Abstract
Orthotopic liver transplantation (OLT) remains the standard treatment option for nonresponsive liver failure. Because ischemia/reperfusion injury (IRI) is an important impediment to the success of OLT, new therapeutic strategies are needed to reduce IRI. We investigated whether blocking the CD47/thrombospondin-1 inhibitory action on nitric oxide signaling with a monoclonal antibody specific to CD47 (CD47mAb400) would reduce IRI in liver grafts. Syngeneic OLT was performed with Lewis rats. Control immunoglobulin G or CD47mAb400 was administered to the donor organ at procurement or to both the organ and the recipient at the time of transplant. Serum transaminases, histological changes of the liver, and animal survival were assessed. Oxidative stress, inflammatory responses, and hepatocellular damage were also quantified. A significant survival benefit was not achieved when CD47mAb400 was administered to the donor alone. However, CD47mAb400 administration to both the donor and the recipient increased animal survival afterward. The CD47mAb400-treated group showed lower serum transaminases, bilirubin, oxidative stress, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling staining, caspase-3 activity, and proinflammatory cytokine expression of tumor necrosis factor α, interleukin-1β, and interleukin-6. Thus, CD47 blockade with CD47mAb400 administered both to the donor and the recipient reduced liver graft IRI in a rat liver transplantation model. This may translate to decreased liver dysfunction and increased survival of liver transplant recipients.
Despite significant efforts to find new therapies to treat liver failure, orthotopic liver transplantation (OLT) remains the standard treatment for acute and chronic liver failure.1,2 Ischemia/reperfusion injury (IRI) reduces the success of OLT, and this is particularly problematic when limited organs are available for transplant.3 The IRI cascade is a result of multiple steps. Initially, the ischemia damages mitochondria and leads to oxidative stress and cellular injury.4 After implantation, reperfusion induces the release of cytokines and production of reactive oxygen species (ROS), and this further damages hepatocytes.5,6 The combination of these factors can lead to microcirculatory impairment, thrombosis, and eventually tissue necrosis.7 IRI with extended cold and/or warm ischemia times plays an important role in the development of early graft dysfunction and postoperative complications and also increases the risk of acute and chronic graft rejection.8,9 In addition, because of the higher sensitivity of marginal donor grafts to ischemic insults,10 IRI contributes to the shortage of suitable livers for transplantation. Therapies that reduce IRI may, therefore, increase the pool of suitable organs and reduce the mortality of patients waiting for liver transplantation.
CD47 is a widely expressed transmembrane receptor of the immunoglobulin superfamily that functions as a receptor for the matricellular protein ligand thrombospondin-1 (TSP-1).11 Through binding with the TSP-1 C-terminal domain,11 CD47 participates in different cellular pathways involving survival and death.12-14 In vivo, soluble TSP-1 binds to CD47 and inhibits the nitric oxide (NO) signaling cascade, and this results in vasoconstriction, platelet aggregation, thrombosis, and ROS production.14,15
In the ischemic state, both TSP-1 and CD47 are up-regulated and linked with ROS induction in vascular cells,16 and this leads to inflammatory reactions, endothelial cell injury accompanied by microcirculatory dysfunction, and extensive tissue necrosis.17 Hence, we postulate that CD47 blockade using antibodies specific to CD47 (CD47mAbs) will provide cyto-protection against IRI during liver transplantation. The aim of this study was to investigate whether CD47mAb blockade of CD47/TSP-1 signaling can reduce hepatocyte injury and inflammatory responses and increase survival after liver transplantation.
MATERIALS AND METHODS
Transplant Model
Male Lewis rats (body weight = 250 ± 20 g) were purchased from Jackson Labs. All animals were maintained in accordance with the National Resource Council guidelines and were supervised by the animal studies committee of Washington University School of Medicine at St. Louis. They were given access to standard rodent food and water ad libitum before and after transplantation except for fasting 12 hours before surgery. All animals were in quarantine for at least 1 week before treatment. Surgical procedures were performed with aseptic conditions approved by the animal studies committee and in accordance with the National Institutes of Health guidelines.
