Abstract
Although NO derived from endothelial NO synthase (eNOS) is thought to be cardioprotective, the role of inducible NO synthase (iNOS) remains controversial. Using mice lacking iNOS (iNOS−/−), we studied (1) whether development of hypertension, cardiac hypertrophy, and dysfunction after deoxycorticosterone acetate (DOCA)–salt would be less severe compared with wild-type controls (WT; C57BL/6J), and (2) whether the cardioprotection attributable to lack of iNOS is mediated by reduced oxidative stress. Mice were uninephrectomized and received either DOCA-salt (30 mg/mouse SC and 1% NaCl+0.2% KCl in drinking water) or vehicle (tap water) for 12 weeks. Systolic blood pressure (SBP) was measured weekly. Left ventricular (LV) ejection fraction (EF) by echocardiography and cardiac response to isoproterenol (50 ng/mouse IV) were studied at the end of the experiment. Expression of eNOS and iNOS as well as the oxidative stress markers 4-hydroxy-2-nonenal (4-HNE, a marker of lipid peroxidation) and nitrotyrosine (a marker for peroxynitrite) were determined by Western blot and immunohistochemical staining, respectively. DOCA-salt increased SBP and LV weight similarly in both strains and decreased EF in WT but not in iNOS−/−. Cardiac contractile and relaxation responses to isoproterenol were greater, 4-HNE and nitrotyrosine levels were lower, and eNOS expression tended to be higher in iNOS−/−. We conclude that lack of iNOS leads to better preservation of cardiac function, which may be mediated by reduced oxidative stress and increased eNOS; however, it does not seem to play a significant role in preventing DOCA-salt–induced hypertension and hypertrophy.
Keywords: nitric oxide synthase, cardiac function, oxidative stress
Nitric oxide is synthesized from l-arginine by 3 isoforms of NO synthase (NOS), described as neuronal (nNOS), inducible (iNOS), and endothelial (eNOS). In the heart, eNOS and nNOS are constitutively expressed in the endocardium, vascular endothelium, and cardiomyocytes, and help regulate vascular tone and cardiac contraction.1–3 In contrast, iNOS is induced in the myocardium in response to various stimuli such as inflammatory mediators, cytokines, and growth factors as well as hypoxia and ischemia,4,5 and its expression correlates positively with the severity of cardiac dysfunction and cytokine expression.6,7 Induction and/or activation of iNOS will produce larger amounts of NO, which reacts with superoxide (O2–) to form the highly cytotoxic oxidant peroxynitrite (ONOO–). Recently, it has been demonstrated that iNOS is also capable of producing O2–, which is transformed to hydrogen peroxide (H2O2) either spontaneously or via an enzymatic reaction with superoxide dismutase.8,9 Both ONOO– and H2O2 have been implicated in tissue injury and organ dysfunction, including the heart.10–12
The role of iNOS in the pathophysiology of hypertension and cardiac hypertrophy remains controversial. A number of studies have shown that iNOS expression and activity are altered in hypertensive patients as well as animal models.13–15 Inhibition of iNOS suppressed development of hypertension in spontaneously hypertensive rats (SHR)16 and cerebral edema resulting from severe hypertension in stroke-prone SHR.17 Furthermore, targeted deletion of the iNOS gene attenuated vascular injury in mice.18 Together, these data suggest that activation of iNOS contributes to the pathogenesis of cardiovascular disease, probably because of excessive release of NO and generation of reactive oxygen species.8–10 However, conflicting findings have been reported. Ni et al showed that salt-induced hypertension was associated with significantly reduced iNOS protein expression in the heart, aorta, and kidney.19,20 Ishimitsu et al21 showed that activation of iNOS by interleukin-2 lowered blood pressure and heart weight in Dahl salt-sensitive rats but not in SHR, suggesting that NO deficiency participates in the pathogenesis of salt-sensitive but not spontaneous hypertension.
To clarify the role of iNOS in salt-induced hypertension, we used iNOS knockout mice (iNOS−/−), testing the hypothesis that lack of iNOS may attenuate the development of hypertension, cardiac hypertrophy, and left ventricular (LV) dysfunction after deoxycorticosterone acetate (DOCA)–salt, and this cardioprotective effect is mediated in part via reduction of oxidative stress.
Materials and Methods
Animals
Eight-week-old male iNOS knockout mice (iNOS−/−) were purchased from Jackson Laboratory (Bar Harbor, Me). Age-matched C57BL/6J mice served as wild-type controls (WT) because iNOS−/− mice were backcrossed to C57BL/6J by Jackson Laboratory until congenic status was achieved. Mice were housed in an air-conditioned room with a 12-hour light/dark cycle and given standard chow and tap water. This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee. All studies in animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Induction of DOCA-Salt Hypertension
One week after adapting to their new environment, mice were anesthetized, uninephrectomized via a retroperitoneal flank incision, and divided into 2 groups: (1) control (placebo+tap water), and (2) DOCA+1% salt. Briefly, a silicone rubber sheet containing DOCA (Sigma) was implanted subcutaneously at a dose of 10 mg/10 g body weight.22 Twenty-four hours after implantation of DOCA-salt, mice started to drink 1% NaCl containing 0.2% KCl, whereas the controls continued to receive tap water. Treatments lasted 12 weeks.
