Abstract
The emergence of resting-state functional connectivity (rsFC) analysis, which examines temporal correlations of low-frequency (<0.1 Hz) blood oxygen level-dependent signal fluctuations between brain regions, has dramatically improved our understanding of the functional architecture of the typically developing (TD) human brain. This study examined rsFC in Down syndrome (DS) compared with another neurodevelopmental disorder, Williams syndrome (WS), and TD. Ten subjects with DS, 18 subjects with WS, and 40 subjects with TD each participated in a 3-Tesla MRI scan. We tested for group differences (DS vs. TD, DS vs. WS, and WS vs. TD) in between- and within-network rsFC connectivity for seven functional networks. For the DS group, we also examined associations between rsFC and other cognitive and genetic risk factors. In DS compared with TD, we observed higher levels of between-network connectivity in 6 out 21 network pairs but no differences in within-network connectivity. Participants with WS showed lower levels of within-network connectivity and no significant differences in between-network connectivity relative to DS. Finally, our comparison between WS and TD controls revealed lower within-network connectivity in multiple networks and higher between-network connectivity in one network pair relative to TD controls. While preliminary due to modest sample sizes, our findings suggest a global difference in between-network connectivity in individuals with neurodevelopmental disorders compared with controls and that such a difference is exacerbated across many brain regions in DS. However, this alteration in DS does not appear to extend to within-network connections, and therefore, the altered between-network connectivity must be interpreted within the framework of an intact intra-network pattern of activity. In contrast, WS shows markedly lower levels of within-network connectivity in the default mode network and somatomotor network relative to controls. These findings warrant further investigation using a task-based procedure that may help disentangle the relationship between brain function and cognitive performance across the spectrum of neurodevelopmental disorders.
Key words: : APOE, Down syndrome, fMRI, resting state functional connectivity, Williams syndrome
Introduction
Down syndrome (DS; trisomy 21) is a neurodevelopmental disorder that is caused by the presence of three copies of chromosome 21. It occurs in one in every 691 live births in the United States (Parker et al., 2010), and it is the most common genetic cause of intellectual disability (Lott and Dierssen, 2010; Mégarbané et al., 2009). The cognitive deficits associated with DS can vary widely across individuals, ranging from mild to severe cognitive impairment, with the mean full-scale intellectual quotient (IQ) of 50 (Lott and Dierssen, 2010). The cognitive profiles can also vary, but individuals with DS typically exhibit deficits in language, verbal short term-memory, and explicit long-term memory (Brown et al., 2003; Clark and Wilson, 2003; Edgin et al., 2010; Hodapp and Dykens, 2005; Nadel, 2003).
In terms of brain abnormalities, volumetric magnetic resonance imaging (MRI) studies have consistently reported that individuals with DS have significantly smaller whole brain, frontal and prefrontal lobe, temporal lobe, amygdalar, cerebellar, and hippocampal volumes compared with individuals with typical development (TD) (Aylward et al., 1999; Kesslak et al., 1994; Pinter et al., 2001; Raz et al., 1995; White et al., 2003). It has also been suggested that individuals with DS experience accelerated or an early onset of brain aging as evidenced by significant age-related reduction in brain volume (Beacher et al., 2010; Koran et al., 2014; Teipel et al., 2004). While numerous volumetric studies have characterized the structural brain anomalies associated with DS, less is known about the functional consequences of such structural anomalies in the DS brain. A task-based functional MRI (fMRI) study showed decreased activation in receptive language areas in DS compared with TD controls during passive story listening (Reynolds Losin et al., 2009). Another fMRI study showed atypical neural activation during an object-recognition task in DS compared with age-matched TD controls (Jacola et al., 2011). While these task-based fMRI studies suggest altered activation patterns in DS, no studies to date have characterized functional connectivity in the DS brain during rest.
The emergence of resting-state functional connectivity (rsFC), which examines temporal correlations of low-frequency (<0.1 Hz) blood oxygen level-dependent (BOLD) signal fluctuations between brain regions, has dramatically improved our understanding of the functional architecture of the human brain and could be used to measure deficits and longitudinal changes in functional brain networks. The most commonly investigated resting-state network is the default mode network (DMN). The DMN refers to a group of brain regions originally characterized by greater activation during control conditions compared with during cognitively demanding goal-oriented tasks in task-based fMRI studies (Raichle et al., 2001). A subsequent study found that regions in the DMN were functionally correlated during rest, confirming the notion that these regions form a network (Greicius et al., 2003). The four core regions consistently identified in the DMN are the medial prefrontal cortex, posterior cingulate (PCC)/precuneus cortex, and bilateral inferior parietal lobules (Gusnard and Raichle, 2001; Raichle et al., 2001); however, additional regions such as medial temporal lobe are sometimes included as a part of the DMN (Buckner et al., 2008; Greicius et al., 2003, 2004). The DMN is hypothesized to mediate task-independent intrinsic stimulus processing (Buckner et al., 2008; Gusnard and Raichle, 2001; Raichle et al., 2001; Whitfield-Gabrieli and Ford, 2012).