Animals were anesthetized with 3% isoflurane (Baxter, IL). Before organ procurement, 1 mL of saline containing 200 u of heparin was given intravenously. After hepatectomy, the donor liver was flushed via the portal vein with 10 mL of cold (4 °C) normal saline, which was followed by 10 mL of cold University of Wisconsin (UW) solution containing either a mouse anti-CD47 monoclonal antibody, CD47mAb400 (Vasculox, Inc., St. Louis, MO), or an isotype matched-control mouse immunoglobulin G 2a (IgG2a) at a dose of 1 μg/g liver weight. After cuff preparation, the graft was then stored in UW solution at 4 °C for 18 hours. After storage, the graft was slowly flushed with 10 mL of cold saline through the portal vein before transplantation. OLT was then performed with a modified version of the cuff technique.18,19 Animals were placed on a circulating warm water pad to keep their body temperature within the normal range of 37.8 °C to 38.7 °C during the surgical procedure. Buprenorphine (0.05 mg/kg) was administered subcutaneously at the end of surgery, immediately after the incision was closed, and before the rat regained consciousness. The animals were divided in 2 groups. In 1 group (n = 20, 10 for each treatment), the animals underwent OLT after the liver graft was flushed with CD47mAb400 or control IgG antibody. In another group (n = 20, 10 for each treatment), the liver graft was flushed with either CD47mAb400 or control IgG antibody, and then after reperfusion of the liver, an additional dose (same amount) of either the CD47mAb400 or control IgG antibody was administered to the recipient intravenously. To investigate expression of CD47 and TSP-1 after cold ischemia, we performed a separate set of experiments. In 2 different groups (n = 6) of rats, after hepatectomy, the livers were flushed with 10 mL of cold UW. In the first group, the samples were collected after this flushing step. In the second group, livers were stored at 4 °C for 18 hours to induce cold ischemia, and then samples were collected and frozen for western blot analysis.
Histopathology
Paraffin-embedded liver tissue samples were prepared, sectioned at 6 μm, and stained with hematoxylin and eosin. A pathologist blinded to the experimental groups examined the slides for infiltration of immune cells, apoptosis, ischemic necrosis, and other evidence of hepatic injury. Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining and immunohistochemistry to detect activated caspase-3 were used to demonstrate apoptosis. Imaging software (NIS-Elements, Nikon) aided in differentiation through utilization of a consistent positive threshold. Centrilobular necrosis was graded on a 0 to 3 scale (reported as histological score) as described by Neil and Hubscher.20
Enzyme-Linked Immunosorbent Assay (ELISA) for Proinflammatory Cytokines and 3-Nitrotyrosine
The liver cytokine concentrations [tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and IL-1β] were measured with 50 mg of rat whole liver protein extracts via ELISA according to the protocol given by the supplier (Invitrogen). The concentrations of 3-nitrotyrosine in the donor livers were measured with ELISA (Invitrogen).
Western Blotting
Liver tissues were homogenized with an ice-cold lysis buffer. Then, the homogenates were centrifuged, and the supernatants were collected. Protein concentrations were determined with the Bradford assay (Bio-Rad, Hercules, CA). Twenty micrograms of total protein was separated with sodium dodecyl sulfate–poly-acrylamide gel electrophoresis and transferred onto nitrocellulose (Bio-Rad). A mouse monoclonal antibody (mAb) to β-actin (1:1000 dilution; Abcam), a goat polyclonal antibody to TSP-1 (1:500 dilution; Santa Cruz Biotechnology, Dallas, TX), a rabbit monoclonal antibody to CD47 (1:5000 dilution; Abcam, Cambridge, MA), and a rabbit polyclonal antibody to inducible nitric oxide synthase (iNOS; 1:1000 dilution; Abcam) were used as primary antibodies along with the respective horseradish peroxidase–conjugated secondary antibodies. The intensity of captured bands was quantified with ImageJ software.
Dihydroethidium (DHE) Staining
Superoxide production was detected with a florescent dye, DHE, or hydroethidine, which detects ROS formation in tissues.21 This compound interacts with intracellular superoxide anion (O2−) and forms oxyethidium,22 which produces a fluorescent signal upon interactions with cellular nucleic acids.23 Frozen sections of livers were stained with 10 mM DHE and then incubated for 30 minutes in a light-protected humidified chamber at 37 °C. After the sections were washed with phosphate-buffered saline, the sections were mounted with fluorescent mounting medium (DAKO). NIS Elements imaging software was used to quantify the images.
Statistics
The data were presented as means and standard errors of the mean (SEMs) unless otherwise indicated. A direct comparison of study groups was carried out with the Student t test for means between 2 groups. P values less than 0.05 were considered to be significant. Animal survival analysis was performed with the Kaplan-Meier analysis and the log-rank test. Statistical analyses were performed with the Prism software (GraphPad Software, San Diego, CA).