Systolic Blood Pressure
Systolic blood pressure (SBP) was measured weekly in conscious mice using a noninvasive computerized tail-cuff system (BP-2000; Visitech).23 Each SBP comprised 3 sets of 10 measurements, with each set including >6 of 10 successful measurements. Weekly SBP was averaged every 4 weeks and expressed as mm Hg monthly.
Echocardiographic Evaluation of Cardiac Morphology and Function
After 12 weeks, LV dimensions and ejection fraction (EF) were evaluated with a Doppler echocardiographic system equipped with a 15-MHz linear transducer (Acuson c256) in awake mice as described previously.24
Response of LV Function to Isoproterenol
After echocardiography, mice were anesthetized, placed on a warm pad (37°C), and ventilated. A left thoracotomy was performed via the fourth intercostal space to expose the heart. A 1.4-French micromanometer pressure catheter (Millar Instruments) was advanced into the left ventricle as described previously.25 LV functional parameters including heart rate, LV systolic pressure (LVSP), maximum and minimum dP/dt (dP/dtmax and dP/dtmin) and instant pressure (iP; LV pressure at maximum) were measured before and after injecting the β-adrenergic agonist isoproterenol (ISO; 50 ng/mouse IV). dP/dt/iP, an indicator of isovolumic contraction, is the maximum rate of rise of ventricular pressure divided by the pressure at the moment when dP/dt reaches a maximum, expressed as s–1. Data were acquired using a Biobench system and computed using PVAN analysis software (Millar).
eNOS and iNOS Protein Expression
Heart tissue was homogenized in lysis buffer and centrifuged at 14 000g for 10 minutes; the supernatant was collected and protein content detected with a protein assay kit (Bio-Rad). Protein (75 μg/lane for eNOS and 120 μg/lane for iNOS) was separated out in 8% SDS-PAGE and electrotransferred to a nitrocellulose membrane (Amervehicle Biosciences). Membranes were blocked with 5% nonfat milk and incubated overnight at 4°C with the primary antibody (anti-eNOS or iNOS; Transduction Laboratories), anti–β-actin (Santa Cruz Biotechnology), and horseradish peroxidase–conjugated secondary antibodies (Amervehicle Biosciences). Blots were subjected to autoradiography (ECL kit; Amervehicle Pharmacia). Results were expressed as the ratio of the density of specific bands to the corresponding β-actin.
Immunohistochemistry for 4-Hydroxy-2-Nonenal and Nitrotyrosine26
Frozen LV sections were fixed in acetone and incubated with 0.3% H2O2. After blocking nonspecific staining with Ig blocking reagent (Vector) and 5% serum, sections were incubated with either a monoclonal antibody to 4-hydroxy-2-nonenal (4-HNE; Oxis; diluted 1:10) or a polyclonal antibody to nitrotyrosine (Sigma; 1:500) and the secondary antibody (biotinylated anti-mouse or anti-rabbit IgG reagent) as well as avidin and biotinylated horseradish peroxidase complex (ABC) reagent. The negative control was processed in similar fashion except for the primary antibody. Immunocomplexes were visualized with 3-amino-9-ethylcarbazole (Vector). Images were obtained from 4 separate randomly selected high-power fields. Results were scored for intensity and area of staining by 3 independent observers over a range of 0 to 3, where 0 indicates no staining, 1 indicates faint staining or small area, 2 indicates moderate staining or medium-sized area, and 3 indicates strong staining or large area.
Data Analysis
All data are expressed as mean±SE. One-way ANOVA and linear models were used to test the time effect of treatment within each strain as well as the time effect between strains per treatment. For heart weight, functional parameters, NOS expression, 4-HNE, and nitrotyrosine, Student's 2-sample t test was used between groups, either between strains or for DOCA-salt treatment within strains. The type 1 error rate was set at α=0.05, considering P<α as significant.
Results
Systolic Blood Pressure
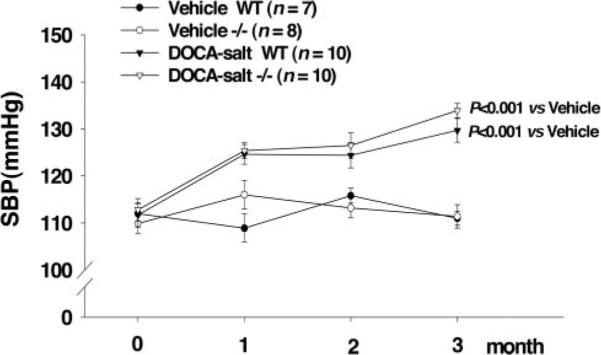
Basal SBP was similar among groups and remained unchanged in control groups of both strains during the study. DOCA-salt caused a significant increase in SBP, and no difference between strains was observed (WT from 111.7±2.6 to 129.7±2.5 mm Hg; iNOS−/− from 112.8±2.4 to 133.9±1.5 mm Hg; Figure 1).
