Abstract
Ebola outbreak-2014 (mainly Zaire strain related Ebola virus) has been declared most widely spread deadly persistent epidemic due to unavailability of rapid diagnostic, detection, and therapeutics. Ebola virus disease (EVD), a severe viral hemorrhagic fever syndrome caused by Ebola virus (EBOV) is transmitted by direct contact with the body fluids of infected person and objects contaminated with virus or infected animals. World Health Organization (WHO) has declared EVD epidemic as public health emergency of international concern with severe global economic burden. At fatal EBOV infection stage, patients usually die before the antibody response. Currently, rapid blood tests to diagnose EBOV infection include the antigen or antibodies capture using ELISA and RNA detection using RT/Q-PCR within 3–10 days after the onset of symptoms. Moreover, few nanotechnology-based colorimetric and paper-based immunoassay methods have been recently reported to detect Ebola virus. Unfortunately, these methods are limited to laboratory only. As state-of-the art (SoA) diagnostics time to confirm Ebola infection, varies from 6 h to about 3 days, it causes delay in therapeutic approaches. Thus developing a cost-effective, rapid, sensitive, and selective sensor to detect EVD at point-of-care (POC) is certainly worth exploring to establish rapid diagnostics to decide therapeutics. This review highlights SoA of Ebola diagnostics and also a call to develop rapid, selective and sensitive POC detection of EBOV for global health care. We propose that adopting miniaturized electrochemical EBOV immunosensing can detect virus level at pM concentration within ∼40 min compared to 3 days of ELISA test at nM levels.
Keywords: Ebola Virus diseases, Ebola therapeutics, Ebola diagnostics, Ebola sensor, Point-of-care sensing
Highlights
-
•
EVD is deadly persistent epidemic due to unavailability of rapid diagnosis and therapeutics.
-
•
Unfortunately, available EVD detection tools are limited to laboratory only.
-
•
Thus developing efficient e sensor to detect EVD at point-of-care is needed.
-
•
For rapid detection, we propose electrochemical Ebola virus sensing as possible solution.
-
•
These sensors can detect virus level at pM concentration within ∼40 min.
1. Introduction
Ebolaviruses (EBOV) belong to the family Filoviridae, a taxonomic group of enveloped, nonsegmented, negative single-strand RNA viruses that includes the genera Marburgvirus and Cuevavirus, and Ebolavirus (Baden et al., 2014, Beeching et al., 2014, Peters and Peters, 1999, Van Kinh Nguyen et al., 2015) ( Fig. 1, Fig. 2). There are 5 species identified for EBOV: Zaire, Bundibugyo, Sudan, Reston and Tai Forest. These species have capability of causing disease but differ in extent of progression and virulence. Fatality rates varies from ∼40% for Bundibugyo, ∼50% for Sudan, 70–90% for Zaire (Feldmann, 2014b, Tan et al., 2014). Bundibugyo, Zaire and Sudan species are associated with outbreaks in Africa.
Fig. 1.
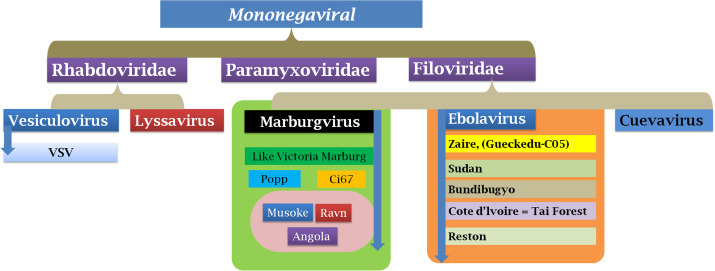
Ebola virus taxonomy and classification.
Fig. 2.
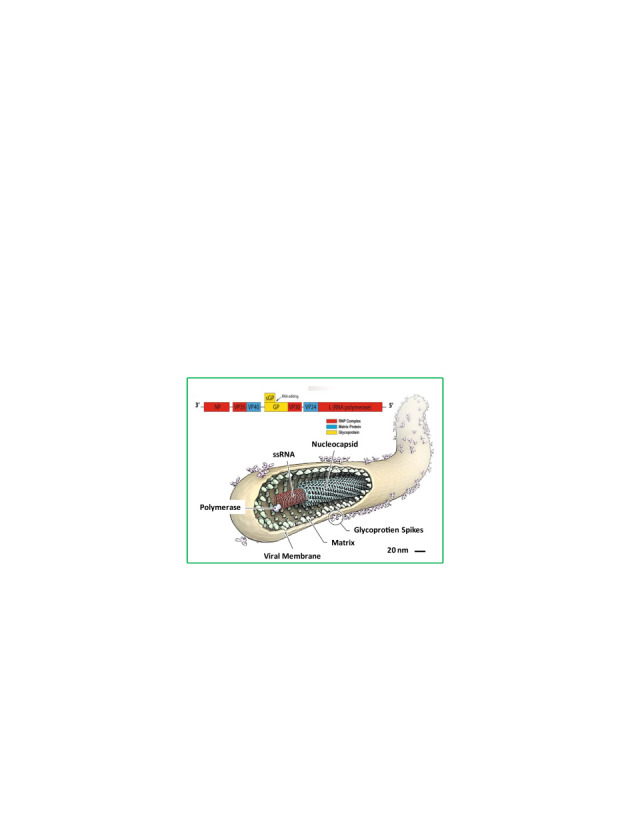
Filamentous structure Ebola virus and Zaire Ebola strain Ebola virus with genomic presentation (Source: www.rcsb.org, www.emdatabank.org). Inset: genomic sequence of Ebola virus.(Choi and Croyle, 2013; Feldmann, 2014a; Nyakatura et al., 2015; Rivera and Messaoudi, 2015; Rougeron et al., 2015; Takada, 2012).
Zaire species of EBOV lead to 2014 West African outbreak (Baize et al., 2014, Fauci, 2014). Ebola virus disease (EVD) first appeared in 1976 in 2 simultaneous outbreaks (Xu et al., 1998, Yang et al., 1998), one in Nzara, Sudan, and other in Yambuku, Democratic Republic of Congo (Breman and Johnson, 2014, Maganga et al., 2014). The latter occurred in a village near the Ebola river, hence the name of the disease. EVD is a zoonosis causing high mortality epidemics in human populations (Baden et al., 2014, Burd, 2015, Feldmann and Geisbert, 2011, Mitman, 2014, Swamy et al., 2014). The recent outbreak in West Africa (March 2014), is the biggest bizarre since its first outbreak, with huge numbers of mortality and morbidity cases (Chan, 2014, Chertow et al., 2014, Drazen et al., 2014). Countries under ruthless attacks are Guinea, Sierra Leone and Liberia. This may be partly due to handicapped health facilities, infrastructural resources, lack of knowledge, dietary habits, funeral rites and the insufficient supply of disposable equipment in hospitals. Ebola epidemic was declared as Public Health Emergency of International Concern health agencies in August 2014 (Carod-Artal, 2015, Cowling and Yu, 2015) (EVD Fact sheet N°103, WHO). As per recent reports by CDC, (as of June 2, 2015) there were a total of 27,255 cases (suspected, probable, and confirmed), 15,052 Laboratory-confirmed cases and 11,169 deaths. (Source: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/). Considerable efforts are being made by health agencies for community awareness, developing test methods, exploring markers for EVD analysis (Briand et al., 2014, Carod-Artal, 2015, Kanapathipillai et al., 2014, MacIntyre et al., 2014, Rivera and Messaoudi, 2015, Rosenbaum, 2015a, Rosenbaum, 2015b).
1.1. EBOV Genome
EBOV appears as a single filamentous particle ( Fig. 3). It has -ssRNA as genetic material along with seven transcriptional units coding for seven structural proteins ( Table 1). Additionally, there are two secreted soluble proteins (sGP and ssGP) which may act as decoys to disrupt the host immune system, by absorbing anti-GP antibodies (Choi and Croyle, 2013, Feldmann, 2014b, Feldmann and Geisbert, 2011, Mohan et al., 2012, Nyakatura et al., 2015, Rivera and Messaoudi, 2015, Rougeron et al., 2015, Takada, 2012).
Fig. 3.
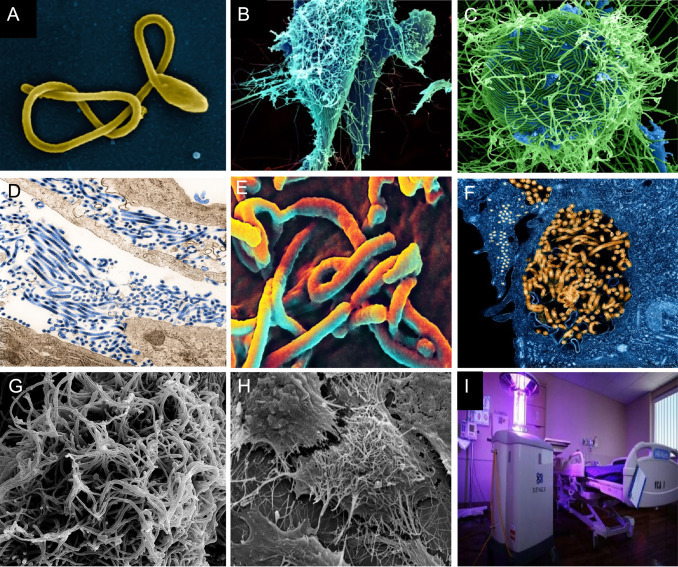
Microscopic view of EBOV Particle: (A) SEM of a single filamentous Ebola virus particle. (B) String-like EBOV particles are shedding from an infected cell in this electron micrograph. (C) SEM of filamentous Ebola virus particles attached to and budding from a chronically infected VERO E6 cell (blue) (25,000x magnification). (D) Ebola virus particles found both as extracellular particles and budding particles from chronically infected African green monkey kidney cells. (E) SEM of Ebola virus budding from the surface of a Vero cell (African green monkey kidney epithelial cell line). (F) EBOV Nucleocapsids and Virus Particles: TEM of EBOV nucleocapsids (small orange circles) and virus particles (larger orange filamentous forms) within infected African green monkey kidney cells. (G) Ebola-infected VERO E6 Cell: SEM of filamentous EBOV particles budding from an infected VERO E6 cell. (H) SEM of Ebola virions on the surface of a tetherin-expressing cell. http://www.sciencedaily.com/releases/2009/01/090127152838.htm. (Source: A-H NIAID of NIH). (I) Can robots help stop the Ebola outbreak? Germ-zapping robot could support war against Ebola (Sagripanti and Lytle, 2011). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Details for seven structural protein of Ebola.
| Nucleoprotein |
|
|
| Virion Protein 35 |
|
|
| Virion Protein 40/"Matrix Protein" |
|
|
| Glycoprotein |
|
|
| Virion Protein 30 |
|
|
| Virion Protein 24/"2nd Matrix Protein" |
|
|
| RNA Replicase |
|
|
1.2. Transmission of EBOV
Fruit bats belonging to the Pteropodidae family are thought to be natural EBOV hosts. EBOV is introduced into the human population through close contact with the blood, secretions, organs or other bodily fluids of infected animals such as chimpanzees, gorillas, fruit bats, monkeys, forest antelopes and porcupines. EBOV then gets transmitted human-to-human via direct contact (through broken skin or mucous membranes) with blood, secretions, organs or other bodily fluids of infected people, or indirectly via contaminated fomites. Health-care workers have frequently been infected while treating patients with suspected or confirmed EVD (Carod-Artal, 2015, Feldmann et al., 2004, Martines et al., 2015, Tan et al., 2014).