RESULTS
CD47mAb400 Increased Survival When Given to Both Donor and Recipient Animals
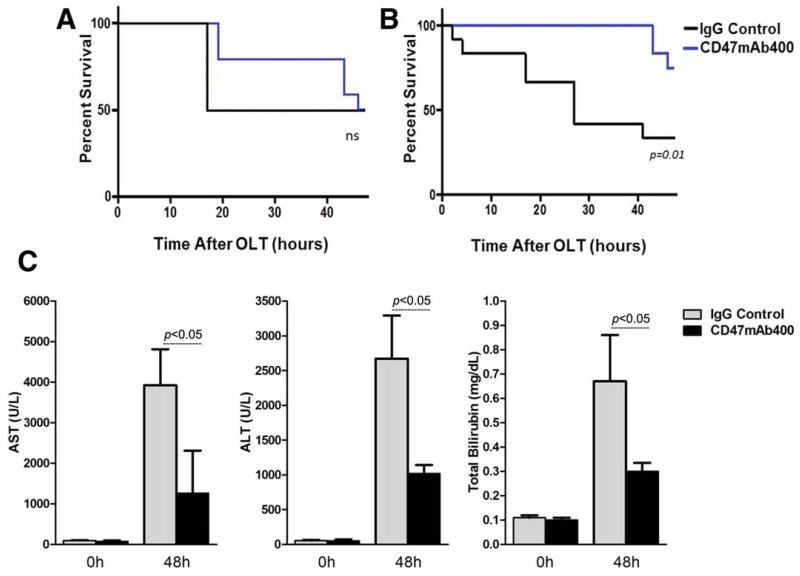
We investigated the effect of CD47 blockade on animal survival when the CD47mAb400 antibody was administered only to the donor liver graft and found that although this tended to increase the survival time, it did not provide a survival benefit to the recipient. The Kaplan-Meier survival analysis suggests that the CD47mAb400 animals initially had improved survival within 20 and 30 hours after OLT; however, this survival benefit was no longer apparent by 48 hours when only 50% of the recipients were alive in both study groups (Fig. 1A). We then investigated the effects of treating both the donor liver graft and the recipient with CD47mAb400 by administering the same dose of the mAbs intravenously after the reperfusion. Survival outcomes were substantially improved in the animals that received CD47mAb400 in comparison with the group given the IgG control. The overall survival at the end of the experiment (48 hours after OLT) was 75% in the CD47mAb400 group and 35% in the IgG control group (P = 0.01; Fig. 1B). In addition, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) levels were also significantly reduced in the CD47mAb400-treated group versus the IgG control group (P < 0.05; Fig. 1C). These data show that blocking TSP-1/CD47 by the administration of CD47mAb400 to both the donor organ before transplantation and to the recipient animal after the reperfusion reduces liver damage and improves overall short-term survival in a syngeneic rat OLT model.
Figure 1.
Animal survival outcome when CD47mAb400 was administered (A) to the donor organ only and (B) to both the donor and recipient. (C) AST, ALT, and TBIL concentrations at 0 and 48 hours after transplantation in animals subjected to OLT. Kaplan-Meier survival curves for both CD47mAb400 and IgG control groups (n = 10 for each group) showed a trend in prolonging survival in the CD47mAb400-treated donor organ group; however, there was no difference between the groups 48 hours after transplantation. In contrast, the treatment of both the donor organ and the recipient with CD47mAb400 significantly increased the survival of the recipients. In addition, liver enzymes and markers were significantly lower in recipients that received the CD47mAb400 injection as well as CD47mAb400-treated donors (P < 0.05).
CD47 Is Up-Regulated by Ischemia and Reperfusion and Reduced by CD47mAb400 Treatment
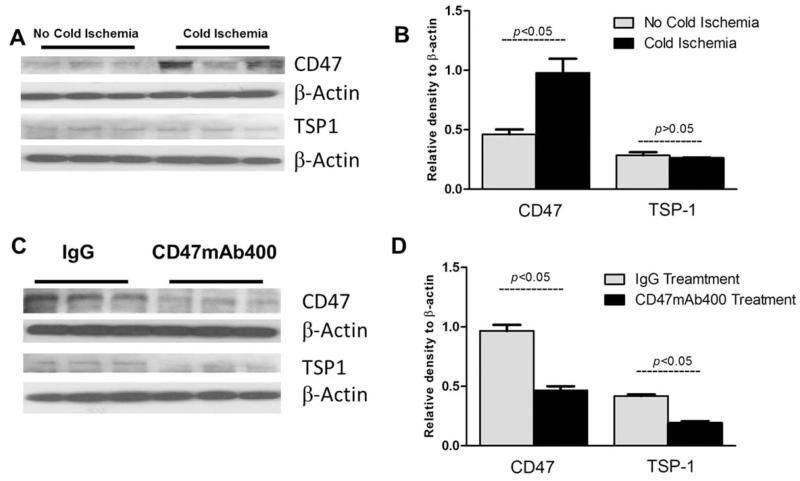
Previous studies have shown that CD47 is up-regulated after renal IRI caused by a transient warm ischemic insult.24 However, hepatic expression of TSP-1 and CD47 has not been investigated after transient warm ischemia, cold ischemia, or transplantation. We compared both CD47 and TSP-1 expression in donor livers under conditions with and without ischemia and reperfusion. Donor livers were flushed with saline and UW solution and frozen immediately (no ischemia) or exposed to 18 hours of cold storage (cold ischemia) and then frozen until evaluated with Western blot analysis. Our data showed that there was a significant induction of CD47 protein but not TSP-1 expression after cold ischemia (Fig. 2A,B). An analysis of transplanted liver tissues 48 hours after OLT showed that the expressions of both TSP-1 and CD47 were increased in livers treated with the control mAb and that both CD47 and TSP-1 protein expression in rats receiving the CD47mAb400 was reduced in comparison with the control IgG group (Fig. 2C,D).