Figure 1.
Effect of iNOS deletion (iNOS−/−) on basal SBP and SBP response to DOCA-salt treatment compared with WT.
Cardiac Hypertrophy
Heart weight in control groups of both strains was similar. DOCA-salt had no effect on right ventricular weight but significantly increased LV weight. There was no difference between strains (Table 1).
TABLE 1.
iNOS Deletion and Its Effect on Heart Weight in Mice With DOCA-Salt Hypertension
| WT |
iNOS |
|||
|---|---|---|---|---|
| Vehicle (n=7) | DOCA-salt (n=8) | Vehicle (n=8) | DOCA-salt (n=9) | |
| RVW (mg/10 g) | 7.1±0.3 | 7.0±0.3 | 6.8±0.5 | 7.1±0.2 |
| LVW (mg/10 g) | 31.0±0.8 | 34.9±0.6† | 29.6±0.7 | 36.6±2.0* |
| THW (mg/10 g) | 40.3±0.7 | 45.0±0.7† | 39.1±0.6 | 47.3±2.3* |
RVW indicates right ventricular weight; LVW, LV weight; THW, total heart weight.
P<0.01
P<0.001 vs vehicle within strains.
Cardiac Function
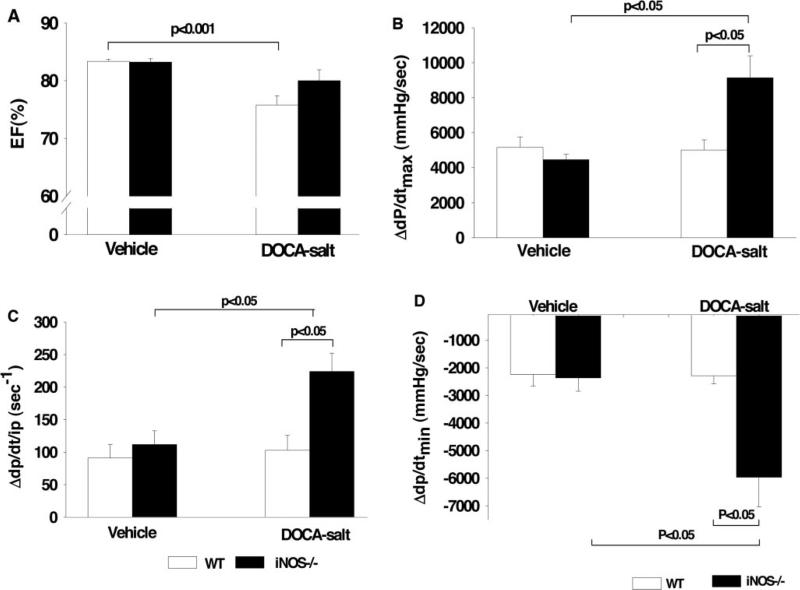
In control groups, EF did not differ between WT and iNOS−/−. DOCA-salt caused a slight but significant decrease in EF in WT, which was not seen in awake iNOS−/−. In anesthetized normotensive controls, basal heart rate, LVSP, dP/dtmax, dP/dt/iP, and dP/dtmin were similar between strains (Table 2). However, in DOCA-salt–treated groups, the contraction and relaxation responses to ISO were significantly enhanced in iNOS−/− compared with WT, indicating that iNOS−/− have better cardiac reserve (Figure 2).
TABLE 2.
Effect of iNOS Deletion on dP/dtmax, dP/dt/iP, and dP/dtmin Response to ISO in Mice With DOCA-Salt Hypertension
| WT |
iNOS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle (n=5) |
DOCA-salt (n=6) |
Vehicle (n=8) |
DOCA-salt (n=5) |
|||||
| Before ISO | After ISO | Before ISO | After ISO | Before ISO | After ISO | Before ISO | After ISO | |
| HR (bpm) | 371±24 | 530±16† | 351±18 | 488±36 | 346±16 | 470±16† | 391±19 | 489±21 |
| LVSP (mm Hg) | 68.0±1.6 | 99.0±4.0* | 74.9±4.5 | 110.5±11.1 | 63.4±3.1 | 120.7±7.3† | 71.8±2.8 | 140.1±18.4† |
| dP/dtmax (mm Hg/s) | 4319±274 | 9488±656† | 4875±448 | 9888±960† | 4449±567 | 8913±631† | 4483±274 | 13621±1120† |
| dP/dt/iP (s−1) | –3326±279 | –5567±526 | –4224±478 | –6513±569* | –3349±341 | –5722±385* | –3729±149 | –697±1178* |
| dP/dtmin (mm Hg/s) | 134.9±10.2 | 226±23* | 143±12 | 246±32† | 152±8 | 264±24† | 155±16 | 378±24* |
P<0.01
P<0.001 vs before ISO within strains.