1.3. Life cycle of EBOV
EBOV undergoes Enzootic or Epizootic life cycle ( Fig. 4, Fig. 5). When it infects the host cells it attaches to the receptors via GP glycoprotein and gets endocytosed in host vesicles. C-type lectins DC-SIGN and DC-SIGNR play crucial role as they bind to Ebola glycoproteins and augment infection. Ebola enters early endosomes via macropinocytosis. In culture cells like dendritic cells, GP glycoprotein modulates to GP1 due to action of Cathepsin L (Cat L) and Cathepsin B (Cat B). Upon GP1 interaction with host NPC1, fusion of virus and vesicle membrane happens. Ribonucleocapsid is released followed by transcription. Replication starts and nucleoprotein encapsidates newly synthetized genomes. There is interaction of ribonucleotide with matrix protein and then virions are released via budding from plasma membrane through ESCRT complexes. (Hatfill et al., 2014, Kawaoka, 2005, Miller et al., 2012, Sullivan et al., 2003a, Sullivan et al., 2003b).
Fig. 4.
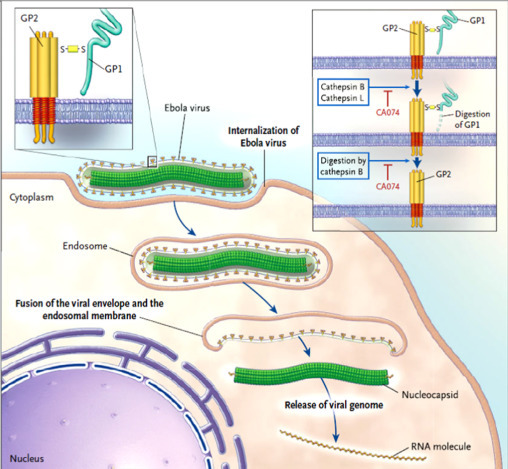
Entry pathway of Ebola Virus into host cell (Kawaoka, 2005). Upon binding to cell-surface receptors, Ebola gets internalized in endosome. Within endosome, endosomal proteases: cathepsin B and cathepsin L, slash the viral GP1 protein into N-terminal fragment and then cathepsin B digests it further into only GP2. GP2 aids in the fusion of viral envelope and endosomal membrane, releasing viral genome into the cytoplasm. Upon release the proteolysis of GP1 is prevented by CA074 (inhibitor) and therefore infection advances.
Fig. 5.
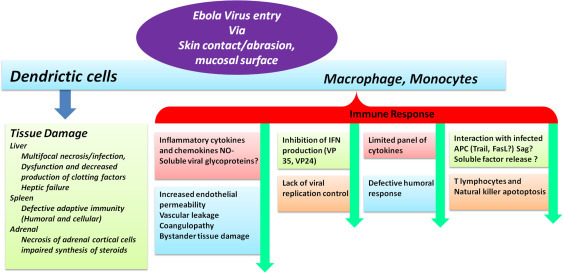
Illustration of Ebola Pathogenesis (Choi and Croyle, 2013), (Feldmann and Geisbert, 2011).
1.4. Clinical symptoms of Ebola
Clinical phase of Ebola can be broadly categorized in Phases: incubation, prodromal and convalescent or deterioration phases. Ebola has incubation period of 2-21days. Clinically, Ebola infection initiates (0–3 days) with symptoms like fever, malaise, fatigue, body aches sore joints, gastrointestinal issues like epigastric pain, nausea, vomiting and diarrhea. Associated issues are conjunctival infection, chest pain, arthralgias, myalgias and hiccups. Late complications (≥10 days) comprise of gastrointestinal hemorrhage, secondary infections, meningoencephalitis, persistent neurocognitive abnormalities, shock and hypotension ( Fig. 6) (Bah et al., 2014, Baize et al., 2002, Burd, 2015, Karwowska, 2015, Lyon et al., 2014, Rosenbaum, 2015a, Rougeron et al., 2015, Saijo et al., 2006)
Fig. 6.
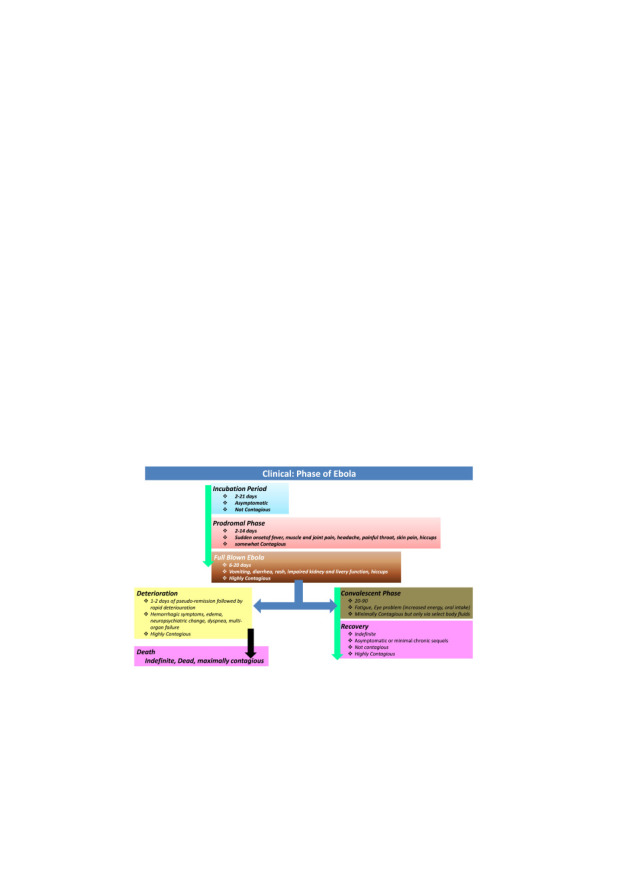
Illustration of clinical phase of EVD.
2. Advancements in diagnostic and detection of EBOV
2.1. SoA of diagnostic tools for EBOV detection
Outcomes of studies related to fundamental and applied research in the field of Ebola confirms that EVD can be controlled ( Fig. 7). Significant efforts have been made by agencies like UN Mission for Ebola Emergency Response, WHO, CDC, NIH, for the awareness to prevent Ebola transmission and possible diagnostics. For this accomplishment, safety training and networking at mass level should be conducted by local and international agencies (Butler, 2014, Cowling and Yu, 2015, Goodman, 2014, Lamontagne et al., 2014, MacIntyre et al., 2014). SoA, need of rapid diagnostics, and diagnostic tool utilized for Ebola Virus outbreak-2014 are summarized in Table 2, Fig. 8, and Fig. 9 respectively. Based on social assessment impact, bottom-up approach (i.e., introduction of new tools such as smart assay for rapid detection), and development of efficient diagnostic tools to increase health quality of Ebola infected patients is important (Farrar and Piot, 2014, Fauci, 2014, Hill et al., 2014, Kanapathipillai, 2014).
Fig. 7.
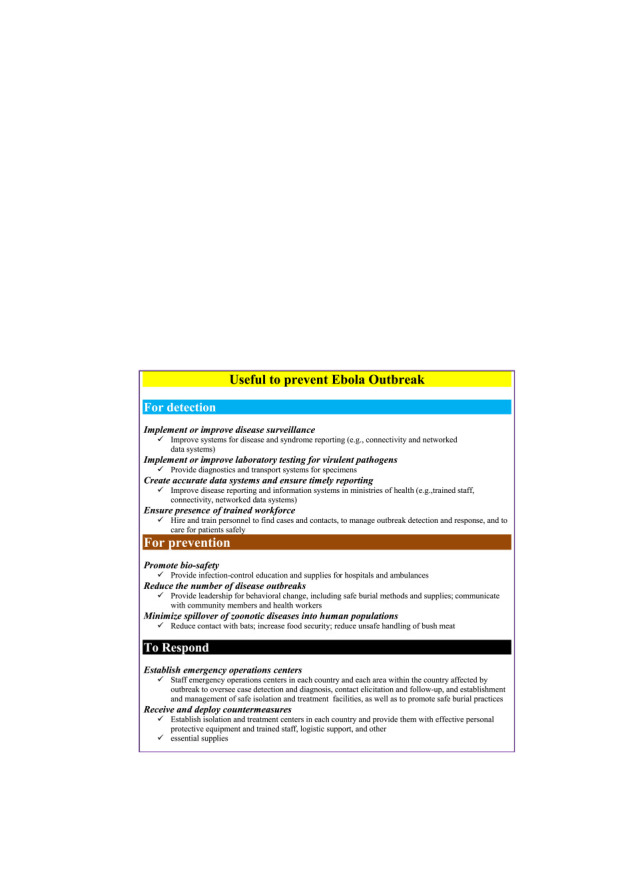
Salient features of Ebola outbreak-2014 (Frieden et al., 2014).
Table 2.
State-of the-Art of Ebola diagnostics at laboratories.
|
Laboratory Diagnostics of Ebola | ||||
|---|---|---|---|---|
| Test | Target | Source | Remarks | Detection Limit |
| Polymerase Chain reaction | Viral nucleic acid | Blood, serum, tissue |
|
Quantifies viral RNA molecules in a wide range from 104 to 1010 per reaction |
| Antigen ELISA | Viral Antigen | Blood, serum, tissue |
|
30 ng of recombinant NP (antigen) |
| Enzyme-linked immunosorbent assay (ELISA) | Virus-specific antibodies | Serum |
|
20 ng of EBOV Zaire GP |
| Immunohistochemistry | Viral Antigen | Tissue (skin & liver) |
|
Qualitative Imaging method |
| Fluorescence Assay | Viral Antigen | Tissue (liver) |
|
Qualitative Imaging method |
| Electron Microscopy | Viral particles | Blood, tissue |
|
Qualitative analysis |
| Indirect immunofluorescence assay (IFA) | Virus-specific antibodies | Serum |
|
Qualitative Imaging method |
| Immuno-blot (western blot) | Virus-specific antibodies | Serum |
|
Qualitative assay |
| Biosensors SPR, QCM, optical | Virus | Blood, Serum |
|
Biosensors, SPR, QCM, optical |
| DNA-based fluorescence nanobarcodes methodology | Viral nucleic acid | Blood tissue |
|
620 attomole |
Fig. 8.

Exploring the need to develop rapid and sensitive Ebola detection methods.
Fig. 9.
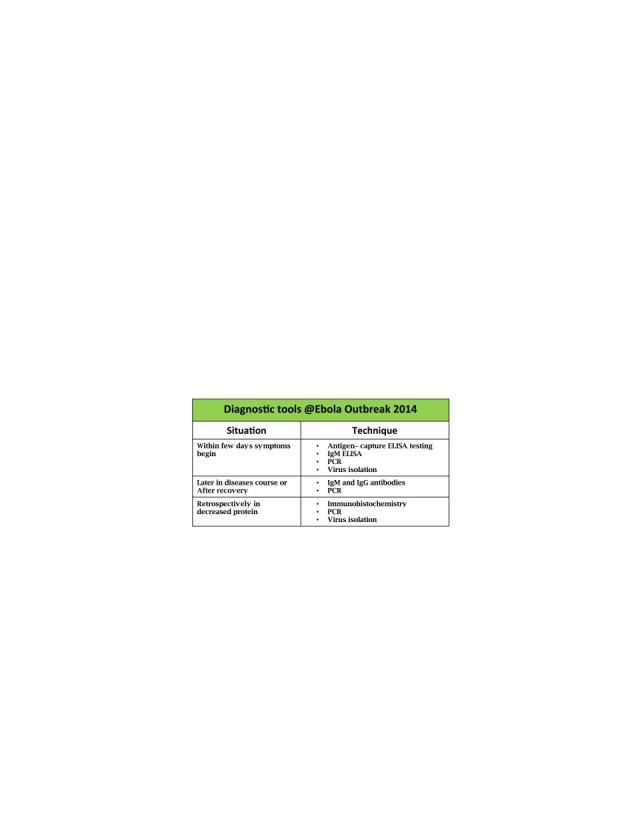
Methodologies used to detect Ebola virus concentration during Ebola outbreak-2014.