Figure 2.
Western blot analysis of CD47 and TSP-1 expression levels (A,B) during cold ischemia and (C,D) 48 hours after OLT. A significant elevation of CD47 protein with little change in TSP-1 expression after cold ischemia was noted after 18 hours of cold ischemia in comparison with livers that were flushed and then immediately frozen. The expression of both CD47 and TSP-1 protein was reduced 48 hours after OLT in CD47mAb400-treated recipients versus the IgG group (P < 0.05).
CD47mAb400 Reduced Liver Cell Injury and Death
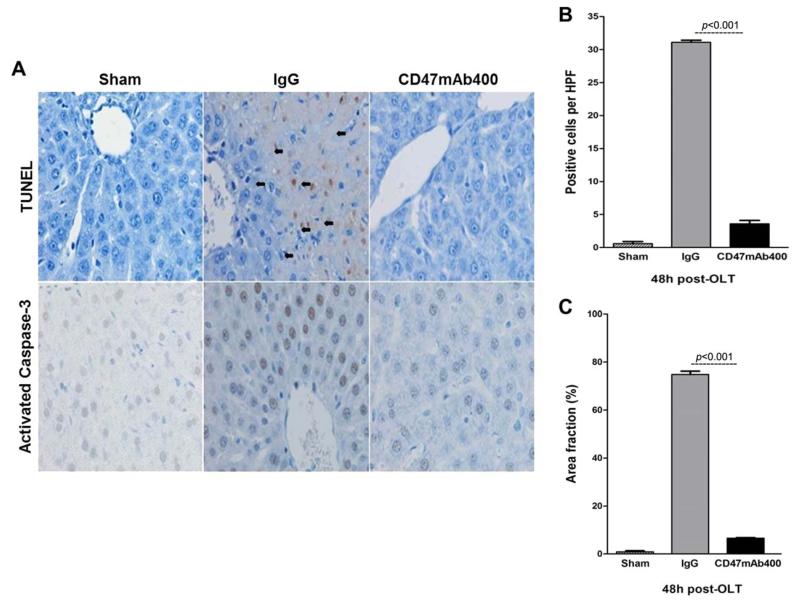
Liver histology showed widespread areas of hepatocyte necrosis in the control IgG-treated rats. However, the histology of liver tissue from CD47mAb400-treated rats was similar to the livers of sham controls in which rat livers did not experience ischemic injury (Fig. 3A). Furthermore, livers from the CD47mAb400-treated group had significantly reduced inflammatory infiltrates in comparison with those from the IgG control group. Although the IgG control group had an average of 32.5 ± 3.6 [standard deviation (SD) = 11.38] inflammatory cells per high-power field (HPF), liver sections from recipients treated with CD47mAb400 had only an average of 1.8 ± 0.3 (SD = 0.94) inflammatory cells per HPF. Liver sections from sham animals had an average of 0.7 ± 0.2 (SD = 0.63) inflammatory cells per HPF (P < 0.001; Fig. 3B). Quantitative assessment of the liver sections also taken 48 hours after OLT revealed that the histological scores of injury were significantly higher in the IgG control group (2.7 ± 0.2, SD = 0.63) versus the CD47mAb400-treated group (1.5 ± 0.2, SD = 0.63) and the sham group (0.14 ± 0.01, SD = 0.03, P < 0.05; Fig. 3C).
Figure 3.
(A) Hematoxylin and eosin staining of liver sections of sham, IgG, and CD47mAb400-treated groups 48 hours after OLT (×200, upper panel; ×400, lower panel). (B) Inflammatory cell counts and (C) histological scores of recipient liver tissues of the sham, IgG, and CD47mAb400-treated groups. Significantly fewer inflammatory cells (P < 0.001) and a significantly lower histological score (P < 0.05) were shown in the CD47mAb400-treated group versus the IgG control group. Data are shown as mean ± SEM. Inflammatory cells are circled.
Both TUNEL staining and caspase-3 activity were assessed in order to detect apoptosis in the liver tissue. Histologic examination showed fewer TUNEL-stained cells in the group treated with CD47mAb400 (Fig. 4A). Quantitative analysis (Fig. 4B) indicated that the control group treated with IgG had an average of 31.1 ± 0.3 (SD = 0.94) TUNEL-positive cells, whereas the CD47mAb400-treated group had an average of 3.6 ± 0.5 (SD = 1.58) positive cells, and the sham group had an average of 0.6 ± 0.3 (SD = 0.93) positive cells (P < 0.001). Similarly, liver histology exhibited a reduction of activated caspase-3 staining in the group treated with CD47mAb400 (Fig. 4A). Quantification of the sections showed that the area fraction of cells stained for caspase-3 in the control group was 74.8% ± 1.4% (SD = 4.42%), whereas the value for the CD47mAb400-treated group was 6.6% ± 0.2% (SD = 0.63%), and the value for the sham group was 0.9% ± 0.4% (SD = 1.26%, P < 0.001; Fig. 4C). These results show that CD47mAb400 treatment can lead to a significant reduction in hepatocyte apoptosis after OLT.