Figure 2.
Effect of iNOS deletion (iNOS−/−) on EF (A), maximum dP/dt (dP/dtmax) (B), maximum dP/dt/iP (C), and negative dP/dt (dP/dtmin) (D) in response to ISO after 3 months of vehicle or DOCA-salt treatment compared with WT.
eNOS and iNOS Protein Expression in the Heart
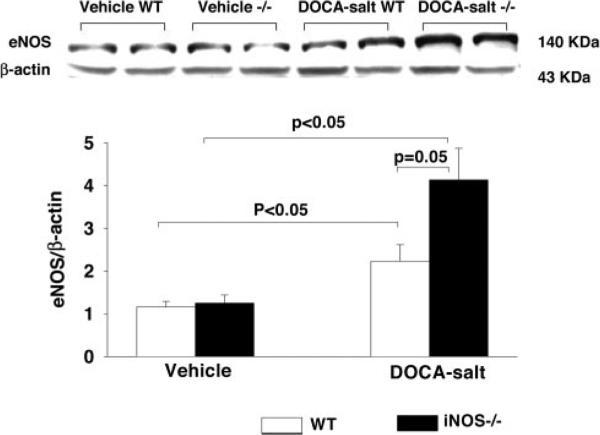
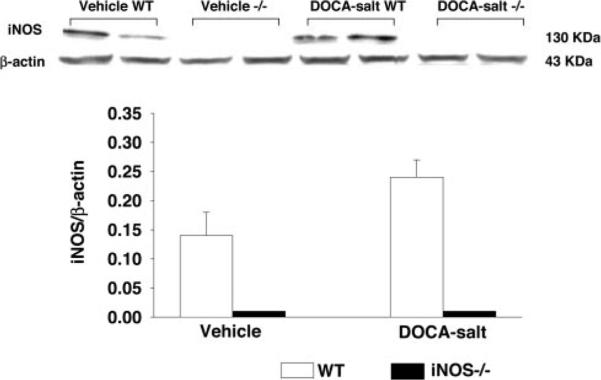
In vehicle-treated controls, eNOS protein expression was similar between strains, suggesting that lack of iNOS does not affect eNOS expression under basal conditions. DOCA-salt increased eNOS expression in both strains, and this increase tended to be greater in iNOS−/− than WT, although the statistical difference was marginal (P=0.05; Figure 3). iNOS protein was undetectable in iNOS−/− with or without DOCA-salt. In WT controls, iNOS protein was expressed in the heart and was not affected by DOCA-salt treatment (P=0.07; Figure 4).
Figure 3.
Representative Western blots (top) and corresponding data (bottom; n=4 in each group) illustrating cardiac eNOS expression in WT and iNOS−/− treated with vehicle or DOCA-salt.
Figure 4.
Representative Western blots (top) and corresponding data (bottom; n=4 in each group) illustrating cardiac iNOS expression in WT and iNOS−/− with vehicle or DOCA-salt treatment.
4-HNE and Nitrotyrosine Expression in the Heart
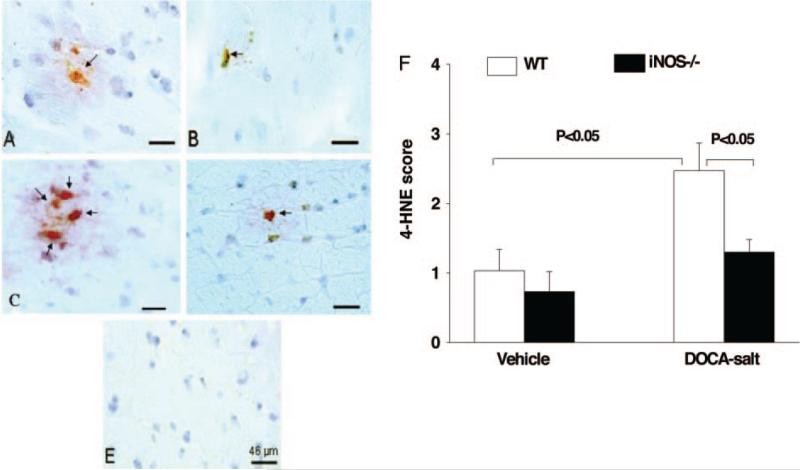
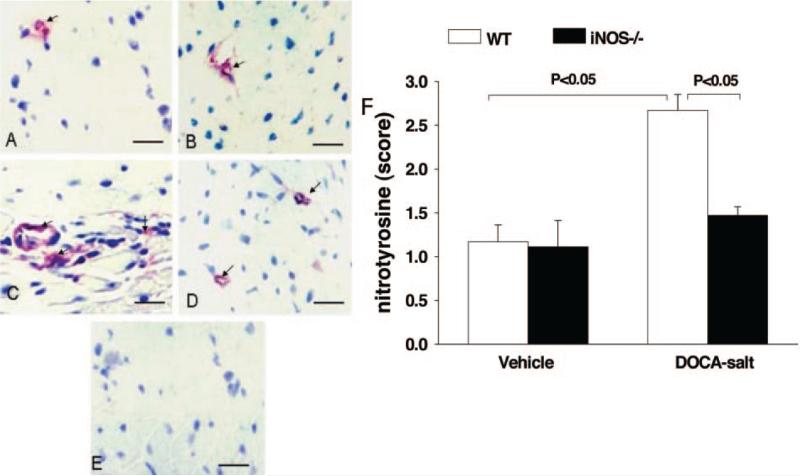
4-HNE, a byproduct of lipid peroxidation and an indicator of oxidative stress, was weakly expressed in normotensive mice of both strains. DOCA-salt significantly increased 4-HNE expres sion in WT, and this effect was diminished in iNOS−/− (Figure 5). Nitrotyrosine, a marker of ONOO– and an indicator of oxidative stress, was expressed weakly in vehicle groups of both strains. As with 4-HNE, DOCA-salt markedly increased nitrotyrosine expression in WT but not in iNOS−/− (Figure 6).
