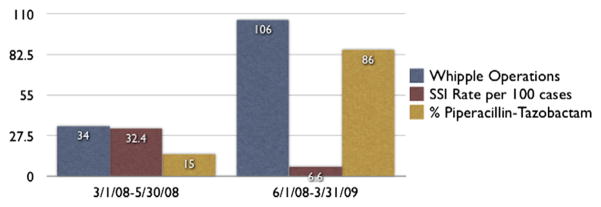
Surgical site infecting (SSI) rate and antibiotic usage. As use of piperacillin-tazobactam increased, the SSI rate decreased dramatically.
Table V.
Pancreaticoduodenectomy (PD) and SSI, UCLA Department of Surgery
| UCLA experience | March 1, 2008–May 31, 2008 | June 1, 2008–March 31, 2009 |
|---|---|---|
| Total no. of Whipple procedures | 34 | 106 |
| Total SSI | 11 | 7 |
| Superficial | 5 | 5 |
| Deep | 6 | 2 |
| SSI rate (per 100 cases) | 32.4 | 6.6 |
| Percentage piptaz/tigecycline | 15% | 86% |
| Culture positive, % (n) | 100% (11) | 71% (5) |
| Culture result | ||
| Enterobacter spp | 55% (6) | 29% (2) |
| Enterococcus spp | 45% (5) | 14% (1) |
| Coagulase-negative staph | 45% (5) | 14% (1) |
| Yeast | 18% (2) | 14% (1) |
| S pneumoniae | 9% (1) | 0% (0) |
SSI, Surgical site infections.
DISCUSSION
As is evident from our experience, use of piperacillin-tazobactam instead of SCIP-approved perioperative antibiotics in PD independently reduces SSI. Patients undergoing PD before implementation of our changed antibiotic regimen had similar demographics and risk factors compared with patients in the piperacillin-tazobactam group. The patients underwent the same operation by the same 2 surgeons, with the same operating room staff. The group receiving the new antibiotic regimen had an 80% reduction in SSI rate. Administration of either piperacillin-tazobactam (or tigecycline) is now the standard of care for any patient undergoing pancreatic resection in our group.
Piperacillin-tazobactam is an appropriate perioperative antibiotic for pancreatic operations in our institution because we found that the most common isolates from SSI after PD were Enterococcus and Enterobacter species, which are not covered by SCIP-approved cephalosporins. In the case of Enterococcus, this is because of both intrinsic and acquired resistance mechanisms.19,20 Furthermore, Enterococcus, once thought of as part the normal flora of the intestinal tract and not prone to causing infection, has become increasingly recognized as a pathogenic organism.21,22 In the case of Enterobacter, although ampicillin-sulbactam does in most cases cover for this microbe and is SCIP approved, it has resistance rates at our institution upwards of 80%. Thus, piperacillin-tazobactam, as a broad-spectrum antibiotic, may be a more appropriate choice to prevent SSI in PD. However, this may be problematic because it is speculated that the reason enterococci have emerged as a pathogen as of late may be owing to overuse of broad-spectrum antibiotics in the first place.23
From March through May 2008, our SSI rate after PD was startlingly high, occurring in nearly 33% of cases, making our initial SSI rate nearly twice as high as rates found in the literature. This observation prompted the initial investigation into our antimicrobial practices. This high rate could have been owing to a number of factors not taken into account by our data collection methodology and might point toward aberrant systems practices in our operating rooms during that time period. A series reported by our same institution less than a decade earlier including >400 PD cases revealed SSI occurring in only 8.2% of cases. The fact that our SSI rate was high before the implementation of our intervention made the SSI rate after that change relatively much lower (and thus statistically significant but possibly not clinically relevant). Another possible explanation would be an endemic outbreak leading to an SSI cluster in the SCIP cohort. Although the patients in this cohort were fact treated in the same ward, the fact that the culture results were polymicrobial led our infectious disease colleagues to essentially exclude a common source as the cause for this SSI cluster.
The mean albumin value in the group receiving SCIP-approved antibiotics was lower than that in the group receiving piperacillin-tazobactam, indicating that the nutritional status of the pre-intervention group was lower, thus putting them at greater risk for SSI. However, when controlled for in the multivariate model, albumin level was not significantly associated with SSI risk in our series, nor was stent placement, EBL, or ASA classification, whereas the treatment effect of piperacillin-tazobactam remained significant. This difference, particularly regarding albumin, may have remained significant if the initial cohort (34 PD cases) had been larger.
With regard to EBL in particular, blood loss was greater in the group receiving SCIP-approved antibiotics (although not statistically significant). This could explain the higher rate of SSI in the SCIP group because increased surgical bleeding and surgical trauma are known risk factor for SSI, and may have been significant had our treatment groups been larger.
Furthermore, we did not conduct a randomized, prospective study, so we cannot make conclusions regarding causality, only that there is a temporal relationship between the change in antibiotics and the decrease in SSI cases, however dramatic that change was.