In addition to the DMN, rsFC studies have identified several other resting-state brain networks associated with higher cognitive functioning (Yeo et al., 2011), such as the dorsal and ventral attention networks (DAN and VAN, respectively) (Fox et al., 2006), the salience network (Seeley et al., 2007), and the frontoparietal/executive control network (Seeley et al., 2007; Vincent et al., 2008). Because rest has no cognitive or behavioral demands, rsFC could be particularly useful for characterizing functional brain network differences in populations with intellectual disabilities.
To date, only one published study has evaluated functional connectivity in individuals with DS. Anderson et al. (2013) compared functional connectivity in 15 individuals with DS to 14 TD control subjects while they viewed 50 min of cartoon video clips and found that individuals with DS exhibited higher levels of connectivity between most brain regions. Brain regions that are typically negatively correlated, such as DMN regions and regions comprising the DAN and frontoparietal network, were found to be less (negatively) correlated in individuals with DS. A functional parcellation of the brain showed a simplified network structure in DS, characterized by increased between-network connectivity, particularly in networks with shorter-range connections, which was not observed in an autism comparison sample with normal IQ (Anderson et al., 2013). The authors suggest that their findings indicate immature development of connectivity in DS with impaired ability to integrate information from distant brain regions into coherent distributed networks. Anderson et al. (2013) has provided one of the first characterizations of functional connectivity across brain networks in the DS population. This study sought to validate those findings when utilizing a conventional resting-state scanning procedure while extending those findings to include a novel comparison group and also investigation of within-network functional connectivity.
For this study, we compared between- and within-network rsFC using a published parcellation of the brain into seven functional networks (Yeo et al., 2011). Given the nature of DS as a neurodevelopmental disorder, we were interested in evaluating whether any abnormalities observed in DS were distinct in different neurodevelopmental disorders. Therefore, we compared between- and within-network rsFC connectivity in our DS group to individuals with Williams syndrome (WS) and TD controls. WS is a neurodevelopmental disorder caused by the hemizygous deletion of a 1–2 Mb contiguous genomic region containing 26–28 genes on chromosome 7q11.23 (Korenberg et al., 2000). As with DS, WS is also associated with cognitive impairment, with average full-scale IQ for individuals ranging from 50 to 60, indicating mild to moderate intellectual disability (Martens et al., 2008). The WS cognitive profile is characterized by deficits in visuospatial and implicit memory and strengths in language, verbal short-term memory, face and object recognition, and music processing skills (Conners et al., 2011; Dykens et al., 2005; Hocking et al., 2011; Tsai et al., 2008). In addition, individuals with WS often demonstrate increased nonsocial anxiety and phobias, paired with hypersociability and heightened empathy (Dykens, 2003; Mervis and John, 2010; Morris, 2010). Work in our own lab has investigated rsFC in WS but focused exclusively on auditory processing networks (Pryweller, 2013). To our knowledge, there are no other published studies of rsFC in WS.
In this study, we first aim at confirming previous findings of increased between-network connectivity in DS compared with TD controls and at establishing whether such differences are specific to DS or are also observed in another developmental disability, WS. If the increased between-network connectivity previously observed in DS is due to atypical neurodevelopment that is unique to DS, then we should observe such between-network pairs connectivity differences between DS versus TD, but not between WS versus TD. If, however, increased between-network connectivity is a generalized, nonspecific feature associated with atypical neurodevelopment, then we should observe between-network connectivity differences between each of the DS and WS groups when compared with TD. Finally, we aim at characterizing how the within-network connectivity profiles of DS and WS could be compared with each other and with TD. Together, these aims will attempt to replicate previous work while providing new insights into resting-state brain function across two different neurodevelopmental disorders.
Materials and Methods
Participants
This study included data from ten participants with DS (4 males; 39±10 years of age), 18 participants with WS (13 males; 26±9 years of age), and 40 participants with TD (24 males; 47±22 years of age), all of whom were recruited for other primary studies in the lab. Participants with DS and WS exhibited the physical, cognitive, and behavioral profile associated with their respective neurodevelopmental disorder and received a clinically confirmed genetic diagnosis before enrolling in the research study. Exclusion criteria for all participants included (1) nonremovable ferromagnetic material on or in the body, (2) claustrophobia, (3) pregnancy, (4) a history of a neurological disorder, and (5) untreated hypothyroidism. The three groups were tested for differences in age and gender, using independent samples t-tests and a chi-square test, respectively. Demographic characteristics and between-group statistics are provided in Table 1. The Vanderbilt University Institutional Review Board approved all study protocols. Informed consent was obtained from TD participants and caregivers of participants with DS and WS, while an assent was obtained from all DS and WS participants.