Figure 5.
Representative immunohistochemical staining (A through E) and qualitative data (F) showing 4-HNE expression in the myocardium in WT and iNOS−/− treated with vehicle or DOCA-salt. A, WT/vehicle. B, iNOS−/−/vehicle. C, WT/DOCA-salt. D, iNOS−/−/DOCA-salt; E, negative control. F, Semiquantitative scoring of cross-reactivity for 4-HNE (n=4 in each group). Red (arrows) indicates positive staining located in the interstitial space and myocytes.
Figure 6.
Representative immunohistochemical staining (A through E) and qualitative data (F) showing nitrotyrosine expression in the myocardium in WT and iNOS−/− treated with vehicle or DOCA-salt. A, WT/vehicle. B, iNOS−/−/vehicle. C, WT/DOCA-salt. D, iNOS−/−/DOCA-salt. E, Negative control. F, Semiquantitative scoring of cross-reactivity for nitrotyrosine (n=4 in each group). Red (arrows) indicates positive staining located in the interstitial space and around vessels.
Discussion
We found that lack of iNOS had no effect on DOCA-salt–induced hypertension and cardiac hypertrophy; however, cardiac contraction and relaxation responses to ISO were greater in iNOS knockout mice (iNOS−/−). Furthermore, iNOS−/− treated with DOCA-salt had reduced 4-HNE and nitrotyrosine expression (markers for oxidative stress) and tended to have enhanced eNOS expression. These data suggest that although iNOS does not play an important role in the development of DOCA-salt hypertension and cardiac hypertrophy, activation of iNOS could have a negative effect on cardiac performance and functional reserve; these adverse effects may be mediated by increased production of reactive oxygen species and therefore oxidative stress.
It has been shown that NO plays a diverse but important role in maintaining hemodynamic homeostasis and contributing to the pathophysiology of cardiovascular diseases. Small amounts of NO constitutively released from eNOS may have a protective effect on the cardiovascular system, because targeted deletion of the eNOS gene causes hypertension, cardiac hypertrophy, and interstitial fibrosis and heart damage,24,27,28 and overexpression of eNOS attenuates development of heart failure after myocardial infarction (MI).29 In contrast, the role of iNOS in regulation of blood pressure and cardiac function under physiological and pathophysiological conditions remains controversial. iNOS is normally expressed at low levels but can be activated in response to stimuli such as tissue injury, inflammation, cytokines, and growth factors, as well as MI and DOCA-salt treatment.13,30–32 In the present study, we found that iNOS protein was expressed in the wild-type mouse heart, and its expression tended to increase after 12 weeks of DOCA-salt treatment, although this increase did not reach statistical significance (P=0.07). The mechanism by which DOCA-salt increases iNOS expression is not fully understood. It has been suggested that DOCA-salt induces inflammatory responses, evidenced by increased vascular cell adhesion molecules and macrophages,33,34 which may be responsible for the increased iNOS expression. Induction of iNOS reportedly produces excessive amounts of NO, which reacts with the O2– anion, generating the highly reactive oxidant OONO–. In addition, iNOS is capable of generating O2– independently of NO production.9,35 O2– is not only toxic to the heart and vessels by itself but leads to formation of H2O2, which, together with its oxidizing metabolites, can promote lipid peroxidation and damage the heart and blood vessels.10–12 Thus, blockade or deletion of iNOS may decrease not only NO and OONO– production but also formation of O2– and H2O2, which could help protect the heart and vasculature. We and others have shown that pharmacological inhibition or deletion of iNOS reduced mortality and infarct size and prevented cardiac dysfunction and heart failure in animals with MI and spontaneous hypertension.14,36–38 In the present study, we found that iNOS knock out mice exhibited better preservation of cardiac function associated with reduced 4-HNE and nitrotyrosine expression, markers for oxidative stress, in the presence of DOCA-salt. Mungrue et al also found that overexpression of iNOS led to increased ONOO– production and sudden death attributable to heart block.10 Together, these data may indicate that increased NO production from iNOS is detrimental to the heart. However, there are conflicting reports showing that deficiency of iNOS neither reduces mortality nor attenuates severity of heart failure in mice with MI;39 nor does over-expression of iNOS have a detrimental effect on the heart.40 These conflicting findings merit further investigation to clarify the role of iNOS in the heart.