Antibiotic prophylaxis in pancreatic surgery is not well-studied. To the knowledge of these authors, there have been no randomized, controlled trials comparing different prophylactic antibiotics regimens in pancreatic surgery, and perioperative antibiotics as recommended by SCIP for “colon/abdominal” surgery may not be appropriate for the subset of operations involving pancreatic resection. The implementation of the Joint Commission SCIP measures has impacted the culture of quality improvement by raising awareness and increasing resources available to implement and study quality improvement interventions. However, adherence to performance measures alone in the absence of demonstrable improvement in patient outcomes is insufficient. This study is an example of how measurement of patient outcomes—SSI rates—can lead to meaningful quality improvement interventions that may directly impact patient outcomes. Continued evaluation of SCIP performance measures in relationship to patient outcomes is integral to sustained quality improvement.
Acknowledgments
Funded by NIH T32 07180-37 and The UCLA Center for Excellence in Pancreatic Disease Grants P01 AT003960 and P01 CA163200.
References
- 1.Consensus paper on the surveillance of surgical wound infections. The Society for Hospital Epidemiology of America; The Association for Practitioners in Infection Control; The Centers for Disease Control; The Surgical Infection Society. Infect Control Hosp Epidemiol. 1992;13:599–605. [PubMed] [Google Scholar]
- 2.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271–4. doi: 10.1016/s0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 3.Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlon KC, Labow D, Leung D, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487–93. doi: 10.1097/00000658-200110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47–55. doi: 10.1097/00000658-200107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–66. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adam U, Makowiec F, Riediger H, et al. Risk factors for complications after pancreatic head resection. Am J Surg. 2004;187:201–8. doi: 10.1016/j.amjsurg.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–25. doi: 10.1001/archsurg.139.7.718. [DOI] [PubMed] [Google Scholar]
- 9.Kazanjian KK, Hines OJ, Eibl G, et al. Management of pancreatic fistulas after pancreaticoduodenectomy: results in 437 consecutive patients. Arch Surg. 2005;140:849–54. doi: 10.1001/archsurg.140.9.849. [DOI] [PubMed] [Google Scholar]
- 10.Kimura F, Shimizu H, Yoshidome H, et al. Increased plasma levels of IL-6 and IL-8 are associated with surgical site infection after pancreaticoduodenectomy. Pancreas. 2006;32:178–85. doi: 10.1097/01.mpa.0000202959.63977.5c. [DOI] [PubMed] [Google Scholar]
- 11.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Kent TS, Sachs TE, Callery MP, et al. The burden of infection for elective pancreatic resections. Surgery. 2012;153:86–94. doi: 10.1016/j.surg.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Rayes N, Seehofer D, Theruvath T, et al. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg. 2007;246:36–41. doi: 10.1097/01.sla.0000259442.78947.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes A, Sauvanet A, Bert F, et al. Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J Am Coll Surg. 2006;202:93–9. doi: 10.1016/j.jamcollsurg.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Ueno T, Yamamoto K, Kawaoka T, et al. Current antibiotic prophylaxis in pancreatoduodenectomy in Japan. J Hepatobiliary Pancreat Surg. 2005;12:304–9. doi: 10.1007/s00534-005-0975-2. [DOI] [PubMed] [Google Scholar]
- 16.National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992–June 2001, issued August 2001. Am J Infect Control. 2001;29:404–21. doi: 10.1067/mic.2001.119952. [DOI] [PubMed] [Google Scholar]
- 17.Jethwa P, Breuning E, Bhati C, et al. The microbiological impact of pre-operative biliary drainage on patients undergoing hepato-biliary-pancreatic (HPB) surgery. Aliment Pharmacol Ther. 2007;25:1175–80. doi: 10.1111/j.1365-2036.2007.03289.x. [DOI] [PubMed] [Google Scholar]
- 18.Soper NJ, Brunt LM, Dunnegan DL, et al. Laparoscopic distal pancreatectomy in the porcine model. Surg Endosc. 1994;8:57–60. doi: 10.1007/BF02909495. [DOI] [PubMed] [Google Scholar]
- 19.Rice LB. Mechanisms of resistance and clinical relevance of resistance to beta-lactams, glycopeptides, and fluoroquinolones. Mayo Clin Proc. 2012;87:198–208. doi: 10.1016/j.mayocp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gholizadeh Y, Courvalin P. Acquired and intrinsic glycopeptide resistance in enterococci. Int J Antimicrob Agents. 2000;16(Suppl 1):S11–7. doi: 10.1016/s0924-8579(00)00300-9. [DOI] [PubMed] [Google Scholar]
- 21.Tendolkar PM, Baghdayan AS, Shankar N. Pathogenic enterococci: new developments in the 21st century. Cell Mol Life Sci. 2003;60:2622–36. doi: 10.1007/s00018-003-3138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513–22. doi: 10.1128/cmr.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt CP. The emergence of enterococci as a cause of nosocomial infection. Br J Biomed Sci. 1998;55:149–56. [PubMed] [Google Scholar]