Table 1.
Within-Group Summary Statistics and Between-Group Comparisons of Sample Demographics
| Summary statistics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age | Verbal IQ | Nonverbal IQ | Composite IQ | DLD-SOS | Gender | APOEɛ4 carrier status | ||
| TD | Mean | 46.95 | 115.00 | 115.73 | 117.86 | n/a | 16 F, 24 M | ɛ4+=3 |
| Std. Dev | ±22.05 | ±15.247 | ±13.491 | ±14.630 | ||||
| Range | 18–89 | 83–141 | 84–132 | 81–141 | ɛ4+=14 | |||
| N | 40 | 22 | 22 | 22 | ||||
| DS | Mean | 38.98 | 47.20 | 45.90 | 45.00 | 5.10 | 5 F, 13 M | ɛ4+=2 |
| Std. Dev | ±10.06 | ±10.518 | ±6.740 | ±7.272 | ±4.00 | |||
| Range | 19–56 | 40–70 | 40–54 | 40–61 | 1–10 | ɛ4+=8 | ||
| N | 10 | 10 | 10 | 10 | 10 | |||
| WS | Mean | 25.89 | 78.65 | 67.24 | 69.76 | n/a | 6 F, 4 M | n/a |
| Std. Dev | ±8.53 | ±15.756 | ±17.852 | ±17.775 | ||||
| Range | 16–48 | 56–108 | 40–96 | 43–93 | ||||
| N | 18 | 17 | 17 | 17 | ||||
| Independent samples t-tests | χ2tests of independence | |||||
|---|---|---|---|---|---|---|
| Age | Verbal IQ | Nonverbal IQ | Composite IQ | Gender | APOEɛ4 carrier status | |
| DS vs. TD | t(48)=1.12 | t(30)=−12.70 | t(30)=−15.42 | t(30)=−14.84 | χ2(2,N=68)=2.8; p>0.05 | χ2(1,N=27)=0.023; p>0.05 |
| p>0.05 | p<0.01** | p<0.01** | p<0.01** | |||
| DS vs. WS | t(26)=3.63 | t(25)=−5.60 | t(25)=−3.607 | t(25)=−4.18 | n/a | |
| p<0.01** | p<0.01** | p≤0.01** | p<0.01** | |||
| WS vs. TD | t(56)=−5.23 | t(37)=−7.28 | t(37)=−9.67 | t(37)=−9.27 | n/a | |
| p<0.01** | p<0.01** | p<0.01** | p<0.01** | |||
p≤0.01 (two-tailed).
DLD, dementia questionnaire for people with learning disabilities; DS, Down syndrome; IQ, intellectual quotient measured by KBIT2; n/a, not applicable since the DLD was only measured in subjects with Down syndrome; SOS, sum of social scores; TD, typically developing; WS, Williams syndrome.
Cognitive testing
IQ was assessed using the Kaufman Brief Intelligence Test, Second Edition (KBIT-2) (Kaufman and Kaufman, 2004), a brief measure of intelligence developed for research or screening purposes in clinical and typically developing populations (TD population: mean=100, SD=15). The KBIT-2 is validated for use in individuals aged 4 years through adulthood, and administration is brief, accommodating functional or behavioral challenges in individuals, which would otherwise preclude the use of a longer intellectual assessment. Standard scores (verbal, nonverbal, and composite IQ) were obtained from 22 of our 40 TD participants, 17 of the 18 WS participants, and all 10 DS participants. KBIT-2 scores correlate highly with other full-scale IQ tests and have successfully been used to estimate intelligence in individuals with neurodevelopmental disorders, including DS and WS (Edgin et al., 2010; Kaufman and Kaufman, 2004; Mervis et al., 2012). Group mean scores from each of the three participant groups were tested for differences using an independent samples t-test (Table 1).
Aging in individuals with DS is associated with an increased risk of developing Alzheimer's disease (AD) (Rohn et al., 2014), with 70 percent developing dementia by age 70 (Evenhuis, 1990). Postmortem studies have revealed that by 40 years of age, nearly all individuals with DS have lesions present in the brain that meet the pathological criteria for AD (Mann, 1988). Caregivers of participants with DS completed the dementia questionnaire for people with learning disabilities (DLD), which is commonly used to assess dementia in individuals with DS (Prasher, 1997; Shultz et al., 2004). The DLD is a 50-item questionnaire that consists of eight subtests to assess cognition and social skills. A sum of cognitive scores is calculated from the short-term memory, long-term memory, and spatiotemporal orientation subtests; whereas a sum of social scores (SOS) is calculated from the speech, practical skills, mood, activity, and interest and behavioral disturbance subtests. Higher scores in either subtest indicate greater impairment. All neuropsychological assessment characteristics along with their corresponding statistical values are detailed in Table 1.