Many international health agencies such as WHO, NIH, Public Health Agency of Canada and Democratic Republic of the Congo provide medical and financial support to Ebola infected remote areas. However, portability of diagnostic tools, and unavailability of power for device operation, and refrigeration to store bio-samples is still a challenge. However, ELISA, immunoassay, genetically modifies genes, proteins, monoclonal antibody, and viral-antigen based EBOV detection approach have been successfully executed. The needs of BSL-4 facility to perform Ebola detection and diagnostics using these methods limit application of these techniques in field. (Saijo et al., 2006)
An update of laboratory diagnosis of Ebola and Marburg hemorrhagic fever was summarized by Grolla et al. (2005). Researchers described that laboratory diagnosis for EBOV is available only at national and international laboratories. This delays diagnostics due to time consuming sample collection, limited storage and complicated transport of sensitive samples. This raises demand to set-up quality diagnosis laboratory at local level. Dhillon et al described that bottom-up, integrated approach, and scaled-up strategies could be of use to monitor EBOV (Dhillon et al., 2014). Iwen et al described that an integrated approach based on automated laboratory instrumentation along with recommendations from companies and regulatory agencies, Ebola can be managed and safely controlled at clinical laboratories (Iwen et al., 2014). A study based on EBOV infected patients conducted as a critical part of the management of epidemic-2014 was proposed by Martin et al. The available diagnostic tastes are capable to rule out patients who experienced symptoms >72 h. Authors suggested the need of a method to detect Ebola at onset of symptoms and also warrant negative Ebola tests within 48–72 h (Martin et al., 2015). The utilized diagnostic protocol and safety process for 2 Ebola infected patients in USA was described by Hiill-2014 et al. These doctors suggested safe management and a menu based procedures to monitor infection related progress during a longer course of EVD treatment. The outcomes of this study will be of use for healthcare professionals to manage and cure other similar infectious diseases. (Hill et al., 2014)
As of now, research groups have highlighted major issues and demands for the development of rapid, selective, sensitive miniaturized Ebola sensing systems with reduced form factors for the recognition, screening, and detection of EBOV level. Such portable sensing platforms could provide to manage Ebola outbreak by monitoring EBOV and increasing the detection limit which will surely improve health quality. The techniques utilized till date to detect Ebola virus diseases/infections/progression are discussed below.
2.1.1. ELISA based assay for EBOV detection
In 1998, Prehaud et al purposed a nucleoprotein (NP) and glycoprotein (GP), Gabon (94 strain) based method to detect EBOV infection in humans (Prehaud et al., 1998). Authors proposed that cloning and sequencing of NP and GP gene of Gabonese strains isolated from Ebola outbreak-1996 found circulating virus in Zaire subtype Ebola strain. GP and NP were synthesized in Sf9 cells and expressed as recombinant proteins to develop ELISA for the detection of IgG antibodies in convalescent human sera from Gabon and Zaire. All the measurements were made using 50 ng of antigen in each well of ELISA plate. NP and GP based ELISA method detected IgG and IgM antibodies successfully and could be used for early diagnosis. However this assay showed issues of false result. Thus developing a selective method to detect EBOV at clinical level was in demand (Prehaud et al., 1998). A sandwich ELISA system based on EBOV antigen-detection using a monoclonal antibody (MAb) prepared via immunization of NP (Zaire subtype) was developed (Niikura et al., 2001). This developed system detected 30 ng of recombinant NP. The utilized Mab was also found reacting with corresponding region of NP derived from the Reston and Sudan subtypes. Researchers claimed that this system can be used at laboratories to detect Ebola virus at very low level (Niikura et al., 2001).
Formenty et al. (2006) proposed ELISA and RT-PCR based detection of Ebola virus in oral fluid during the Ebola-outbreak 2003 hemorrhagic fever (EVHF) in the Republic of Congo (Formenty et al., 2006). They used serum and oral fluid specimens of 24 Ebola virus infected patients along with 10 normal patients and confirmed EVHF. In this study, author failed to detect IgG against Ebola in oral fluid sample of patients whose serum samples were seropositive. Besides this, patients with positive serum, RT-PCR results were also found positive in oral fluid specimens (Formenty et al., 2006). An ELISA assay was developed by Nakayama et al. (2010) to detect filovirus species-specific antibodies. His-tagged, a secreted form of the transmembrane GPs of five different EBOV species was used as an antigen for the detection filovirus species specific antibodies. An assay was performed on antisera collected from mice immunized with virus-like particles and from humans and nonhuman primates infected with virus. A little cross-reactivity of IgG antibodies was observed in most of the mouse antisera. Results suggest that virus specific IgM antibodies were specifically detected in acute infected patients and developed method could be of use for Ebola diagnostics and management (Nakayama et al., 2010).
Lucht et al. (2003) proposed the need of a Mab to develop an Ebola diagnostic ELISA kit to analyze Zaire strain- EBOV (Lucht et al., 2003). Authors produced a specific Mab from mice immunized with inactivated viral particles and directed against viral structural protein VP40. The efficiency of developed Mab was tested using ELISA-based antigen capture protocol. This assay exhibited detection of VP40 in all virus infected samples. Moreover, SDS inactivated material can also be detected using this antigen capture ELISA method (Lucht et al., 2003). Later, this research group developed an immunofiltration based assay to detect VP40 for rapid diagnostic of EBOV infection ( Fig. 10) (Lucht et al., 2007). This was an antigen capture based approach to detect Ebola virus in chemically inactivated clinical samples. Developed assay was tested using known virus stocks. This assay can be acknowledged as the first-generation on-site tool to detect Ebola virus within 30 min. Author proposed that developed assay can be used for EBOV progression among animals and further for human (Lucht et al., 2007).
Fig. 10.
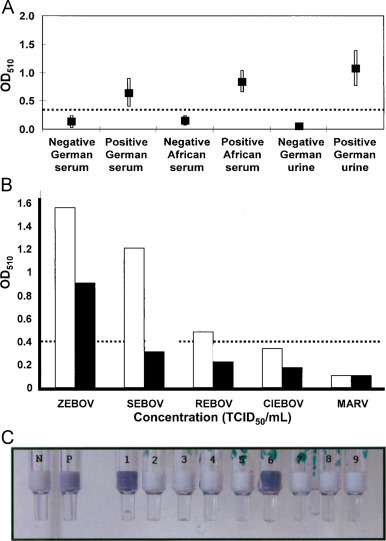
(A) Comparison of the results obtained in serum and urine samples infected by Zaire Ebola virus Mayinga (TCID50 1105 /mL), 1105/mL), and results were compared with those for corresponding unspiked samples. (B) Cross-reactivity with antigens for other Ebola virus species and Lake Victoria marburg virus Musoke. (C) Demonstration of Immuno-filtration assay based on photometric integrated with immune-filtration column at diseases location (Lucht et al., 2007).
2.1.2. Immunofluorescence based assay for EBOV detection
Saijo et al developed an immunofluorescent (IF) method to detect IgG of EBOV. Author used HeLa cells to be expressed with a baculovirus recombinant NP ( Fig. 11) (Saijo et al., 2001). Selected NP found to express Ebola virus under cytomegalovirus immediately, which is an early stage promoter. To conduct all related experiments, selected cell line was infected with the baculovirus expressed EBOV NP to perform IF based on antigen to detect Ebola virus IgG. This method is very sensitive for Ebola detection and an alternate of highly complicated procedures performed only under BSL-4 facilities (Saijo et al., 2001). Ikegami et al also promoted IF assay to detect Reston subtype EBOV subtype using HeLa cell line expressed by related EBOV NP ( Fig. 12) (Ikegami et al., 2002). Author performed IF detection of selected virus specific IgG in both hyperimmune rabbit sera and monkey. Result showed that developed assay is more sensitive for Reston subtype than Zaire subtype Ebola virus samples. Authors believe that developed method has potential for seroepidemiological studies of virus infected animals (Ikegami et al., 2002).
Fig. 11.
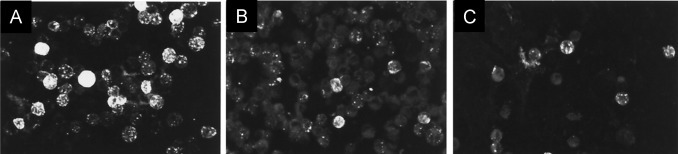
IF staining patterns of Ebola virus NP-expressing HeLa cells. (a) anti-Ebola virus NP rabbit serum. (b) Positive staining with serum collected from a patient with Ebola virus infected convalescent phase, and (c) Positive IF staining with serum collected from an Ebola infected monkey.(Saijo et al., 2001).
Fig. 12.
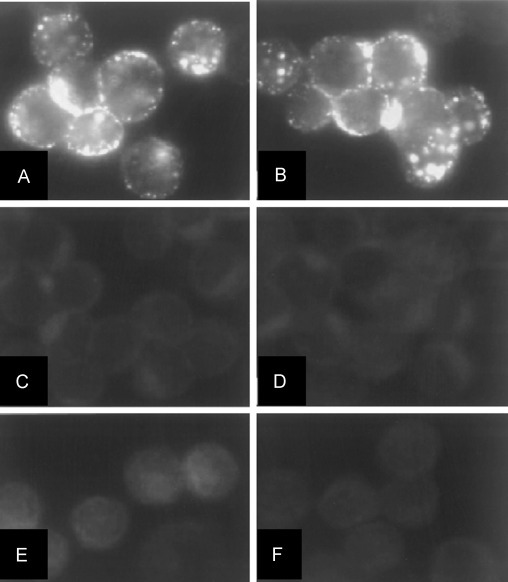
(A) IF staining of HeLa cells expressed by Ebola virus NP with anti-Ebola virus-NP rabbit serum case No. 1, (B) EBO-R infected monkey serum, (C) normal rabbit serum, (D) Ebola virus uninfected monkey serum case 2, (E), normal HeLa cells treated anti-Ebola virus-NP rabbit serum case 1, and (F) Ebola virus infected monkey serum. (Ikegami et al. 2002).
Recently Martin et al 2015 published a review to highlight EBOV pathogenesis in humans using histopathology (Martin et al. 2015). Results showed that virus infection affect liver significantly. Histopathology was successful to differentiate normal cell and virus such as Ebola virus, hemorrhagic, and filovirus fever effected cells in most of the organ ( Fig.13). This group also summarized that the pathogenesis affect mononuclear phagocytic system activation, pro-inflammatory cytokines release, immune impairment, dysregulation in endothelial on virus progression, and viral replication. These outcomes are significant for understanding viral pathogenesis, epidemics, transmission, and histopathological analysis of host organisms. However, pathogenesis of virus infection has many unanswered issues and future research should be directed to explore pathways and mechanism for better diagnosis.
Fig. 13.