The role of iNOS in the development of salt-induced hypertension is not fully understood. Using Dahl salt-sensitive rats, investigators have shown that iNOS expression was significantly downregulated in the heart,20 which was further reduced by a high-salt diet, and that selective inhibition of iNOS accelerated development of hypertension.13 Ihrig et al reported that blood pressure was significantly higher in iNOS-deficient mice than in WT on a high-salt diet.41 These data suggest that NO produced by iNOS plays an important role in preventing salt-sensitive hypertension. However, results have been controversial. In the present study, we found that deficiency of iNOS neither affected basal blood pressure nor exaggerated or diminished the blood pressure response to DOCA-salt; however, iNOS knockout mice had better preserved cardiac function, arguing against the hypothesis that iNOS plays an important role in the pathophysiology of DOCA-salt hypertension. On the other hand, Horinaka et al13 showed that in Dahl salt-sensitive rats, iNOS expression in the heart increased progressively along with the severity of hypertension, cardiac hypertrophy, and heart failure, suggesting that iNOS plays an important role in the transition from compensated hypertrophy to heart failure. Hong et al16 showed that in SHR, inhibition of iNOS with aminoguanidine suppressed the development of hypertension, improved the vascular response to acetylcholine, and reduced expression of nitrotyrosine, a marker for oxidative stress. Takemori et al17 reported that the iNOS inhibitor S-methylisothiourea reduced hypertensive brain injury in stroke-prone SHR, associated with reduction of intercellular adhesion molecule-1 and fibrinogen expression in the brain. Although the precise mechanism responsible for the detrimental effect of NO derived from iNOS is not fully understood, it is possible that enhanced oxidative stress and activation of the inflammatory response play an important role, because iNOS expression correlated positively with severity of cardiac dysfunction and expression of oxidative stress and inflammatory markers.42,43
In this study, we also found that iNOS−/− mice had better systolic function and greater contraction and relaxation responses to β-adrenergic stimulation after DOCA-salt treatment. These results were similar to Ullrich's observation that after 7 hours of endotoxin challenge, iNOS−/− had a higher shortening fraction, dP/dtmax, and dP/dtmin44 and to our recent report that iNOS−/− mice with MI had a greater LV contractile response to ISO when treated with NG-nitro-l-arginine methyl ester.31 Kobayashi et al reported that cardiac contractile function was protected when the iNOS inhibitor aminoguanidine was given to Dahl salt-sensitive rats on high salt.45 Sam also found that peak LV developed pressure was higher in iNOS−/− than WT after MI, suggesting improved contractile function in iNOS−/− with MI.38 Overall, our data and others support the hypothesis that induction of iNOS in the heart contributes to impaired cardiac contractility. Although the precise mechanisms are not understood, activation of iNOS may produce large quantities of NO, in turn augmenting the second messenger cGMP. cGMP accumulation has been shown to decrease cardiac contractility via reduction of myofilament sensitivity to calcium.46,47 In addition, we observed increased eNOS expression in both strains treated with DOCA-salt, which tended to be greater in iNOS−/− (P=0.05). Increased eNOS expression may be a compensatory mechanism for increased vascular resistance and decreased myocardial perfusion. It has been suggested that upregulation of eNOS enhances myocardial relaxation and reduces myocardial O2 consumption.48 Our finding that iNOS−/− mice had a better response to ISO may also be attributable in part to increased NO derived from eNOS, which helps preserve cardiac function.
Perspectives
The role of iNOS in the pathophysiology of hypertension and heart failure remains controversial. Our study shows that lack of iNOS had no effect on development of hypertension and cardiac hypertrophy caused by DOCA-salt; however, iNOS−/− mice responded more favorably to β-adrenergic stimulation, indicating better preservation of cardiac function. iNOS−/− mice also had reduced expression of 4-HNE and nitrotyrosine (markers for oxidative stress) and tended to have increased expression of eNOS. Our data suggest that the beneficial cardiac effect observed in iNOS−/− mice may be partially mediated by decreased oxidative stress and increased eNOS expression. These mechanisms require further study.
Acknowledgments
This work was supported by National Institutes of Health grants HL-28982 and HL-078951.