MRI image acquisition
Neuroimaging data were acquired from all participants using a 3T Philips Achieva MRI scanner (Philips Medical Systems, Inc.) housed in the Vanderbilt University Institute of Imaging Science. During acquisition, participants wore foam earplugs and Philips headphones to attenuate noise. In an effort to minimize anxiety and create a comfortable scanner environment, participant-selected music or a movie was presented during acquisition of structural sequences. Resting-state fMRI was collected in the absence of external stimuli using a T2*-weighted echo planar imaging (EPI) sequence (FOV=240 mm2, 1.875×1.875×3.85 mm3 voxels, TR=2000 msec, TE=35 msec, flip angle=79°, 0.35 gap, 33 axial slices, 150 volumes). A high-resolution T1-weighted (T1W) 3D anatomical image (FOV=256 mm2, 1 mm isotropic voxels, TR=9 msec, TE=4.6 msec, flip angle=8°, 170 sagittal slices) was collected to provide a template for image registration.
fMRI preprocessing
All functional images underwent quality assurance and standard preprocessing in SPM software (version 8, Wellcome Department of Imaging Neuroscience; www.fil.ion.ac.uk/spm), which included slice-time correction, motion correction, T1W-EPI co-registration, spatial normalization, and spatial smoothing (8 mm FWHM). Individual T1W images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid tissue maps; then, the T1W image, functional EPI, and tissue maps were normalized to MNI152 space (Montreal Neurological Institute). Afterward, functional images underwent artifact and motion regression in the Artifact Detection Tools (ART) toolbox (www.nitrc.org/projects/artifact_detect) using the following threshold criteria to define outliers: global signal; z≥2; translation ≥2 mm, rotation ≥0.0349 radians. A matrix of SPM movement and threshold outliers was generated for each subject and subsequently included as covariates in connectivity analyses.
Functional connectivity analysis
To evaluate resting-state brain networks, we used a publicly available parcellation of the brain obtained from Yeo et al. (2011), which includes seven distributed brain networks projected into MNI152 space. We used these networks (Visual, Somatomotor, DAN, VAN, Limbic, Frontoparietal, and DMN) as user-defined regions of interest (ROIs) in rsFC analyses performed using the MATLAB-based functional connectivity toolbox “Conn” (www.nitrc.org/projects/conn) (Whitfield-Gabrieli, 2010). Each subject's normalized structural and functional images and T1W tissue maps were used as input into Conn. Importantly, the Conn toolbox first implements an anatomical, component-based, noise correction strategy (CompCor) to identify and reduce physiological and other noise signals that are unlikely to be related to neural activity (Whitfield-Gabrieli and Nieto-Castanon, 2012). After regressing out Compcor-identified noise, the resulting BOLD time series were band-pass filtered (0.008–0.09 Hz) to further reduce noise and increase sensitivity. The output matrices of SPM movement and threshold outliers generated by the ART toolbox were entered into Conn as first-level covariates.
APOE genotyping
Altered rsFC has been observed in APOE ɛ4 carriers compared with noncarriers (Buckner et al., 2005; Filippini et al., 2009; Fleisher et al., 2009). Genotyping was performed at the Vanderbilt DNA Resources Core on a portion of TD (n=17) and on all participants with DS (n=10). APOE was directly genotyped using premade TaqMan single nucleotide polymorphism (SNP) genotyping assays C_3084793-20 (rs429358) and C_904973_10 (rs7412) from Applied Biosystems. Positive controls (DNA samples with known genotypes from Coriell Institute for Medical Research, Camden, NJ) and negative controls (no template) were included on the plate for assay validation. Genotyping was performed in a research laboratory that is not CLIA certified; therefore, genotyping results were not returned to participants or their clinicians. APOE genotypes available for TD and DS participants are detailed in Table 1. Quality control checks for SNP and sample genotyping efficiency, sample duplications, sex discrepancies, and tests of Hardy–Weinberg equilibrium were performed in PLINK (http://pngu.mgh.harvard.edu/purcell/plink/) (Purcell et al., 2007).
Statistical analyses for between- and within-network connectivity
The following rsFC analysis procedures were conducted using the MATLAB-based Conn toolbox (Kesler et al., 2014; Redcay et al., 2013; Whitfield-Gabrieli and Nieto-Castanon, 2012). To evaluate between-network connectivity, for each subject, the BOLD time course was averaged across all voxels in each of the seven network ROIs, and the Fisher-transformed correlation was calculated for each pair of networks (for a total of 21 network pairs). Independent samples t-tests were used to assess group differences in between-network connectivity for all 21 network pairs (Table 2). We used a Bonferroni correction for multiple comparisons. Next, we conducted Spearman correlation analyses to assess the relationships between age, IQ, and between-network connectivity for each of the participant groups (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/brain) using SPSS (IBM, www-01.ibm.com/software/analytics/spss). To control for Type I error, we restricted our age and IQ correlation analyses to network pairs that were statistically significant for any of the group comparisons.