Figure 4.
(A) TUNEL assay and caspase-3 associated activity for localization of apoptotic cells in liver sections of sham, IgG, and CD47mAb400-treated groups 48 hours after OLT. Graphs quantifying (B) TUNEL-positive cells per HPF and (C) caspase-3–associated activity in rats subjected to OLT. Data are shown as mean ± SEM. TUNEL-positive cells were significantly reduced in the CD47mAb400-treated group (P < 0.001). Activated caspase-3 was markedly lower in the CD47mAb400 group (P < 0.001). TUNEL-positive cells are indicated by black arrows.
CD47mAb400 Reduces Expression of Inflammatory Mediators in Liver
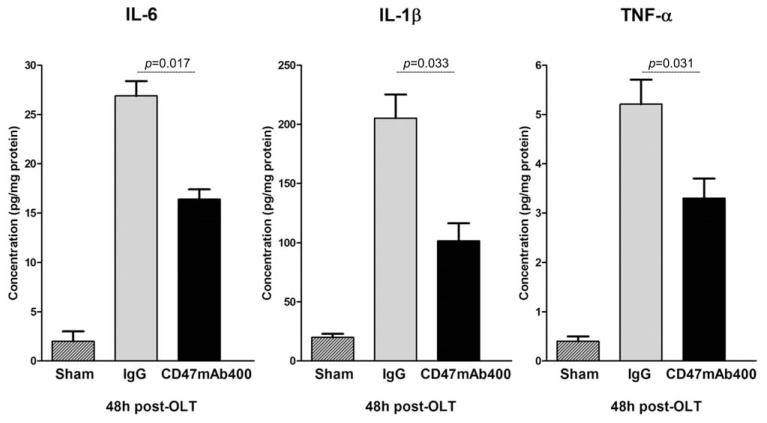
The proinflammatory response was assessed in the liver tissues 48 hours after OLT. Concentrations of IL-6, IL-1β, and TNF-α were significantly elevated in the livers of animals treated with the IgG antibody in comparison with those treated with CD47mAb400 or the sham animals (Fig. 5): IL-6 (26.9 ± 1.5 pg/mg of protein for control IgG versus 16.4 ± 1.1 pg/mg of protein for CD47mAb400, P = 0.017), IL-1β (205.33 ± 20.2 versus 101.78 ± 15.1 pg/mg of protein, P = 0.033), and TNF-α (5.21 ± 0.4 versus 3.30 ± 0.5 pg/mg of protein, P = 0.031). These results indicate that treatment of the donor graft and the recipient animal at the time of reperfusion with CD47mAb400 results in a reduction of the proinflammatory response in the transplanted liver.
Figure 5.
ELISA results for proinflammatory cytokines in the liver homogenate of the recipients. Concentrations of the proinflammatory cytokines IL-6, IL-1β, and TNF-α were significantly reduced in the CD47mAb400-treated group versus the IgG group. Data are shown as mean ± SEM.
CD47mAb400 Treatment Reduces Oxidative Stress
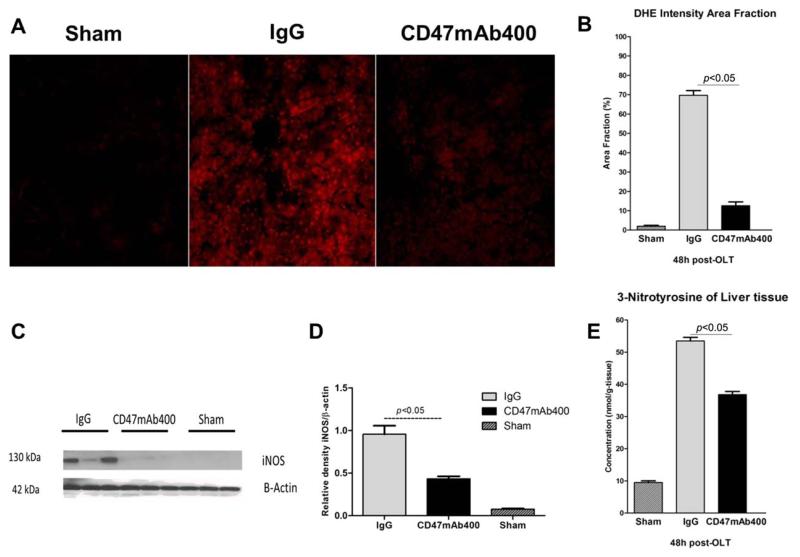
Mitochondrial dysfunction and resulting oxidative stress are key factors in IRI.10 IRI is associated with increased production of ROS and rising levels of , which is a major mediator of cellular injury. We used the fluoroprobe DHE to visualize the intracellular generation of superoxide anions. Sections of liver from IgG- and CD47mAb400-treated rats were stained with DHE and examined with confocal microscopy, and the intensity of the immunofluorescence signal was quantified. DHE staining was significantly increased in the IgG control group versus the group treated with CD47mAb400 (Fig. 6A). The area fraction of staining in the IgG control group was 69.7% ± 2.4% (SD = 7.58%), whereas the area fraction in the CD47mAb400-treated group was 12.6% ± 2% (SD = 7.2%), and the area fraction in the sham group was 2% ± 0.5% (SD = 1.58%; P < 0.05; Fig. 6B).