References
- 1.Arnolda LF. Inducible nitric oxide synthase and cardiac dysfunction in salt-sensitive hypertension. J Hypertens. 2002;20:2355–2356. doi: 10.1097/00004872-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Damy T, Ratajczak P, Robidel E, Bendall JK, Oliviéro P, Boczkowski J, Ebrahimian T, Marotte F, Samuel J-L, Heymes C. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J. 2003;17:1934–1936. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- 3.MacCarthy PA, Shah AM. Impaired endothelium-dependent regulation of ventricular relaxation in pressure-overload cardiac hypertrophy. Circulation. 2000;101:1854–1860. doi: 10.1161/01.cir.101.15.1854. [DOI] [PubMed] [Google Scholar]
- 4.Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- 5.Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. doi: 10.1016/s0163-7258(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 6.Shinmura K, Tang X-L, Wang Y, Xuan Y-T, Liu S-Q, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu KK. Cycloooxygenase-2 induction in congestive heart failure. Friend or foe? Circulation. 1998;98:95–96. doi: 10.1161/01.cir.98.2.95. [DOI] [PubMed] [Google Scholar]
- 8.Escames G, Khaldy H, León J, González L, Acuña-Castroviejo D. Changes in iNOS activity, oxidative stress and melatonin levels in hypertensive patients treated with lacidipine. J Hypertens. 2004;22:629–635. doi: 10.1097/00004872-200403000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh M, Nakamura M, Tamura G, Makita S, Segawa I, Tashiro A, Satodate R, Hiramori K. Inducible nitric oxide synthase and tumor necrosis factor-alpha in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol. 1997;29:716–724. doi: 10.1016/s0735-1097(96)00567-0. [DOI] [PubMed] [Google Scholar]
- 12.Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–1155. doi: 10.1016/s0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 13.Horinaka S, Kobayashi N, Mori Y, Yagi H, Onoda M, Matsuoka H. Expression of inducible nitric oxide synthase, left ventricular function and remodeling in Dahl salt-sensitive hypertensive rats. Int J Cardiol. 2003;91:25–35. doi: 10.1016/s0167-5273(02)00587-9. [DOI] [PubMed] [Google Scholar]
- 14.Abe K, Tokumura M, Ito T, Murai T, Kimoto S, Takashima M, Ibii N. Involvement of iNOS in post-ischemic heart dysfunction of stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2001;280:H668–H673. doi: 10.1152/ajpheart.2001.280.2.H668. [DOI] [PubMed] [Google Scholar]
- 15.Manning RD, Jr, Hu L, Tan DY, Meng S. Role of abnormal nitric oxide systems in salt–sensitive hypertension. Am J Hypertens. 2001;14:68S–73S. doi: 10.1016/s0895-7061(01)02072-6. [DOI] [PubMed] [Google Scholar]
- 16.Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemori K, Ito H, Suzuki T. Effects of inducible nitric oxide synthase inhibition on cerebral edema in severe hypertension. Acta Neurochir Suppl. 2000;76:335–338. doi: 10.1007/978-3-7091-6346-7_69. [DOI] [PubMed] [Google Scholar]
- 18.Chyu K-Y, Dimayuga P, Zhu J, Nilsson J, Kaul S, Shah PK, Cercek B. Decreased neointimal thickening after arterial wall injury in inducible nitric oxide synthase knockout mice. Circ Res. 1999;85:1192–1198. doi: 10.1161/01.res.85.12.1192. [DOI] [PubMed] [Google Scholar]
- 19.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155–163. doi: 10.1016/s0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 20.Ni Z, Oveisi F, Vaziri ND. Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension. 1999;34:552–557. doi: 10.1161/01.hyp.34.4.552. [DOI] [PubMed] [Google Scholar]
- 21.Ishimitsu T, Uehara Y, Numabe A, Tsukada H, Ogawa Y, Yagi S. Antihypertensive effect of interleukin-2 in salt-sensitive Dahl rats. Hypertension. 1994;23:68–73. doi: 10.1161/01.hyp.23.1.68. [DOI] [PubMed] [Google Scholar]
- 22.Rhaleb N-E, Peng H, Alfie M, Shesely EG, Carretero OA. Effect of ACE inhibitor on DOCA-salt- and aortic coarctation-induced hypertension in mice. Do kinin B2 receptors play a role? Hypertension. 1999;33:329–334. doi: 10.1161/01.hyp.33.1.329. [DOI] [PubMed] [Google Scholar]
- 23.Alfie ME, Sigmon DH, Pomposiello SI, Carretero OA. Effect of high salt intake in mutant mice lacking bradykinin-B2 receptors. Hypertension. 1997;29:483–487. doi: 10.1161/01.hyp.29.1.483. [DOI] [PubMed] [Google Scholar]
- 24.Yang X-P, Liu Y-H, Rhaleb N-E, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 25.Cingolani OH, Yang X-P, Cavasin MA, Carretero OA. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension. 2003;41:249–254. doi: 10.1161/01.hyp.0000052832.96564.0b. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Yang F, Yang X-P, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-mediated vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 27.Yang X-P, Liu Y-H, Shesely EG, Bulagannawar M, Liu F, Carretero OA. Endothelial nitric oxide gene knockout mice. Cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischemia/reperfusion injury. Hypertension. 1999;34:24–30. doi: 10.1161/01.hyp.34.1.24. [DOI] [PubMed] [Google Scholar]