Table 2.
Between-Group Comparison of Within-Network Connectivity
| (a) DS vs. TD | |||||||
|---|---|---|---|---|---|---|---|
| t | df | Sig. (two-tailed) | Group | Mean r | Std. deviation | Direction for significant networks | |
| DMN | 0.940 | 48 | 0.3519 | DS | 0.50 | 0.09 | |
| TD | 0.46 | 0.10 | |||||
| Frontoparietal network | 1.218 | 48 | 0.2291 | DS | 0.46 | 0.06 | |
| TD | 0.43 | 0.07 | |||||
| Limbic network | 2.265 | 48 | 0.0280* | DS | 0.38 | 0.09 | |
| TD | 0.32 | 0.08 | |||||
| VAN | −1.202 | 48 | 0.2351 | DS | 0.46 | 0.08 | |
| TD | 0.50 | 0.10 | |||||
| DAN | 0.535 | 48 | 0.5952 | DS | 0.46 | 0.08 | |
| TD | 0.44 | 0.11 | |||||
| Somatomotor network | −1.221 | 48 | 0.2280 | DS | 0.50 | 0.11 | |
| TD | 0.56 | 0.13 | |||||
| Visual network | 0.834 | 48 | 0.4082 | DS | 0.56 | 0.10 | |
| TD | 0.52 | 0.16 | |||||
| (b) DS vs. WS | |||||||
|---|---|---|---|---|---|---|---|
| t | df | Sig. (two-tailed) | Group | Mean r | Std. deviation | Direction for significant networks | |
| DMN | 3.381 | 26 | 0.0023** | DS | 0.50 | 0.09 | DS>WS |
| WS | 0.36 | 0.10 | |||||
| Frontoparietal network | 1.790 | 26 | 0.0850 | DS | 0.46 | 0.06 | |
| WS | 0.40 | 0.10 | |||||
| Limbic network | 2.289 | 26 | 0.0305* | DS | 0.38 | 0.09 | |
| WS | 0.30 | 0.09 | |||||
| VAN | 1.248 | 26 | 0.2232 | DS | 0.46 | 0.08 | |
| WS | 0.41 | 0.11 | |||||
| DAN | 1.557 | 26 | 0.1315 | DS | 0.46 | 0.08 | |
| WS | 0.40 | 0.11 | |||||
| Somatomotor network | 1.976 | 26 | 0.0589 | DS | 0.50 | 0.11 | |
| WS | 0.41 | 0.12 | |||||
| Visual network | 3.394 | 26 | 0.0022** | DS | 0.56 | 0.10 | DS>WS |
| WS | 0.41 | 0.12 | |||||
| (c) WS vs. TD | |||||||
|---|---|---|---|---|---|---|---|
| t | df | Sig. (two-tailed) | Group | Mean r | Std. deviation | Direction for significant networks | |
| DMN | −3.467 | 56 | 0.0010** | WS | 0.36 | 0.10 | WS<TD |
| TD | 0.46 | 0.10 | |||||
| Frontoparietal network | −1.459 | 56 | 0.1502 | WS | 0.40 | 0.10 | |
| TD | 0.43 | 0.07 | |||||
| Limbic network | −0.767 | 56 | 0.4464 | WS | 0.30 | 0.09 | |
| TD | 0.32 | 0.08 | |||||
| VAN | −3.101 | 56 | 0.0030** | WS | 0.41 | 0.11 | WS<TD |
| TD | 0.50 | 0.10 | |||||
| DAN | −1.312 | 56 | 0.1948 | WS | 0.40 | 0.11 | |
| TD | 0.44 | 0.11 | |||||
| Somatomotor network | −3.984 | 56 | 0.0002** | WS | 0.41 | 0.12 | WS<TD |
| TD | 0.56 | 0.13 | |||||
| Visual network | −2.671 | 56 | 0.0099* | WS | 0.41 | 0.12 | |
| TD | 0.52 | 0.16 | |||||
p<0.05 (uncorrected).
p<0.05 (Bonferroni corrected).
DAN, dorsal attention network; DMN, default mode network; VAN, ventral attention network.
To evaluate within-network connectivity, we calculated Fisher-transformed correlations between each voxel within each network ROI and that ROI's mean time course. We then calculated the mean correlation value within each ROI as proxy of total within-network connectivity. Independent samples t-tests were used to assess group differences (DS vs. TD, DS vs. WS, and WS vs. TD) in within-network connectivity for all seven networks (Table 3). Spearman correlation analyses were conducted to evaluate the relationships between age, IQ, and within-network connectivity in all seven networks for each of the participant groups (Supplementary Table S2).
Table 3.