Figure 6.
(A) DHE staining of liver sections visualized with confocal microscopy. (B) Quantitative analysis of immunofluorescence signals. (C,D) Western blot and quantitative analysis of iNOS and (E) expression of 3-nitrotyrosine in the recipient liver tissues collected 48 hours after OLT. The DHE staining signal was significantly lower in the CD47mAb400-treated group. Similarly, a quantitative analysis of immunofluorescence signals revealed a lower percentage of the area fraction in tissues from the CD47mAb400-treated group (P < 0.05). Expression of iNOS was also significantly reduced in the group treated with CD47mAb400 versus the IgG control group (P < 0.05). Oxidative stress was markedly reduced in the group treated with CD47mAb400 as 3-nitrotyrosine expression versus the IgG group (P < 0.05). Data are shown as mean ± SEM.
Under physiological conditions, liver perfusion is mainly regulated by endothelial nitric oxide synthase (eNOS) expression along with small amounts of NO. However, IRI leads to increased iNOS expression in the liver, which leads to the production of large amounts of NO, which can interact with superoxide to form peroxynitrite that can damage liver tissue. We measured the iNOS expression in the liver tissues 48 hours after OLT. The results showed that the level of iNOS was elevated in the IgG control group and was reduced in the CD47mAb400-treated group (P < 0.05; Fig. 6C,D).
We also measured the tissue expression level of 3-nitrotyrosine 48 hours after OLT with ELISA. The mean concentration of 3-nitrotyrosine in the control group was 53.5 ± 1.1 nmol/g of tissue (SD = 3.47), whereas the average concentration in the treatment group was significantly less at 36.8 ± 1 nmol/g of tissue (SD = 3.16), and the value for the sham group was 9.5 ± 0.5 nmol/g of tissue (SD = 1.58, P < 0.05; Fig. 6E). The reduction of proinflammatory cytokines triggered by CD47mAb400 treatment was associated with a significant decline in oxidative stress in the grafts. These results suggest that CD47mAb400 protects the graft against ROS production during OLT.
DISCUSSION
During OLT, IRI leads to a number of pathological changes, including an inflammatory response and a rise in ROS resulting in oxidative stress that can ultimately damage cells and lead to cell death. This study implicates CD47 as a potential target for reducing IRI and improving patient outcomes for the first time in the setting of liver transplantation. Interestingly, when administered solely to the donor, CD47mAb400 resulted in no significant survival benefit. However, when the antibody was given to both the donor and the recipient, a significant survival benefit was observed in the group that received the CD47mAb400. These results suggest that administration of the antibody to both the recipient and the donor graft effectively reduces IRI and improves overall survival outcomes.
NO is synthesized enzymatically from L-arginine in numerous cell types by distinct isoforms of the enzyme nitric oxide synthase (NOS). Under normal physiologic conditions, only the eNOS isoform is constitutively expressed by the liver and generates low transient concentrations of NO (picomolar to nanomolar) in response to agonist stimulation to regulate blood flow because of its potent vasodilatory effects.25-27 The NO generated by eNOS also has antiinflammatory properties28 and is thus beneficial in reducing ischemic insults and improves tissue survival.29 Blockade of the TSP-1/CD47 interaction enhances the production of NO generated by eNOS.30,31 However, under stressful conditions such as ischemia and transplantation, the inducible form of iNOS expression is induced, and this generates high sustained amounts (nanomolar to micromolar) of NO.32 The subsequent interaction of iNOS-derived NO with the superoxide anions that are generated by the ongoing oxidative stress following liver transplantation results in the production of peroxynitrite molecule and can elicit cytotoxicity and tissue damage. In addition, it has been reported in other studies that inhibition of iNOS can attenuate IRI in the ischemia-insulted tissues.33 The reduction in iNOS expression in the CD47mAb400-treated livers appears to result from the enhanced NO signaling and its antiinflammatory effects, one of which is to reduce the expression of inflammatory enzymes such as iNOS.