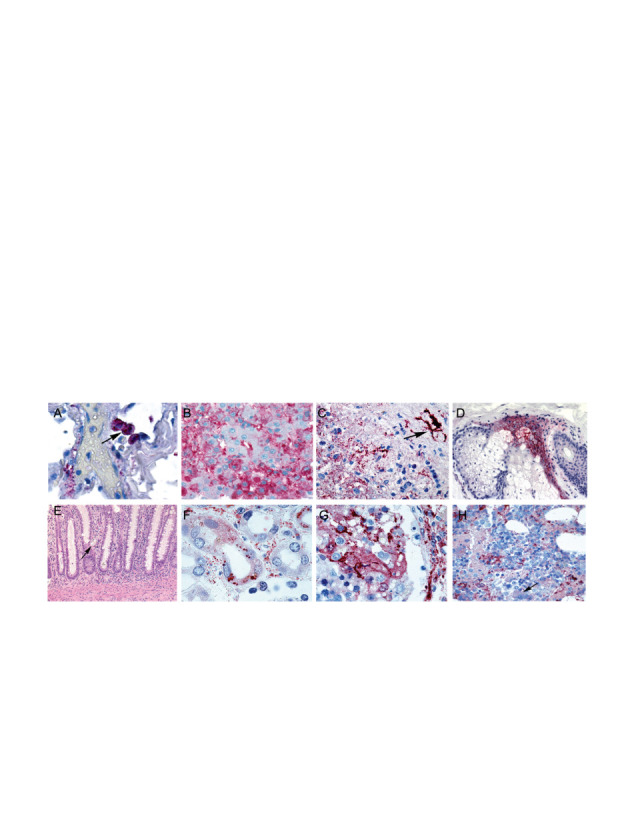
(A) Immunohistopathological analysis of in-situ hybridization and ultrastructural characteristics of fatal Ebola hemorrhagic fever. (B) Hepatic histopathological features and viral immunostaining in a fatal case of Ebola virus infection, (C) Splenic histopathological features and immunohistochemical characteristics in fatal cases of filovirus infection, (D) Cutaneous immunohistochemical and ultrastructural features in fatal cases of Ebola virus, (E) Gastrointestinal tract histopathological features and immunohistochemistry in a fatal case of Ebola hemorrhagic fever, (F) Renal histopathological features and immunohistochemistry in Ebola and Marburg hemorrhagic fevers, (G) Histopathological and immunohistochemical features in the testis of a fatal case of Ebola virus infection, (H) Bone marrow in a fatal case of Ebola virus infection (Martin et al. 2015).
2.1.3. RT-PCR based assay for EVD
In 1999, for the first time, Sanchez et al demonstrated RT-PCR for detection and characterization of EBOV infected samples collected from human and non-human premises (Sanchez et al. 1999). Authors employed RT-PCR method for the detection of viral RNA in infected body fluids and tissues. It is well known that during infection a little change take place in nucleotide or amino acid sequences of GP Ebola virus of any subtypes in comparison of uninfected original representatives. Such nonstructural secreted GP and SGP was successfully detected at acute infection in humans (Sanchez et al. 1999). Additionally, RT-PCR method to detect and quantify RNA of Ebola and Marburg Viruses, Lassa Virus, Crimean-Congo Hemorrhagic Fever Virus, Rift Valley Fever Virus, Dengue Virus, and Yellow Fever Virus was developed by Drosten et al 2002 (Drosten et al. 2002). These mentioned diseases are clinically difficult to detect, diagnose, and screen out. Novel primers and 5-nuclease detection probes were designed for specific related virus using DNA database entries. Assay sensitivity for all measurement was optimized and validated using an in vitro-transcribed RNA. A detection limit of >95% was estimated using probit regression analysis and average measurement time was within 3 hrs after receiving samples of patient. Developed PCR method was suggested to be an important part of a diagnostic procedure to detect virus-specific IgG and IgM (Drosten et al. 2002)
Trombley et al., 2010 designed 48 TaqMan™-RT-PCR assays for the detection and quantification of multiple hemorrhagic fever viruses. (Trombley et al. 2010) A set of 46 virus specific assay were analyzed and only 2 were designated for assays to measure Marburg virus. TaqMan™-RT-PCR exhibited a detection range from 10 to 0.001 plaque-forming units (PFU)/PCR. Results of presented report suggest that TaqMan™-based viral RNA real-time qRT-PCR assays can be adopted as a diagnostic tool for the identification and analysis of EVD (Trombley et al. 2010) A rapid detection of EBOV and Marburg virus via targeting NP genes of Zaire strain using RT-TaqMan-PCR as an assay tool was reported by Huang-2014.(Huang et al. 2012) Corresponds to the threshold of a standard RNA transcript, RT-TaqMan-PCR reported in this work detected virus copies widely ranging from 103 to 109 among 1010 RNA copies/mL of virus culture supernatant which is equivalent to 10,000 RNA molecules per infection. Outcomes presented in this report also clearly indicated the presence of many non-infectious particles. Authors suggest that qRT-PCR is one amongst the main analytical tool utilized which is very sensitive, selective, rapid against EBOV (Huang et al. 2012).
2.1.4. Gene Guided Approach to Detect EBOV
A multiplexed approach to detect pathogen using DNA with DNA-based fluorescence nanobarcodes methodology was described by Li et al. (2005). Authors synthesized a dendrimer-like (DL) DNA based fluorescence intensity coded nanobarcodes containing an in-built code and a probe for molecular recognition ( Fig.14). These nanobarcodes were utilized for multiplexed detection of several pathogens DNA using a fluorescence microscopy, dot blotting, and flow cytometry. Authors used unknown DNA sampled isolated from three pathogenic species namely anthracis, Ebola and SARS coronavirus for analysis. Presented method exhibited a detection limit of 6.2x10-16 mole or 620 attomole within 30s. Further, DNA from 4 multiple pathogens were detected simultaneously using nanobarcode methodology. Authors claimed that DL-DNA can be used as structural scaffolding and a functional probe. Multiplexed molecular sensing relies on the detection of precise fluorescent color ratios, and can be used to detect other deadly viruses. (Li et al., 2005)
Fig. 14.
(A) Schematic illustration of nanobarcodes 4G1R, 2G1R, 1G1R, 1G2R and 1G4R decoded based on fluorescence intensity ratio. (B) Nanobarcode colors (2-6 bar codes are 4G1R, 2G1R, 1G1R, 1G2R and 1G4R, respectively while 1 & 7 are 488–labeled starting oligonucleotide component and Bodipy 630/650–labeled starting oligonucleotide component) in an agarose gel on illumination using strong UV light. (C) Multiple target detection, (D) Dot-blotting detection of multiple DNA targets with nanobarcodes. (Li et al., 2005).
Bar et al raised an issue that the direct role of Ebola virus-GP in membrane fusion and need of low-pH activation is not clear (Bar et al., 2006). Authors developed an assay based on galactosidase (lacZ), a reporter gene in target cell for the detection of cytoplasmic exchanges which is an indication of membrane fusion with EBOV infected cell (Zaire species) ( Fig. 15). The outcomes of this study demonstrated the direct role of EBOV in progression and inhibition by anti-GP IgG (Bar et al. 2006). Kurosaki-2007 et al developed a one-step reverse transcription-loop mediated isothermal amplification (RT-LAMP) assay to detect Zaire EBOV via targeting trailer region of the viral genome (Kurosaki et al. 2007). This reported assay detected 20 copies of artificial Zaire EBOV RNA within 26 mins. Real time-monitoring detection was also performed to detect 10−3 FFU of propagated virus. RT-LAMP assay is found selective to Zaire EBOV. At good side, this method does not need sophisticated instrumentation and seems suitable for EBOV diagnosis at clinics and in-field (Kurosaki et al. 2007).
Fig. 15.
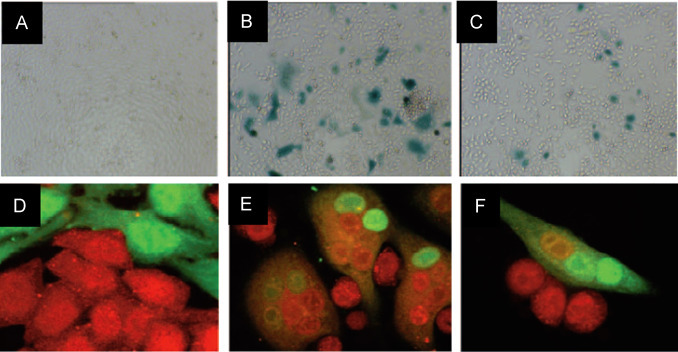
Immunofluorescence image of staining of co-cultured HeLa-CD4-LTRlacZ target cells with HeLa cells transfected with respect to HIV-1 Tat (A), Tat and HIV-1 Env (B), Tat and Ebola virus-GP (C). Confocal microscopy fields of co-cultures of CMTMR (red) labeled HeLa-CD4-LTRlacZ cells and CTG labeled (green) HeLa cells (D), HIV-1 Env (E), Ebola virus- GP (F) expression vectors. (Bar et al. 2006) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
2.1.5. Biosensors based Ebola detection
Yu et al reported that polyclonal (pAb) and Mab against Ebola virus-GP can be used to detect Ebola virus and studying related diversities. Authors developed a surface plasma resonance (SPR) and quartz crystal microbalance (QCM) immunosensor-based on pAb and mAb to detect Ebola virus produced by different strains. Besides this, Mab was also able to recognize and differentiate EBOV GP among human and non-human primates. Both immunosensor strategy were found selective (Yu et al., 2006). Recently, a paper based synthetic gene network was investigated by Pardee et al. (2014) to detect EBOV ( Fig. 16) (Pardee et al., 2014). Authors explored an in-vitro paper-based platform which is capable of detecting virus smartly using engineered gene circuits and visual transduction. Small-molecule and RNA actuation of genetic switches, rapid prototyping of complex gene circuits, and programmable in vitro diagnostics, including glucose sensors and strain-specific EBOV sensors has been successfully demonstrated using this approach. This test method is very rapid, cost-effective, sensitive (2.5–3.53), and performed detection and diagnostics based on antibody or sequences. Detail of sensor preparation and methodology is illustrated in Fig. 19 C-D. (Pardee et al., 2014)
Fig. 16.
(A) Schematic illustration of paper based genosensor consists of enzyme transcription and translation combined with engineered gene circuits to detect Ebola virus. (B) Prototype of Ebola RNA Sensors based Sudan and Zaire Strains sequencing of the Ebola Virus. 24 toehold switch-based RNA sensors were fabricated and tested-based on RNA segment windows (A–L) to detect Sudan and Zaire strains of Ebola virus at 570 nm absorption within 90 min at 30 °C (C) 240 paper-based reactions used to test the 24 sensors, yellow(control and un-triggered) and purple (activated toehold sensors). (D) Sequence specificity tested for 4 Sudan and 4 Zaire sensors.(E) Fold change of the color output rate of sensors SD and ZH over a titration of RNA concentrations.(Pardee et al. 2014).
Fig. 19.
Illustration of utilized specific Rx for Ebola
Yanik et al developed ( Fig. 17) a label free biosensor based on optofluidic nanoplasmonic to detect live viruses such as VSV, PT-Ebola, and Vaccinia in biological specimen at clinically relevant concentration (Yanik et al., 2010). This sensor due to high signal throughput and without complicated mechanical or optical isolation is proposed as efficient tool to detect small and high virus level. The direct detection of VSV, PT-Ebola, and Vaccinia is also an added advantage. A low detection range of 105 PFU/mL and a detection range from 106 to 109 PFU/mL along with a dynamic magnitude of order 3 have successfully been demonstrated. Moreover, these sensors can be developed in multiplexed manner wherein, various viral antibodies can be immobilized for selective detection of pathogens in desired body fluids. Authors claimed that extraordinary light transmission phenomena for detecting broad range of pathogens can be used immediately at clinics (Yanik et al., 2010).