Between-Group Comparison of Between-Network Connectivity
| DS vs. TD | |||||||
|---|---|---|---|---|---|---|---|
| Network pairs | t | df | Sig. (two-tailed) | Group | Mean | Std. deviation | Direction for significant network pairs |
| Visual-frontoparietal | 4.829 | 48 | <0.0001** | DS | 0.57 | 0.27 | DS>TD |
| TD | 0.07 | 0.29 | |||||
| Somatomotor-VAN | 2.999 | 48 | 0.0043* | DS | 0.96 | 0.29 | |
| TD | 0.66 | 0.28 | |||||
| Somatomotor-frontoparietal | 3.453 | 48 | 0.0012** | DS | 0.33 | 0.31 | DS>TD |
| TD | −0.03 | 0.30 | |||||
| Somatomotor-DMN | 3.678 | 48 | 0.0006** | DS | 0.37 | 0.23 | DS>TD |
| TD | 0.00 | 0.30 | |||||
| DAN-limbic | 2.654 | 48 | 0.0108* | DS | 0.48 | 0.27 | |
| TD | 0.19 | 0.32 | |||||
| DAN-frontoparietal | 4.950 | 48 | <0.0001** | DS | 0.87 | 0.24 | DS>TD |
| TD | 0.47 | 0.23 | |||||
| DAN-DMN | 4.297 | 48 | 0.0001** | DS | 0.47 | 0.30 | DS>TD |
| TD | 0.01 | 0.30 | |||||
| VAN-frontoparietal | 2.098 | 48 | 0.0412* | DS | 0.48 | 0.26 | |
| TD | 0.27 | 0.29 | |||||
| VAN-DMN | 2.671 | 48 | 0.0103* | DS | 0.19 | 0.28 | |
| TD | −0.07 | 0.28 | |||||
| Limbic-frontoparietal | 2.778 | 48 | 0.0078* | DS | 0.40 | 0.28 | |
| TD | 0.13 | 0.28 | |||||
| Limbic-DMN | 3.197 | 48 | 0.0025** | DS | 0.78 | 0.23 | DS>TD |
| TD | 0.51 | 0.24 | |||||
| Frontoparietal-DMN | 2.236 | 48 | 0.0301* | DS | 0.66 | 0.43 | |
| TD | 0.40 | 0.31 | |||||
| DS vs. WS | |||||||
|---|---|---|---|---|---|---|---|
| Network pairs | t | df | Sig. (two-tailed) | Group | Mean | Std. deviation | Direction for significant network pairs |
| Visual-DAN | 2.864 | 26 | 0.0082* | DS | 0.61 | 0.37 | |
| WS | 0.28 | 0.25 | |||||
| Visual-frontoparietal | 2.983 | 26 | 0.0061* | DS | 0.57 | 0.27 | |
| WS | 0.23 | 0.30 | |||||
| Somatomotor-DAN | 2.297 | 26 | 0.0300* | DS | 0.60 | 0.30 | |
| WS | 0.32 | 0.31 | |||||
| Somatomotor-limbic | 2.094 | 26 | 0.0462* | DS | 0.46 | 0.21 | |
| WS | 0.27 | 0.24 | |||||
| Somatomotor-frontoparietal | 2.084 | 26 | 0.0471* | DS | 0.33 | 0.31 | |
| WS | 0.08 | 0.31 | |||||
| Somatomotor-DMN | 3.338 | 26 | 0.0026* | DS | 0.37 | 0.23 | |
| WS | 0.06 | 0.24 | |||||
| DAN-frontoparietal | 2.627 | 26 | 0.0143* | DS | 0.87 | 0.23 | |
| WS | 0.64 | 0.22 | |||||
| DAN-DMN | 2.123 | 26 | 0.0434* | DS | 0.47 | 0.30 | |
| WS | 0.24 | 0.26 | |||||
| WS vs. TD | |||||||
|---|---|---|---|---|---|---|---|
| Network pairs | t | df | Sig. (two-tailed) | Group | Mean | Std. deviation | Direction for significant network pairs |
| Visual-Somatomotor | −2.895 | 56 | 0.0054* | WS | 0.15 | 0.27 | |
| TD | 0.42 | 0.35 | |||||
| Somatomotor-VAN | 2.596 | 56 | 0.0120* | WS | 0.87 | 0.30 | |
| TD | 0.66 | 0.28 | |||||
| DAN-frontoparietal | 2.674 | 56 | 0.0098* | WS | 0.64 | 0.22 | |
| TD | 0.47 | 0.23 | |||||
| DAN-DMN | 2.790 | 56 | 0.0072* | WS | 0.24 | 0.26 | |
| TD | 0.01 | 0.30 | |||||
| VAN-DMN | 2.790 | 56 | 0.0072* | WS | 0.15 | 0.30 | |
| TD | −0.07 | 0.28 | |||||
| Limbic-frontoparietal | 3.187 | 56 | 0.0024* | WS | 0.38 | 0.29 | |
| TD | 0.13 | 0.28 | |||||
| Frontoparietal-DMN | 3.477 | 56 | 0.0010** | WS | 0.72 | 0.38 | WS>TD |
| TD | 0.40 | 0.31 | |||||
p<0.05 (Uncorrected).
p<0.05 (Bonferroni corrected).