Studies have shown that the downstream effects of the inhibitory CD47/TSP-1 signaling pathway exacerbates IRI by reducing eNOS-derived NO signaling and that blocking this inhibitory interaction reduces IRI and increases the viability of various ischemic tissues after reperfusion. In a mouse renal IRI warm transient ischemia model, CD47 knockout mice were protected against tubular damage and renal dysfunction. In our previous work with a syngeneic rat model of renal transplantation, administration of CD47mAb before transplantation led to a marked improvement in post-transplant survival and lower levels of renal injury.34 In this study, CD47 expression was significantly up-regulated after cold ischemia and OLT. However, TSP-1 expression was not elevated during cold ischemia, and this could be due to the elimination of blood cells, including platelets, by the donor flush after procurement. Furthermore, our data showed that the administration of CD47mAb400 reduced both CD47 and TSP-1 expression 48 hours after OLT. Hence, this blocking effect of CD47/TSP-1 interaction reduced IRI and improved survival in the experimental rat model of OLT with a long period of cold ischemia. Moreover, the data showed that giving CD47mAb400 to both donors and recipients reduced hepatocellular injury, inflammatory cell influx, the production of inflammatory mediators, and necrosis.
During IRI, IL-1β plays a critical role in recruiting neutrophils,35 whereas TNF-α is a key mediator of the immune response and is involved in both the inflammatory response as well as necrosis.36 We showed that IL-1β, IL-6, and TNF-α expression were reduced in livers treated with CD47mAb400, and this correlated with the reduction in liver injury. The impaired sinusoidal blood flow is also reported to be linked to decreased intrahepatic energy (adenosine triphosphate depletion) and subsequent hepatocyte dysfunction.37-39 This phenomenon is considered to be the predominant form of cell death in transplanted livers.40 The significant reduction in hepatocyte apoptosis after OLT in the CD47mAb400 group highlights the possible therapeutic effects of CD47 blockade in OLT.
IRI leads to oxidative stress and the production of ROS, which damages liver tissue.9,41 Our data indicate that CD47mAb400 treatment leads to a significant reduction in iNOS expression and ROS production, and this suggests the ability of this antibody to diminish oxidative stress during IRI. Peroxynitrite (ONOO−), which is a product of iNOS-derived NO and super oxygen radical interactions, can lead to severe molecular and cellular damage upon its production. We evaluated the intensity of protein damage caused by peroxynitrite anion by measuring the presence of 3-nitrotyrosine in tissue extracts. The administration of CD47mAb400 caused a marked reduction in the concentration of 3-nitrotyrosine in liver tissues of donors versus the control group.
In conclusion, we demonstrated that the blockade of the CD47 receptor and thus the CD47/TSP-1 inhibitory pathway resulted in the protection of hepatocytes during transplantation and decreased liver injury after an extended cold ischemic period. Additionally, we provided evidence that the administration of a CD47 antibody to both the recipient and the donor enhanced the overall outcomes in comparison with the treatment of the donor organ alone in a liver transplantation model. Together, the data suggest that CD47mAb400 administration to the liver graft and also to recipients may be useful for improving patient outcomes in liver transplantation, particularly with longer preservation time periods.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (award number R44HL097521). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DHE
dihydroethidium
- ELISA
enzyme-linked immunosorbent assay
- eNOS
endothelial nitric oxide synthase
- HPF
high-power field
- IgG
immunoglobulin G
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IRI
ischemia/reperfusion injury
- mAb
monoclonal antibody
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2−
superoxide anion
- OLT
orthotopic liver transplantation
- ROS
reactive oxygen species
- SD
standard deviation
- SEM
standard error of the mean
- TBIL
total bilirubin
- TNF-α
tumor necrosis factor α
- TSP-1
ligand thrombospondin-1
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
- UW
University of Wisconsin.
Footnotes
Potential conflicts of interest: Pamela T. Manning and Ronald R. Hiebsch are employees and shareholders of Vasculox. William A. Frazier is a shareholder of Vasculox. All other authors declare no conflicts of interest.