Fig. 17.
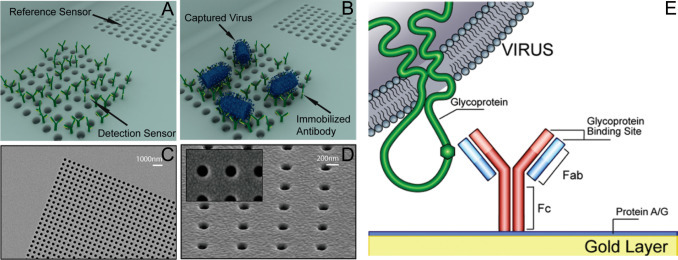
3D schematic illustration of resonance transmissions based opto-fluidic nanoplasmonic biosensors. (A) Immobilization and capturing of antibody to target virus, (B) VSV attaches only to the antibody immobilized sensor. (C) SEM of patterned SiNx membrane after Au deposition. (D) Au deposition result in suspended plasmonic nano-hole sensors without any lift-off process (inset: nanohole openings without clogging). (E) Immunosensing surface functionalization.(Yanik et al., 2010).
Daaboul-et al purposed a specific and sensitive virus/nanoparticles (60 nm)-imaging sensing assay i.e, Single Particle Interferometric Reflectance Imaging Sensor (SP-IRIS)(Daaboul et al., 2014). This nanoparticles/antibody microarray based assay possessed high throughput and rapid size variation on interaction with target virus. The detection of virus particles such as replication-competent (1) wild-type vesicular stomatitis virus (VSV), (2) defective VSV, (3), Ebola and Marburg VSV has been demonstrated using SP-IRIS ( Fig. 18). During sensing, the interaction of nanoparticles-antibody with virus changes size of particles which differentiate modified virus according to genome length. A low detection limit of 5x10-3 pfu/mL in blood within 2 hrs with respect to Ebola and Marburg VSV pseudotypes has been achieved. Authors claim that the detection of viruses expressing hemorrhagic fever virus GP using SP-IRIS has significance as a point-of-need device to detect infectious virus for diagnostics. (Daaboul et al., 2014)
Fig. 18.
Illustration of microarray configuration and detection mechanism to detect Ebola virus using single particle interferometric reflectance imaging sensor (SP-IRIS). Specific antibodies immobilized onto microarray to detect virus via colorimetric based imaging as illustrated green, red, and blue spots represent anti-VSV, anti-EBOV, and anti-MARV probes, respectively (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
2.2. Towards EVD Detection at point-of-care (POC)
Currently, rapid blood tests used to diagnose EBOV infection include the antigen or antibodies capture ELISA and RNA detection by RT-PCR within 3-10 days after the onset of symptoms. However ELISA test procedure is very cumbersome to the health care workers based on the high infectivity nature of the virus that includes several washes of the ELISA plates over a period of time. Further ELISA is difficult to perform at POC facility, while RT-PCR is complicated by genetic diversity due to rapidly changing nature of the genomic sequence of the virus. Further, these methods are limited to laboratory only. Thus the turn-around time for a confirmed laboratory Ebola diagnostics results is about 3 days and this causes delay in therapeutic approaches.
The need of an effective Ebola testing kit and smart diagnostics tool for POC EBOV sensing to identify disease, rapid infections monitoring, easy operation, and preventing epidemics are highlighted by Vogel (2014). In brief, available Corgenix and Senova testing-based on blood-antibody analysis have demonstrated EBOV detection in human patients. However, longer detection time, traveling with biosample is still an obstacle. However, both testing method are based on sophisticated device and demand need of high expertize in performing test. Experts explained both good and bad side of these devices but also due to rapid and on-site detection they recommend this method for screening tool at POC in remote area. Experts believe that, once optimized, Senova could potentially produce thousands of sensing unit per day and assay-cost would be $1 or $2 each. Recently, WHO, USAID, and Gates foundation has funded various programs to promote research for the development of new diagnostic methods to improve state-of-the-art of Ebola diagnostics. Experts suggest that WHO should increase PCR based labs to manage Ebola diagnostics of large volume of population. (Vogel, 2014)
In conclusion, developing a cost-effective, rapid, sensitive, and selective sensor to detect EBOV at POC is the need to diagnose Ebola at filed and clinics. We believe this can be achieved using electrochemical immune-sensing methodology. Electrochemical sensors have proven potential for sensitive and selective detection of markers at pM level. Recently these sensors are being integrated with microfluidic systems (sample automation) and miniaturized devices for POC target analyte detection (Cruz et al., 2014; Kaushik et al., 2014a, 2014b; Vasudev et al., 2013). However, this methodology is not yet explored for EVD. We at College of Medicine and College of engineering of FIU propose that development of a POC system consists of an electrochemical Ebola imunosensor fabricated using a specific monoclonal antibody can detect Ebola virus as low as pM levels within ∼40 min compared to 3 days of ELISA test at nM levels. If developed, such devices can be used for rapid screening of Ebola strains using available specific Anti-Ebola virus antibodies based immunosensing and can also estimate Ebola virus levels enabling a faster therapeutic approach.
2.3. Towards vaccine development against EVD
Tremendous efforts have been made by scientists to develop appropriate vaccines against EBOV infection (Fig. 19). Scientists, public health organizations, regulatory bodies and pharma-industries attended an urgent meeting namely “Ebola Vaccine-An International priority” was organized by WHO, Geneva. The aim of this meeting was to discuss SOA and possible future prospects for developing vaccines to treat EVD. The potentials of two vaccines utilized for Ebola outbreak-2014 i.e., cAd3 (approved by Glaxo Smithline and National Institute of Allergy and Infectious Diseases) and rCSVΔG-Ebola Virus-GP (-Approved by New Link Genetic and Public Health agency of Canada) was discussed at WHO. Kanapathipillai et al. reported that except these two drugs, other vaccines are only in preliminary stage and only monovalent form of cAd3 vaccine-based on Zaire strain found effective in Ebola outbreak-2014 in West Africa ( Fig. 20). The cAd3 vaccine demonstrated safety and immunogenecity but enough production of this vaccine is still an existing challenge. WHO and other public health related agencies requested Pharma companies to increase vaccines production for safety in advance and immediate therapy, in case of future Ebola Epidemic.
Fig. 20.
Genomic structures of Ebola vaccine candidates-based on rVSV (Panel A) and cAd3 (Panel B) (Kanapathipillai, 2014, Kanapathipillai et al., 2014).
Recently, Li et al. (2014) and De Clercq (2015) described advancements in development of therapeutics/vaccine to prevent and control EBOV infection. Authors suggested that EBOV positive patients should be isolated immediately after confirmation. Further, medical experts, volunteers, and inhabitants must be immunized with prophylactic vaccines to avoid all possible infections to break transmission chain. This group reported that multiple drugs against EVD are under development and only available potential antivirals are ZMapp and favipiravir against Anti-Ebola. ZMapp targets the viral entry and favipiravir targets RNA replication steps. For complete eradication of EBOV, the development of powerful drugs and vaccines is urgently required (De Clercq, 2015, Li et al., 2014).
Carette et al. (2011) explored features of EBOV entry pathway used by filoviruses to investigate potential antiviral strategies. Authors described filovirus GP based viral membrane fusion and escape from the vesicular compartment (Carette et al., 2011) which requires NPC1 protein, a cholesterol transporter. A global gene associated with human cells was utilized to explore components related with unusual entry pathway ( Fig. 21). Authors explored that aspects of lysosome function found associated with identified genes. This indicated that filoviruses exploit this organelle differently. Authors purposed that investigated role of hereditary disease gene NPC1 in viral entry, infection and pathogenesis will be of use to develop therapy to cure filovirus (Carette et al., 2011).
Fig. 21.
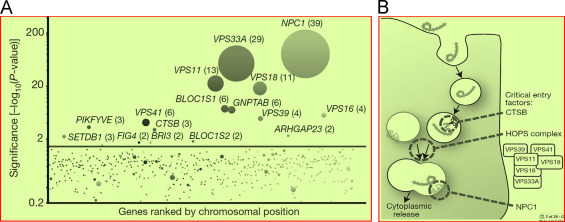
(A) Illustration of gene-trap insertions in the rVSV-GP-Ebola virus selected cell population. (B) Presentation of hypothetical model to understand the roles of CTSB, the HOPS complex and NPC1 during Ebola virus entry.(Carette et al. 2011).
Bornholdt et al. (2013) ( Fig. 22) explored structural rearrangement of EBOV VP40 begets multiple functions during viral life cycle (Bornholdt et al., 2013). It is well known VP40 protein performs multiple distinct roles in the EBOV life cycle. Authors used multiple crystal structures to explain biochemistry, and cellular microscopy related to VP40 rearrangement with respect to distinct function of EBOV life cycle. Variations in structure-shifting properties of VP40 provide added informatics to develop antiviral drugs for therapeutics. Moreover, VP40 provides a way to establish correlations with EBOV genomes with the essential functions targeted proteins (Bornholdt et al., 2013).
Fig. 22.
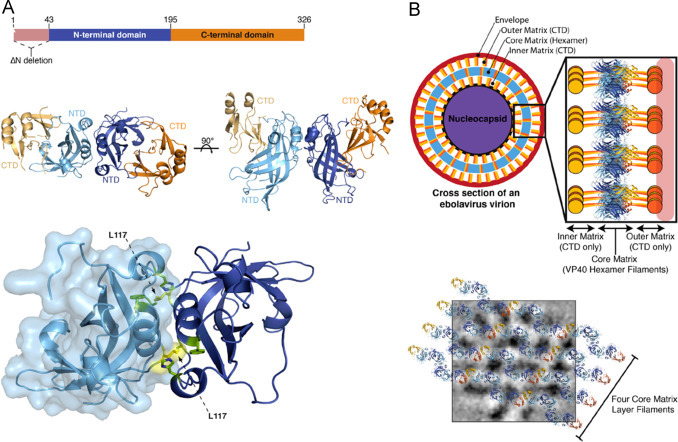
(A) The Dimeric Structure of VP40 and related changed after Ebola virus infection. Dimeric crystal structure of VP40 EDN displayed with NTDs. This dimeric interface displayed A55, H61, F108, A113, and M116 (green) and L117 (yellow). (B) A cross-section of an Ebola virus virion is modeled by assembly of VP40 (NTD is in blue), And (B)2D modeling of 4 individual VP40 filaments derived from the assembly of VP40 hexamers.(Bornholdt et al., 2013) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Among four major main strains of EBOV responsible for hemorrhagic fever, Zaire subtype related Ebola outbreak exhibited highest lethality. It was uncontrolled due to rapid virus progression that did not allow developing immunity and lack of effective anti-viral therapy. Sullivan-2000 et al developed of a vaccine to cure Ebola virus infection. Authors demonstrated an immunization and boosting of DNA/combinations by adenoviral vectors that encode viral proteins generated cellular and humoral immunity in cynomolgus macaques. Authors observed that T-cell mediated and humoral immunity based system exhibited therapeutics significance to clear virus. Moreover, antibody titer and cellular proliferative response also exhibited improved immunity for protection (Sullivan et al., 2003a, Sullivan et al., 2003b, Sullivan et al., 2000). These researchers have also explored EBOV infection pathway, progression, need of receptors, role of GP, and genetic variability during infection and transmission. The outcomes of this study provide a pathway to develop vaccine for EVD therapeutics. Chandran et al explored that CatB and CatL reduces multiplication of EBOV of Zaire subtype using selective protease inhibitors and protease-deficient cell lines (Chandran et al., 2005). A detailed antiviral study of these inhibitors is necessary to develop therapeutics against EBOV.