Secondary analyses addressing age as potential confounder
Given that the range of ages differs substantially between groups and that connectivity can vary with age (Grady et al., 2006), it is possible that age is a confounder in our between-group analyses. To address this question, we repeated our between-network and within-network analyses after restricting the TD age range to more closely match that of both the DS and WS groups. In addition, as recommended by Faresjö and Faresjö (2010), we also conducted univariate regression analyses, using age as a covariate and testing for significance of the Group term beta coefficient (Faresjö and Faresjö, 2010).
Exploratory analyses in DS
To further characterize rsFC patterns specific to DS, we performed several analyses to test for an association between rsFC and other cognitive and genetic risk factors. Spearman correlation analyses were used to assess the relationship between DLD-SOS score and between-network connectivity (included in Supplementary Table S1), and between DLD-SOS score and within-network connectivity for each of the seven networks (included in Supplementary Table S2). To control for Type I error, we restricted our DLD-SOS correlation analyses to network pairs that were statistically significant in any of the group comparisons.
Next, we performed an exploratory analysis to determine the relationship between rsFC and APOE genotype in the TD and DS participants for whom genotyping was performed. The participants with APOE genotype data were divided into two groups based on the absence (ɛ4−; n=22) or presence (ɛ4+; n=5) of an APOE ɛ4 allele. For each of the seven networks, the relationship between APOE ɛ4 and within-network connectivity or between-network connectivity was assessed using independent samples t-tests. These analyses were restricted to network pairs that were statistically significant in the DS versus TD comparison.
Results
Between-network connectivity results
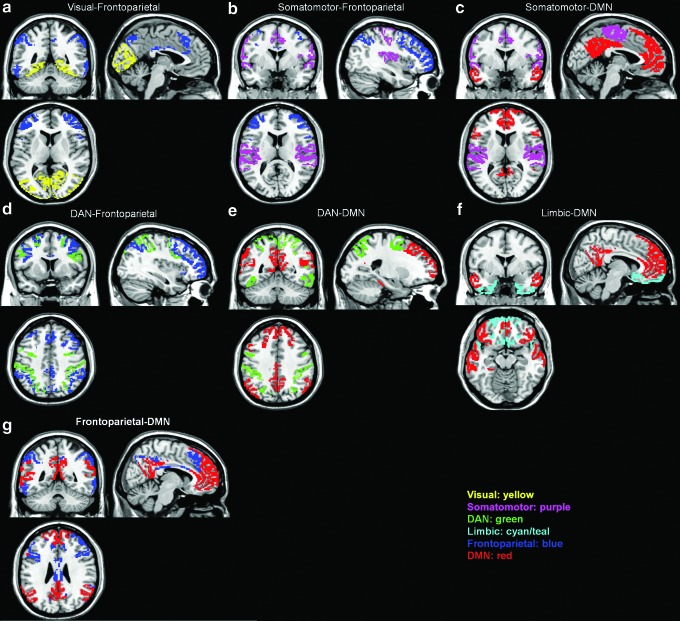
We first tested for group differences in connectivity between brain networks. The networks were defined a priori by using the seven-network brain parcellation obtained from Yeo et al. (2011). Consistent with previous findings (Anderson et al., 2013), the DS group showed greater between-network connectivity for 12 out of 21 network pairs compared with TD. After correcting for multiple comparisons, 6 out of 21 network pairs remained statistically significant (Table 2a and Fig. 1a). In particular, DS showed significantly greater between-network connectivity compared with TD for the following network pairs: (1) Visual-Frontoparietal (t(48)=4.83, p<0.0001), (2) Somatomotor-Frontoparietal (t(48)=3.45, p=0.0012), (3) Somatomotor-DMN (t(48)=3.68, p=0.0006), (4) DAN-Frontoparietal (t(48)=4.95, p<0.0001), (5) DAN-DMN (t(48)=4.30, p=0.0001), and (6) Limbic-DMN (t(48)=3.18, p=0.0025).
FIG. 1.
Visualization of the approximate anatomical location of between-network pairs whose interconnectivity was significantly different for DS versus TD (a–f), and WS versus TD (g). No between-network pairs were significantly different for DS versus WS. DAN, dorsal attention network; DMN, default mode network; DS, Down syndrome; TD, typically developing; WS, Williams syndrome.