REFERENCES
- 1.Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3–18. doi: 10.1055/s-0029-1192052. [DOI] [PubMed] [Google Scholar]
- 2.Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. doi: 10.1097/01.sla.0000109146.72827.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehrau RC, Mas VR, Dumur CI, Ladie DE, Suh JL, Luebbert S, Maluf DG. Regulation of molecular pathways in ischemia-reperfusion injury after liver transplantation. Transplantation. 2013;96:926–934. doi: 10.1097/TP.0b013e3182a20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 5.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 6.Lichtman SN, Lemasters JJ. Role of cytokines and cytokine-producing cells in reperfusion injury to the liver. Semin Liver Dis. 1999;19:171–187. doi: 10.1055/s-2007-1007108. [DOI] [PubMed] [Google Scholar]
- 7.Porte RJ. Coagulation and fibrinolysis in orthotopic liver transplantation: current views and insights. Semin Thromb Hemost. 1993;19:191–196. doi: 10.1055/s-2007-994025. [DOI] [PubMed] [Google Scholar]
- 8.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury—a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 9.Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11:1031–1047. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 10.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 11.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, –2, and –4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung J, Wang XQ, Lindberg FP, Frazier WA. Thrombospondin-1 acts via IAP/CD47 to synergize with collagen in alpha2beta1-mediated platelet activation. Blood. 1999;94:642–648. [PubMed] [Google Scholar]
- 13.Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res. 2011;63:13–22. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, et al. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res. 2012;93:682–693. doi: 10.1093/cvr/cvr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar-Melero P, Luque A, Machuca MM, Pérez de Obanos MP, Navarrete R, Rodríguez-García IC, et al. Cardiotrophin-1 reduces ischemia/reperfusion injury during liver transplant. J Surg Res. 2013;181:e83–e91. doi: 10.1016/j.jss.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 19.Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93(pt 1):64–69. [PubMed] [Google Scholar]
- 20.Neil DA, Hubscher SG. Are parenchymal changes in early post-transplant biopsies related to preservation-reperfusion injury or rejection? Transplantation. 2001;71:1566–1572. doi: 10.1097/00007890-200106150-00014. [DOI] [PubMed] [Google Scholar]
- 21.Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, et al. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093–H2100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 23.Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 24.Rogers NM, Yao M, Novelli EM, Thomson AW, Roberts DD, Isenberg JS. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol. 2012;303:1117–1125. doi: 10.1152/ajprenal.00359.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Murad F. Cyclic GMP: synthesis, metabolism, and function. Introduction and some historical comments. Adv Pharmacol. 1994;26:1–5. [PubMed] [Google Scholar]
- 27.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi H, Granger DN, Saito H, Kurose I. Assay of antioxidant and antiinflammatory activity of nitric oxide in vivo. Methods Enzymol. 1999;301:424–436. doi: 10.1016/s0076-6879(99)01106-4. [DOI] [PubMed] [Google Scholar]
- 29.Topp SG, Zhang F, Chatterjee T, Lineaweaver WC. Role of nitric oxide in surgical flap survival. J Am Coll Surg. 2005;201:628–639. doi: 10.1016/j.jamcollsurg.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Rogers NM, Thomson AW, Isenberg JS. Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:1538–1550. doi: 10.1681/ASN.2012020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: friend, foe, or just passerby. Ann N Y Acad Sci. 2002;962:275–295. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 33.Takamatsu Y, Shimada K, Yamaguchi K, Kuroki S, Chijiiwa K, Tanaka M. Inhibition of inducible nitric oxide synthase prevents hepatic, but not pulmonary, injury following ischemia-reperfusion of rat liver. Dig Dis Sci. 2006;51:571–579. doi: 10.1007/s10620-006-3172-5. [DOI] [PubMed] [Google Scholar]
- 34.Lin Y, Manning PT, Jia J, Gaut JP, Xiao Z, Capoccia BJ, et al. CD47 blockade reduces ischemia-reperfusion injury and improves outcomes in a rat kidney transplant model. Transplantation. 2014;98:394–401. doi: 10.1097/TP.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CJ, Li RW, Kahl S, Elsasser TH. Alpha-tocopherol alters transcription activities that modulates tumor necrosis factor alpha (TNF-α) induced inflammatory response in bovine cells. Gene Regul Syst Bio. 2012;6:1–14. doi: 10.4137/GRSB.S8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berthiaume F, Barbe L, Mokuno Y, MacDonald AD, Jindal R, Yarmush ML. Steatosis reversibly increases hepatocyte sensitivity to hypoxia-reoxygenation injury. J Surg Res. 2009;152:54–60. doi: 10.1016/j.jss.2007.12.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Mosbah I, Roselló-Catafau J, Alfany-Fernandez I, Rimola A, Parellada PP, Mitjavila MT, et al. Addition of carvedilol to University Wisconsin solution improves rat steatotic and nonsteatotic liver preservation. Liver Transpl. 2010;16:163–171. doi: 10.1002/lt.21968. [DOI] [PubMed] [Google Scholar]
- 39.Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the micro-circulation of the steatotic liver of the Zucker rat. Transplantation. 2001;72:1625–1631. doi: 10.1097/00007890-200111270-00008. [DOI] [PubMed] [Google Scholar]
- 40.Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 41.Connor HD, Gao W, Nukina S, Lemasters JJ, Mason RP, Thurman RG. Evidence that free radicals are involved in graft failure following orthotopic liver transplantation in the rat—an electron paramagnetic resonance spin trapping study. Transplantation. 1992;54:199–204. doi: 10.1097/00007890-199208000-00002. [DOI] [PubMed] [Google Scholar]