- 28.Brede M, Roell W, Ritter O, Wiesmann F, Jahns R, Haase A, Fleischmann BK, Hein L. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension. 2003;42:1177–1182. doi: 10.1161/01.HYP.0000100445.80029.8E. [DOI] [PubMed] [Google Scholar]
- 29.Jones SP, Greer JJM, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci U S A. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funakoshi H, Kubota T, Kawamura N, Machida Y, Feldman AM, Tsutsui H, Shimokawa H, Takeshita A. Disruption of inducible nitric oxide synthase improves b-adrenergic inotropic responsiveness but not the survival of mice with cytokine-induced cardiomyopathy. Circ Res. 2002;90:959–965. doi: 10.1161/01.res.0000017632.83720.68. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y-H, Carretero OA, Cingolani OH, Liao T-D, Sun Y, Xu J, Li LY, Pagano PJ, Yang JJ, Yang X-P. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2005 Jul 29; doi: 10.1152/ajpheart.00546.2005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Obst M, Gross V, Bonartsev A, Janke J, Müller DN, Park J-K, Kärgel E, Luft FC. Nitric oxide synthase expression in AT2 receptor-deficient mice after DOCA-salt. Kidney Int. 2004;65:2268–2278. doi: 10.1111/j.1523-1755.2004.00646.x. [DOI] [PubMed] [Google Scholar]
- 33.Ogata T, Miyauchi T, Sakai S, Takanashi M, Irukayama-Tomobe Y, Yamaguchi I. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol. 2004;43:1481–1488. doi: 10.1016/j.jacc.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 34.Kagitani S, Ueno H, Hirade S, Takahashi T, Takata M, Inoue H. Tranilast attenuates myocardial fibrosis in association with monocyte/macrophage infiltration in DOCA/salt hypertensive rats. J Hypertens. 2004;22:1007–1015. doi: 10.1097/00004872-200405000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BSS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Yang X-P, Liu Y-H, Carretero OA, LaPointe MC. Reduction of myocardial infarct size by inhibition of inducible nitric oxide synthase. Am J Hypertens. 1999;12:174–182. doi: 10.1016/s0895-7061(98)00235-0. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Hu F, Tayara L, Fahas L, Shennib H, Giaid A. Inhibition of NOS II prevents cardiac dysfunction in myocardial infarction and congestive heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H339–H345. doi: 10.1152/ajpheart.00596.2001. [DOI] [PubMed] [Google Scholar]
- 38.Sam F, Sawyer DB, Xie Z, Chang DLF, Ngoy S, Brenner DA, Siwik DA, Singh K, Apstein CS, Colucci WS. Mice lacking inducible nitric oxide synthase have improved left ventricular contractile function and reduced apoptotic cell death late after myocardial infarction. Circ Res. 2001;89:351–356. doi: 10.1161/hh1601.094993. [DOI] [PubMed] [Google Scholar]
- 39.Jones SP, Greer JJM, Ware PD, Yang J, Walsh K, Lefer DJ. Deficiency of iNOS does not attenuate severe congestive heart failure in mice. Am J Physiol Heart Circ Physiol. 2005;288:H365–H370. doi: 10.1152/ajpheart.00245.2004. [DOI] [PubMed] [Google Scholar]
- 40.Heger J, Gödecke A, Flögel U, Merx MW, Molojavyi A, Kühn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 41.Ihrig M, Dangler CA, Fox JG. Mice lacking inducible nitric oxide synthase develop spontaneous hypercholesterolemia and aortic atheromas. Atherosclerosis. 2001;156:103–107. doi: 10.1016/s0021-9150(00)00636-5. [DOI] [PubMed] [Google Scholar]
- 42.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 43.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, Zapol WM, Quezado ZMN. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation. 2000;102:1440–1446. doi: 10.1161/01.cir.102.12.1440. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi N, Horinaka S, Mita S, Yoshida K, Honda T, Kobayashi T, Hara K, Nishikimi T, Matsuoka H. Aminoguanidine inhibits mitogen-activated protein kinase and improves cardiac performance and cardiovascular remodeling in failing hearts of salt-sensitive hypertensive rats. J Hypertens. 2002;20:2475–2485. doi: 10.1097/00004872-200212000-00028. [DOI] [PubMed] [Google Scholar]
- 46.Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quignard J-F, Frapier JM, Harricane M-C, Albat B, Nargeot J, Richard S. Voltage-gated calcium channel currents in human coronary myocytes: regulation by cyclic GMP and nitric oxide. J Clin Invest. 1997;99:185–193. doi: 10.1172/JCI119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie YW, Shen W, Zhao G, Xu X, Wolin MS, Hintze TH. Role of endothelium-derived nitric oxide in the modulation of canine myocardial mitochondrial respiration in vitro. Implications for the development of heart failure. Circ Res. 1996;79:381–387. doi: 10.1161/01.res.79.3.381. [DOI] [PubMed] [Google Scholar]