The genomic surveillance elucidates EBOV origin and transmission during Ebola outbreak-2014 by Gire et al. (2014) via sequencing 99 Ebola virus genomes isolated from 78 patients ( Fig. 23) (Gire et al. 2014). An inter-host and intra-host genetic variation accumulation characterizes viral patterns of epidemic. Ebola outbreak-2014 exhibited a genetic variability and changes distinct to lineage. In this study, a catalog of 395 mutations, including 50 fixed non synonymous changed with 8 at positions with high risk of EBOV infection was used to conduct other related studies. Authors released genomic surveillance to promote Ebola research globally to identify viral determinants of transmission dynamics, monitor viral changes and adaptation. This investigated genomic surveillance can be helpful to develop therapy and diagnostic tools for health care (Gire et al. 2014).
Fig. 23.
The top row shows the type of mutation of patient/raw identified with the Kissidougou Guinean sequence, with genomic locations indicated above and Cluster assignments are shown at the left. This informatics confirmed that Sierra Leone outbreak stemmed by the introduction of two genetically distinct viruses from Guinea around the same time. (Gire et al., 2014).
Uebelhoer et al explored for the first time the MARV and EBOV reverse genetic system-based on secreted Gaussia luciferase (gLuc) to establish two screening methods for the development of antiviral therapeutics ( Fig. 24) (Uebelhoer et al., 2014). First method was based on mini-genome replicon in BSL-2 facility exhibited screening of virus replication inhibitors using gLuc level. Another method based on gLuc utilized as a reporter gene product encoded in recombinant infection. Thus variation in gLuc level is correlated with viral growth quantification during antiviral effect. Researchers characterized these systems for proof of the concept and proposed that mini-genome and infectious virus platform can be used as bioinformatics needed to develop anti-virus therapies (Uebelhoer et al., 2014).
Fig. 24.
Illustration of filovirus genome in anti-genome (viral complementary) sense. 7 viral genes separated by intergenic regions and flanked by 50 and 30 UTRs (A), mini-genome and viral support protein plasmids used to drive the gLuc minigenome systems (B), and gLuc-expressing filovirus genomes (C)(Uebelhoer et al., 2014).
Immunogenicity, antibody neutralizing activity, and antigenic determining domain of Zaire strain EBOV fragment MFL (aa393–556) containing furin site and internal loop has been explored to develop a single rVSV-based polyvalent vaccine ( Fig. 25) (Wang et al., 2014). Outcomes suggested that recombinant protein and plasmid of MFL fragment of Zaire EBOV GP showed high levels of neutralizing antibody and induced cellular immune response in mice. It was also observed that internal fusion loop and the furin site containing domain showed significant contribution in immunogenicity of MFL. Such investigated vaccines are found effective and can be used as an antigen to produce neutralizing antibody to cure EBOV (Wang et al., 2014).
Fig. 25.
Illustration of GP monomer and MFL fragments.(Wang et al., 2014).
Choi et al for the first time investigated a single dose respiratory recombinant adenovirus-based vaccine and its long-term protection from lethal Ebola infection in non-human primates (Choi et al., 2014). A dose of respiratory and sublingual 1.4 × 109 infectious virus particles (ivp)/kg of Ad-CAGoptZGP strongly induced the response of CD8+ and CD4+ T cell specifically to EBOV GP antibodies. This vaccine was investigated in three primates and showed improved immune response to Ebola in rodents. Significantly, such formulated vaccine exhibited protection against challenge, 21 weeks after immunization. However, in case of sublingual, the developed antibodies were unable to neutralize that makes vaccine un-protective (Choi et al., 2014). The role of humoral versus cellular immunity in recombinant vesicular stomatitis virus (rVSV) vaccine for the protection against Zaire strain EBOV has been investigated using a model of EBOV infected animals (Marzi et al., 2014). The developed rVSV-Zaire Ebola GP vaccine was tested in groups of cynomolgus macaques depleted of CD4+T, CD8+T, or CD20+B cells. Detailed analysis of the results suggested that the role of antibodies was crucial for rVSV-based vaccine therapeutics against EBOV infection (Marzi et al., 2014). Mohan et al explored the phenomena of antigenic subversion, a novel EBOV escape strategy via improving host immunity (Mohan et al., 2012). Authors reported that DNA immunized GP1, 2 and/or truncated glycoprotein isoform (sGP) and among them only sGP has ability to compete with anti-GP1 2 antibodies. sGP is secreted during EBOV infection and it quantity has correlated with virus progression. Authors correlated outcomes with EBOV vaccinology and claimed its importance to elicit immunity for rapid clearing of EBOV infection before antigenic subversion. Antigenic subversion represents a novel virus escape strategy that likely helps EBOV evade host immunity, and may represent an important obstacle to EBOV vaccine design (Mohan et al., 2012).
It has been demonstrated that human adenovirus type 5 vectors (rAd5) is capable for encoding of Ebola virus GP to generate protective immunity against acute Zaire Ebola virus infection (Stanley et al., 2014). But therapeutics of rAd5 is unable to protect animals. Stanley et al showed for the first time that chimpanzee derived replication defective adenovirus (ChAd3) vaccine modified with MVA generated longer protection against EBOV infection in animals (Stanley et al., 2014). Macrophages and dendritic cells, antigen presenting cells (APCs) exhibit early and in-vivo sustained targets of EBOV infection. Recently, the effect of pyridinyl imidazole inhibitors of p38 MAPK on EBOV infection on human APCs and on virus mediated cytokine production has been demonstrated by Jackson et al using an EBOV like particles (VLP). Results suggested that utilized p38 MAPK inhibitor blocked EBOV infection and replication and thus may be proposed as potential therapeutics (Johnson et al., 2014).
Above discussion highlighted the efforts made by researchers in direction to explore EBOV infection pathway, host-cell target, possible antibodies/antigen, and genomic sequencing to develop vaccine against EVD. However, no potential vaccine is available yet in market that could be of use against EBOV infection. Thus current review is also a call to increase significant efforts in advance to develop an effective vaccine to cure EVD.
3. Challenges and future prospects
As deliberated above, Ebola expert (Dr. Aileen Marty at Department of Medicine, FIU) claimed that lack of appropriate safety, work stations, and efficient selective rapid Ebola diagnostics methodologies are the main challenges to fight against EBOV in future. An effective EVD monitoring and treatment demands for the establishment of BSL-4 level laboratories internationally to explore more fundamental and applied research in EBOV for development of effective diagnostics system. As of now, enough tools to prevent EBOV transmission, pathogenesis, replication, and health effects are being explored. RNA/protein, ELISA, immunofluorescent assays, RT/Q-PCR, disposable immunoassays, SPR, and QCM based Ebola detection are serving good at clinical level only for recognition and detection of EBOV. Unfortunately, random false results exhibited by these methods are still unsolved issues. However, smart miniaturized and POC Ebola sensing devices or diagnostic kits are not explored yet for selective rapid detection of EBOV level need for timely therapeutics.
Thus, developing a cost-effective, rapid, sensitive, and selective sensor to detect EBOV at POC will be worth exploring. This can possibly be achieved using electrochemical immunosensing methodology. Nano-electronics and nanoplatform based electrochemical immunosensors based miniaturized devices are capable of detecting target analytes (pM level) at clinics and at disease location. The integration of such miniaturized sensing devices with me NEMS/MEMS is also increasing for automation of sample and for accurate and precise measurement. We hypothesize that development of specific Ebola monoclonal antibody based electrochemical immunosensor can detect EBOV selectively at pM level within ∼40 min. These devices can be used for rapid screening and estimation of EBOV level especially at early stage. The investigation of miniaturized potentiostat for rapid, sensitive, and selective detection of EBOV at POC will help to decide the therapeutic decision of Ebola infected patients. Moreover, rapid assessment of EBOV using miniaturized electrochemical ebola sensing systems will improve the EBOV progression and personalized health care of patients ( Fig. 26). In future a fully optimized POC Ebola sensing device can be used for on-site diagnostics of infected patients. This would be of great importance to decide therapeutics.
Fig. 26.
Schematic illustration of possible future prospects of miniaturized nano-enabling electrochemical Ebola sensor for POC application.
4. Conclusion
This review highlights EBOV associated health effects, pathogenesis, Ebola outbreak-2014, and SOA of Ebola diagnostics for health care to decide therapeutics. An impactful research has been conducted to explore the strategies to control the transmission and epidemic. Efforts have also been made to improve detection performance of Ebola diagnostic tools in particular to achieve rapid and selective detection to decide therapies. The challenge of discrimination among symptoms of Ebola and other deadly fever like malaria and diarrhea is remain unresolved and raise the demand to develop highly sensitive diagnostic tools at early stage. The available diagnostic tools are capable of detecting Ebola virus concentration but the longer analysis time and portability limit their applications in field. According to SOA of miniaturized sensing technology, POC Ebola detection can be achieved via adopting electrochemical Ebola immunosensing methodologies. This review is a request to initiate high level multidisciplinary research in collaboration of biologist, nanotechnologist, and engineers to developed smart compartments to integrate POC Ebola sensing devices in BSL-4 environment. The successful demonstration of Ebola sensing devices in-field for Ebola virus detection at pM level within 30 minutes in all virus strain at diseases site to decide therapeutics should be the aim of future research approach.
Acknowledgments
This work was supported by NIH grants namely RO1-DA027049, R21-MH 101025, RO1-MH085259, and RO1-DA 034547.
The Public Health & Security Interface (HSI) of the Global Outbreak Alert and Response Network (GOARN), WHO and NSF-AIR IIP-1237818 "PFI-AIR: CREST-I/UCRC-Industry Ecosystem to Pipeline Research" are also acknowledged.
References
- Baden L.R., Kanapathipillai R., Campion E.W., Morrissey S., Rubin E.J., Drazen J.M. Ebola - an ongoing crisis. N. Engl. J. Med. 2014;371(15):1458–1459. doi: 10.1056/NEJMe1411378. [DOI] [PubMed] [Google Scholar]
- Bah E.I., Lamah M.-C., Fletcher T., Jacob S.T., Brett-Major D.M., Sall A.A., Shindo N., Fischer W.A., Lamontagne F., Saliou S.M., Bausch D.G., Moumié B., Jagatic T., Sprecher A., Lawler J.V., Mayet T., Jacquerioz F.A., Mendez Baggi M.F., Vallenas C., Clement C., Mardel S., Faye O., Faye O., Soropogui B., Magassouba N., Koivogui L., Pinto R., Fowler R.A. Vol. 372. 2014. Clinical Presentation of Patients with Ebola Virus Disease in Conakry, Guinea; pp. 40–47. (N. Engl. J. Med.). [DOI] [PubMed] [Google Scholar]
- Baize S., Leroy E., Georges A., GEORGES†COURBOT M.C., Capron M., Bedjabaga I., LANSOUD†SOUKATE J.,, Mavoungou E. Inflammatory responses in Ebola virus†infected patients. Clin. Exp. Immunol. 2002;128(1):163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N.F., Soropogui B., Sow M.S., Keïta S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traoré A., Kolié M., Malano E.R., Heleze E., Bocquin A., Mély S., Raoul H., Caro Vr, Cadar Dn, Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A.K., Formenty P., Van Herp M., Günther S. Emergence of Zaire Ebola Virus Disease in Guinea. N. Engl. J. Med. 2014;371(15):1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- Bar S., Takada A., Kawaoka Y., Alizon M. Detection of cell-cell fusion mediated by Ebola virus glycoproteins. Journal of virology. 2006;80(6):2815–2822. doi: 10.1128/JVI.80.6.2815-2822.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeching N.J., Fenech M., Houlihan C.F. Ebola virus disease. Br. Med. J. 2014;349:g7348. doi: 10.1136/bmj.g7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornholdt Z.A., Noda T., Abelson Dafna M., Halfmann P., Wood Malcolm R., Kawaoka Y., Saphire Erica O. Structural Rearrangement of Ebola Virus VP40 Begets Multiple Functions in the Virus Life Cycle. Cell. 2013;154(4):763–774. doi: 10.1016/j.cell.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman J.G., Johnson K.M. Ebola Then and Now. N. Engl. J. Med. 2014;371(18):1663–1666. doi: 10.1056/NEJMp1410540. [DOI] [PubMed] [Google Scholar]
- Briand S., Bertherat E., Cox P., Formenty P., Kieny M.-P., Myhre J.K., Roth C., Shindo N., Dye C. The International Ebola Emergency. N. Engl. J. Med. 2014;371(13):1180–1183. doi: 10.1056/NEJMp1409858. [DOI] [PubMed] [Google Scholar]
- Burd E.M. Ebola Virus: a Clear and Present Danger. J. Clin. Microbiol. 2015;53(1):4–8. doi: 10.1128/JCM.03115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. Ebola experts seek to expand testing. Nature. 2014:516. doi: 10.1038/516154a. [DOI] [PubMed] [Google Scholar]
- Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal F.J. Illness due the Ebola virus: epidemiology and clinical manifestations within the context of an international public health emergency. Rev. Neurol. 2015;60(6):267–277. [PubMed] [Google Scholar]
- Chan M. Ebola Virus Disease in West Africa — No Early End to the Outbreak. N. Engl. J. Med. 2014;371(13):1183–1185. doi: 10.1056/NEJMp1409859. [DOI] [PubMed] [Google Scholar]
- Chandran K., Sullivan N.J., Felbor U., Whelan S.P., Cunningham J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308(5728):1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow D.S., Kleine C., Edwards J.K., Scaini R., Giuliani R., Sprecher A. Ebola Virus Disease in West Africa — Clinical Manifestations and Management. N. Engl. J. Med. 2014;371(22):2054–2057. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Croyle M.A. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. BioDrugs. 2013;27(6):565–583. doi: 10.1007/s40259-013-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Jonsson-Schmunk K., Qiu X., Shedlock D.J., Strong J., Xu J.X., Michie K.L., Audet J., Fernando L., Myers M.J., Weiner D., Bajrovic I., Tran L.Q., Wong G., Bello A., Kobinger G.P., Schafer S.C., Croyle M.A. A single dose respiratory recombinant adenovirus-based vaccine provides long-term protection for non-human primates from lethal Ebola infection. Mol. Pharmaceut. 2014 doi: 10.1021/mp500646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling B.J., Yu H. Ebola: worldwide dissemination risk and response priorities. The Lancet. 2015;385(9962):7–9. doi: 10.1016/S0140-6736(14)61895-X. [DOI] [PubMed] [Google Scholar]
- Cruz A.F.D., Norena N., Kaushik A., Bhansali S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014;62:249–254. doi: 10.1016/j.bios.2014.06.053. [DOI] [PubMed] [Google Scholar]
- Daaboul G.G., Lopez C.A., Chinnala J., Goldberg B.B., Connor J.H., Unlu M.S. Digital sensing and sizing of vesicular stomatitis virus pseudotypes in complex media: a model for ebola and Marburg detection. ACS nano. 2014;8(6):6047–6055. doi: 10.1021/nn501312q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Ebola virus (EBOV) infection: Therapeutic strategies. Biochem. Pharmacol. 2015;93(1):1–10. doi: 10.1016/j.bcp.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon R.S., Srikrishna D., Sachs J. Controlling Ebola: next steps. The Lancet. 2014;384(9952):1409–1411. doi: 10.1016/S0140-6736(14)61696-2. [DOI] [PubMed] [Google Scholar]
- Drazen J.M., Campion E.W., M.D. E.J.R., Morrissey S., Baden L.R. Ebola in West Africa at One Year - From Ignorance to Fear to Roadblocks. N. Engl. J. Med. 2014;0(0) doi: 10.1056/NEJMe1415398. null. [DOI] [PubMed] [Google Scholar]
- Drosten C., Göttig S., Schilling S., Asper M., Panning M., Schmitz H., Günther S. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 2002;40(7):2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J.J., Piot P. The Ebola emergency - immediate action, ongoing strategy. N. Engl. J. Med. 2014;371(16):1545–1546. doi: 10.1056/NEJMe1411471. [DOI] [PubMed] [Google Scholar]
- Fauci A.S. Ebola-underscoring the global disparities in health care resources. N. Engl. J. Med. 2014;371(12):1084–1086. doi: 10.1056/NEJMp1409494. [DOI] [PubMed] [Google Scholar]
- Feldmann H. Ebola-A growing threat? N. Engl. J. Med. 2014;371(15):1375–1378. doi: 10.1056/NEJMp1405314. [DOI] [PubMed] [Google Scholar]
- Feldmann H. Ebola -A Growing Threat? N. Engl. J. Med. 2014;371(15):1375–1378. doi: 10.1056/NEJMp1405314. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Geisbert T.W. Ebola hemorrhagic fever. The Lancet. 2011;377(9768):849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Wahl-Jensen V., Jones S.M., Ströher U. Ebola virus ecology: a continuing mystery. Trends Microbiol. 2004;12(10):433–437. doi: 10.1016/j.tim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Formenty P., Leroy E.M., Epelboin A., Libama Fo, Lenzi M., Sudeck H., Yaba P., Allarangar Y., Boumandouki P., Nkounkou V.B. Detection of Ebola virus in oral fluid specimens during outbreaks of Ebola virus hemorrhagic fever in the Republic of Congo. Clin. Infect. Dis. 2006;42(11):1521–1526. doi: 10.1086/503836. [DOI] [PubMed] [Google Scholar]
- Frieden T.R., Damon I., Bell B.P., Kenyon T., Nichol S. Ebola 2014 - New Challenges, New Global Response and Responsibility. N. Engl. J. Med. 2014;371(13):1177–1180. doi: 10.1056/NEJMp1409903. [DOI] [PubMed] [Google Scholar]
- Gire S.K., Goba A., Andersen K.G., Sealfon R.S., Park D.J., Kanneh L., Jalloh S., Momoh M., Fullah M., Dudas G. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345(6202):1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J.L. Studying Secret Serums- Toward Safe, Effective Ebola Treatments. N. Engl. J. Med. 2014;371(12):1086–1089. doi: 10.1056/NEJMp1409817. [DOI] [PubMed] [Google Scholar]
- Grolla A., Lucht A., Dick D., Strong J., Feldmann H. Laboratory diagnosis of Ebola and Marburg hemorrhagic fever. Bull. Soc. Pathol. Exotique. 2005;98(3):205. [PubMed] [Google Scholar]
- Hatfill S.J., Nordin T., Shapiro G.L. Ebola Virus Disease. J. Am. Phys. Surgeons. 2014;19:4. [Google Scholar]
- Hill C.E., Burd E.M., Kraft C.S., Ryan E.L., Duncan A., Winkler A.M., Cardella J.C., Ritchie J.C., Parslow T.G. Laboratory test support for Ebola patients within a high-containment facility. Lab Med. 2014;45(3):e109–e111. doi: 10.1309/LMTMW3VVN20HIFS. [DOI] [PubMed] [Google Scholar]
- Huang Y., Wei H., Wang Y., Shi Z., Raoul H., Yuan Z. Rapid detection of filoviruses by real-time TaqMan polymerase chain reaction assays. Virol. Sin. 2012;27(5):273–277. doi: 10.1007/s12250-012-3252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T., Saijo M., Niikura M., Miranda M.E., Calaor A.B., Hernandez M., Manalo D.L., Kurane I., Yoshikawa Y., Morikawa S. Development of an immunofluorescence method for the detection of antibodies to ebola virus subtype reston by the use of recombinant nucleoprotein†expressing HeLa Cells. Microbiol. Immunol. 2002;46(9):633–638. doi: 10.1111/j.1348-0421.2002.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Iwen P.C., Garrett J.L., Gibbs S.G., Lowe J.J., Herrera V.L., Sambol A.R., Stiles K., Wisecarver J.L., Salerno K.J., Pirruccello S.J. An integrated approach to laboratory testing for patients with ebola virus disease. Lab Med. 2014;45(4):e146–e151. doi: 10.1309/LMTULFM62W3RKMYI. [DOI] [PubMed] [Google Scholar]
- Johnson J.C., Martinez O., Honko A.N., Hensley L.E., Olinger G.G., Basler C.F. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. 2014:102–109. doi: 10.1016/j.antiviral.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapathipillai R. Ebola Virus Disease: Current Knowledge. N. Engl. J. Med. 2014;371(13):e18. doi: 10.1056/NEJMp1410741. [DOI] [PubMed] [Google Scholar]
- Kanapathipillai R., Henao Restrepo A.M., Fast P., Wood D., Dye C., Kieny M.-P., Moorthy V. Ebola Vaccine- An Urgent International Priority. N. Engl. J. Med. 2014;371(24):2249–2251. doi: 10.1056/NEJMp1412166. [DOI] [PubMed] [Google Scholar]
- Karwowska K. Ebola virus disease. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2015;38(223):42–45. [PubMed] [Google Scholar]
- Kaushik A., Ruiz A., Bhansali S., Nair M. Miniaturized Sensing Devices for Biomarker Detection. J. Biosens. Bioelectron. 2014;5(4):1000e1132. [Google Scholar]
- Kaushik A., Vasudev A., Arya S.K., Pasha S.K., Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y. How Ebola Virus Infects Cells. N. Engl. J. Med. 2005;352(25):2645–2646. doi: 10.1056/NEJMcibr051754. [DOI] [PubMed] [Google Scholar]
- Kurosaki Y., Takada A., Ebihara H., Grolla A., Kamo N., Feldmann H., Kawaoka Y., Yasuda J. Rapid and simple detection of Ebola virus by reverse transcription-loop-mediated isothermal amplification. J. Virol. Methods. 2007;141(1):78–83. doi: 10.1016/j.jviromet.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Lamontagne Fo, Clement C., Fletcher T., Jacob S.T., Fischer W.A., Fowler R.A. Doing Today Work Superbly Well — Treating Ebola with Current Tools. N. Engl. J. Med. 2014;371(17):1565–1566. doi: 10.1056/NEJMp1411310. [DOI] [PubMed] [Google Scholar]
- Li H., Ying T., Yu F., Lu L., Jiang S. Development of therapeutics for treatment of Ebola virus infection. Microbes and Infection. 2014 doi: 10.1016/j.micinf.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Li Y., Cu Y.T.H., Luo D. Multiplexed detection of pathogen DNA with DNA-based fluorescence nanobarcodes. Nature biotechnology. 2005;23(7):885–889. doi: 10.1038/nbt1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht A., Formenty P., Feldmann H., Götz M., Leroy E., Bataboukila P., Grolla A., Feldmann F., Wittmann T., Campbell P. Development of an immunofiltration-based antigen-detection assay for rapid diagnosis of Ebola virus infection. J. Infect. Dis. 2007;196(Suppl. 2):S184–S192. doi: 10.1086/520593. [DOI] [PubMed] [Google Scholar]
- Lucht A., Grunow R., Möller P., Feldmann H., Becker S. Development, characterization and use of monoclonal VP40-antibodies for the detection of Ebola virus. J. Virol. Methods. 2003;111(1):21–28. doi: 10.1016/s0166-0934(03)00131-9. [DOI] [PubMed] [Google Scholar]
- Lyon G.M., Mehta A.K., Varkey J.B., Brantly K., Plyler L., McElroy A.K., Kraft C.S., Towner J.S., Spiropoulou C., Ströher U., Uyeki T.M., Ribner B.S. Clinical care of two patients with Ebola virus disease in the United States. N. Engl. J. Med. 2014;371(25):2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- MacIntyre C.R., Chughtai A.A., Seale H., Richards G.A., Davidson P.M. Vol. 51. 2014. Respiratory protection for healthcare workers treating Ebola virus disease (EVD): Are facemasks sufficient to meet occupational health and safety obligations? pp. 1421–1426. (Int. J. Nurs. Stud.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganga Gl.D., Kapetshi J., Berthet N., Kebela Ilunga Bt, Kabange F., Mbala Kingebeni P., Mondonge V., Muyembe J.-J.T., Bertherat E., Briand S., Cabore J., Epelboin A., Formenty P., Kobinger G., González-Angulo L., Labouba I., Manuguerra J.-C., Okwo-Bele J.-M., Dye C., Leroy E.M. Ebola Virus Disease in the Democratic Republic of Congo. N. Engl. J. Med. 2014;371(22):2083–2091. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- Martin P., Laupland K.B., Frost E.H., Valiquette L. Laboratory diagnosis of Ebola virus disease. Intens. Care Med. 2015:1–4. doi: 10.1007/s00134-015-3671-y. [DOI] [PubMed] [Google Scholar]
- Martines R.B., Ng D.L., Greer P.W., Rollin P.E., Zaki S.R. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J. Pathol. 2015;235(2):153–174. doi: 10.1002/path.4456. [DOI] [PubMed] [Google Scholar]
- Marzi A., Engelmann F., Feldmann F., Haberthur K., Shupert W.L., Brining D., Scott D.P., Geisbert T.W., Kawaoka Y., Katze M.G. Antibodies are necessary for rVSV/ZEBOV-GP–mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc. Natl. Acad. Sci. 2014;110(5):1893–1898. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.H., Obernosterer G., Raaben M., Herbert A.S., Deffieu M.S., Krishnan A., Ndungo E., Sandesara R.G., Carette J.E., Kuehne A.I. Ebola virus entry requires the host†programmed recognition of an intracellular receptor. EMBO J. 2012;31(8):1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitman G. Ebola in a Stew of Fear. N. Engl. J. Med. 2014;371(19):1763–1765. doi: 10.1056/NEJMp1411244. [DOI] [PubMed] [Google Scholar]
- Mohan G.S., Li W., Ye L., Compans R.W., Yang C. Antigenic subversion: a novel mechanism of host immune evasion by Ebola virus. PLoS Pathogens. 2012;8(12):e1003065. doi: 10.1371/journal.ppat.1003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama E., Yokoyama A., Miyamoto H., Igarashi M., Kishida N., Matsuno K., Marzi A., Feldmann H., Ito K., Saijo M. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin. Vaccine Immunol. 2010;17(11):1723–1728. doi: 10.1128/CVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura M., Ikegami T., Saijo M., Kurane I., Miranda M.E., Morikawa S. Detection of Ebola viral antigen by enzyme-linked immunosorbent assay using a novel monoclonal antibody to nucleoprotein. J. Clin. Microbiol. 2001;39(9):3267–3271. doi: 10.1128/JCM.39.9.3267-3271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakatura E.K., Frei J.C., Lai J.R. Chemical and structural aspects of Ebola virus entry inhibitors. ACS Infect. Dis. 2015;1(1):42–52. doi: 10.1021/id500025n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee K., Green A.A., Ferrante T., Cameron D.E., DaleyKeyser A., Yin P., Collins J.J. Paper-based synthetic gene networks. Cell. 2014;159(4):940–954. doi: 10.1016/j.cell.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., Peters J. An introduction to Ebola: the virus and the disease. J. Infect. Dis. 1999;179(Suppl. 1) doi: 10.1086/514322. ix-xvi. [DOI] [PubMed] [Google Scholar]
- Prehaud C., Hellebrand E., Coudrier D., Volchkov V., Volchkova V., Feldmann H., Le Guenno B., Bouloy M. Recombinant Ebola virus nucleoprotein and glycoprotein (Gabon 94 strain) provide new tools for the detection of human infections. J. Gen. Virol. 1998;79(11):2565–2572. doi: 10.1099/0022-1317-79-11-2565. [DOI] [PubMed] [Google Scholar]
- Rivera A., Messaoudi I. Pathophysiology of Ebola virus infection: Current challenges and future hopes. ACS Infect. Dis. 2015 doi: 10.1021/id5000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum L. Communicating Uncertainty - Ebola, Public Health, and the Scientific Process. N. Engl. J. Med. 2015;372(1):7–9. doi: 10.1056/NEJMp1413816. [DOI] [PubMed] [Google Scholar]
- Rosenbaum L. License to Serve - U.S. Trainees and the Ebola Epidemic. N. Engl. J. Med. 2015;372(6):504–506. doi: 10.1056/NEJMp1415192. [DOI] [PubMed] [Google Scholar]
- Rougeron, V., Feldmann, H., Grard, G., Becker, S., Leroy, E.M., 2015. Ebola and Marburg hemorrhagic fever. [DOI] [PMC free article] [PubMed]
- Sagripanti J.-L., Lytle C.D. Sensitivity to ultraviolet radiation of Lassa, vaccinia, and Ebola viruses dried on surfaces. Arch. Virol. 2011;156(3):489–494. doi: 10.1007/s00705-010-0847-1. [DOI] [PubMed] [Google Scholar]
- Saijo M., Niikura M., Ikegami T., Kurane I., Kurata T., Morikawa S. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin. Vaccine Immunol. 2006;13(4):444–451. doi: 10.1128/CVI.13.4.444-451.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M., Niikura M., Morikawa S., Kurane I. Immunofluorescence method for detection of Ebola virus immunoglobulin G, using HeLa cells which express recombinant nucleoprotein. J. Clin. Microbiol. 2001;39(2):776–778. doi: 10.1128/JCM.39.2.776-778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A., Ksiazek T.G., Rollin P.E., Miranda M.E., Trappier S.G., Khan A.S., Peters C.J., Nichol S.T. Detection and molecular characterization of Ebola viruses causing disease in human and nonhuman primates. J. Infect. Dis. 1999;179(Suppl. 1):S164–S169. doi: 10.1086/514282. [DOI] [PubMed] [Google Scholar]
- Stanley D.A., Honko A.N., Asiedu C., Trefry J.C., Lau-Kilby A.W., Johnson J.C., Hensley L., Ammendola V., Abbate A., Grazioli F., Foulds K.E., Cheng C., Wang L., Donaldson M.M., Colloca S., Folgori A., Roederer M., Nabel G.J., Mascola J., Nicosia A., Cortese R., Koup R.A., Sullivan N.J. Nat Med advance online publication; 2014. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. [DOI] [PubMed] [Google Scholar]
- Sullivan N., Yang Z.-Y., Nabel G.J. Ebola virus pathogenesis: implications for vaccines and therapies. J. Virol. 2003;77(18):9733–9737. doi: 10.1128/JVI.77.18.9733-9737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Geisbert T.W., Geisbert J.B., Xu L., Yang Z.-y, Roederer M., Koup R.A., Jahrling P.B., Nabel G.J. Accelerated vaccination for Ebola virus hemorrhagic fever in non-human primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.-y, Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- Swamy M.A., Nair M.P., Saxena S.K. Current scenario of therapeutics for Ebola virus disease. Am. J. Infect. Dis. 2014;10(3):100. [Google Scholar]
- Takada A. Filovirus tropism: cellular molecules for viral entry. Front. Microbiol. 2012;3:34. doi: 10.3389/fmicb.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D.X., Korkmaz A., Reiter R.J., Manchester L.C. Ebola virus disease: Potential use of melatonin as a treatment. J. Pin. Res. 2014;57(4):381–384. doi: 10.1111/jpi.12186. [DOI] [PubMed] [Google Scholar]
- Trombley A.R., Wachter L., Garrison J., Buckley-Beason V.A., Jahrling J., Hensley L.E., Schoepp R.J., Norwood D.A., Goba A., Fair J.N. Comprehensive Panel of Real-Time TaqManâ„¢ Polymerase Chain Reaction Assays for Detection and Absolute Quantification of Filoviruses, Arenaviruses, and New World Hantaviruses. Am. J. Trop. Med. Hyg. 2010;82(5):954–960. doi: 10.4269/ajtmh.2010.09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelhoer, L.S., Albariño, Cs.G., McMullan, L.K., Chakrabarti, A.K., Vincent, J.P., Nichol, S.T., Towner, J.S., 2014. High-throughput luciferase-based reverse genetics systems for identifying inhibitors of Marburg and Ebola viruses. 86–94. [DOI] [PubMed]
- Van Kinh Nguyen S.C.B., Boianelli A., Meyer-Hermann M., Hernandez-Vargas E.A. Ebola virus infection modeling and identifiability problems. Front. Microbiol. 2015:6. doi: 10.3389/fmicb.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudev A., Kaushik A., Tomizawa Y., Norena N., Bhansali S. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sens. Actuators B: Chem. 2013;182:139–146. [Google Scholar]
- Vogel G. Testing new Ebola tests. Science. 2014;345(6204):1549–1550. doi: 10.1126/science.345.6204.1549. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Z., Dai Q. A highly immunogenic fragment derived from Zaire Ebola virus glycoprotein elicits effective neutralizing antibody. 2014:254–261. doi: 10.1016/j.virusres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Xu L., Sanchez A., Yang Z.-Y., Zaki S.R., Nabel E.G., Nichol S.T., Nabel G.J. Immunization for Ebola virus infection. Nat. Med. 1998;4(1):37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]
- Yang Z.-y, Delgado R., Xu L., Todd R.F., Nabel E.G., Sanchez A., Nabel G.J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279(5353):1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- Yanik A.A., Huang M., Kamohara O., Artar A., Geisbert T.W., Connor J.H., Altug H. An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett. 2010;10(12):4962–4969. doi: 10.1021/nl103025u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.-S., Liao H.-X., Gerdon A.E., Huffman B., Scearce R.M., McAdams M., Alam S.M., Popernack P.M., Sullivan N.J., Wright D. Detection of Ebola virus envelope using monoclonal and polyclonal antibodies in ELISA, surface plasmon resonance and a quartz crystal microbalance immunosensor. J. Virol. Methods. 2006;137(2):219–228. doi: 10.1016/j.jviromet.2006.06.014. [DOI] [PubMed] [Google Scholar]