The DS group showed a trend toward greater between-network connectivity compared with the WS group for 8 out of 21 network pairs; however, none of these network pairs remained statistically significant after correcting for multiple comparisons (Table 2b). Similarly, the WS group showed a trend toward greater between-network connectivity for seven out of 21 network pairs compared with TD, but only one out of 21 network pairs remained statistically significant (Table 2b and Fig. 1b). WS showed significantly greater between-network connectivity compared with TD for the Frontoparietal-DMN (t(56)=3.48, p=0.001) network pair.
We then tested for associations between age and IQ and between-network connectivity. We limited our correlation analysis to the seven network pairs that were shown to be statistically significant in the DS versus TD and the WS versus TD comparisons. Results for each group are shown in Supplementary Table S1. In the Spearman correlation analyses, age showed a significant negative correlation with the Limbic-DMN pair (ρ(40)=−0.49, p=0.001; corrected p<0.05) for the TD group (Supplementary Fig. S1), such that connectivity was lower for higher ages. Neither age nor IQ showed a significant association with any of the significant between-network pairs in the DS or WS group.
Within-network connectivity results
We next tested for group differences in connectivity within each of the seven brain networks. Results are shown in Table 3 for each group comparison. For the within-network connectivity analyses, the DS group showed a trend for greater within-network connectivity in the Limbic network compared with the TD group. However, no differences in within-network connectivity for the DS versus TD comparison remained significant after correction for multiple testing. When examining differences in within-network connectivity between DS and WS, DS showed a trend for greater within-network connectivity in three out of seven networks compared with WS. After correcting for multiple comparisons, the following two networks remained statistically significant for the DS versus WS comparison: (1) DMN (t(26)=3.38, p<0.05) and (2) Visual network (t(26)=3.39, p<0.05). When examining group differences in within-network connectivity between WS and TD, TD showed a trend for greater within-network connectivity in four out of seven networks compared with WS. After correcting for multiple comparisons, the following three networks remained statistically significant for the WS versus TD comparison: (1) DMN (t(56)=−3.47, p<0.05), (2) VAN (t(56)=3.10, p<0.05), and (3) Somatomotor network (t(56)=−3.98, p<0.05).
We then tested for associations between age and IQ measures and within-network connectivity. Results for each group are shown in Supplementary Table S2. In the Spearman correlation analyses, composite IQ showed a significant positive correlation with DMN connectivity (rs(22)= 0.63, p=0.002; Bonferroni-corrected p<0.05) for the TD group (Supplementary Fig. S2), such that DMN connectivity was higher in individuals with higher composite IQ. No associations were seen between IQ and age with any of the seven networks for either the DS or WS groups.
Secondary analyses addressing age as potential confounder
Age-restricted t-test results
For the DS versus TD comparisons, the TD age range was restricted to ≤56 years (the age of the oldest DS participant), thereby reducing the TD sample size to n=21, and for the WS versus TD comparisons, the TD age range was restricted to ≤48 (the age of the oldest WS participant), thereby reducing the TD sample size to n=20. For the between-network connectivity comparisons, out of the seven that were significant in the full dataset, two were no longer significant when using the age-restricted TD dataset—the Limbic-DMN for DS versus TD (Supplementary Table S3a) and the Frontoparietal-DMN for WS versus TD (Supplementary Table S3b). For the within-network connectivity comparisons, one of the three networks that were significant in the full dataset no longer reached significance—the VAN for the WS versus TD group (Supplementary Table S4b). However, there also was one within-network connectivity comparison that reached significance using the age-restricted TD dataset that was only showing a trend in the full dataset—the Limbic network, suggesting DS within-newtwork connectivity was greater compared with TD (Supplementary Table S4a).
Age-adjusted regression results
For the between-network connectivity comparisons, out of the seven that were significant in the full dataset, two were no longer significant when using age as a covariate and testing for significance of the Group term beta coefficient—the Limbic-DMN for DS versus TD (Supplementary Table S5a) and the Frontoparietal-DMN for WS versus TD (Supplementary Table S6b). For the within-network connectivity comparisons, one of the three networks that were significant in the full dataset no longer reached significance—the VAN for the WS versus TD group (Supplementary Table S6b). The results for the DS versus TD within-network comparison remained unchanged from the original analysis (Supplementary Table S6a).
Exploratory analyses in DS
In the DLD-SOS post hoc Spearman correlation analysis, which was performed only in the relevant DS group, no relationship between DLD-SOS score and any of the seven significant between-network pairs was found (included in Supplementary Table S1). In the Spearman correlation analysis for DLD-SOS score and within-network connectivity, no relationship was found for any of the seven networks after correcting for multiple comparisons (included in Supplementary Table S2). However, there was a trend toward higher DLD-SOS scores being associated with lower VAN (rs(10)=−0.70, p=0.02, uncorrected) and DAN (rs(10)=−0.65, p=0.04, uncorrected) within-network connectivity.
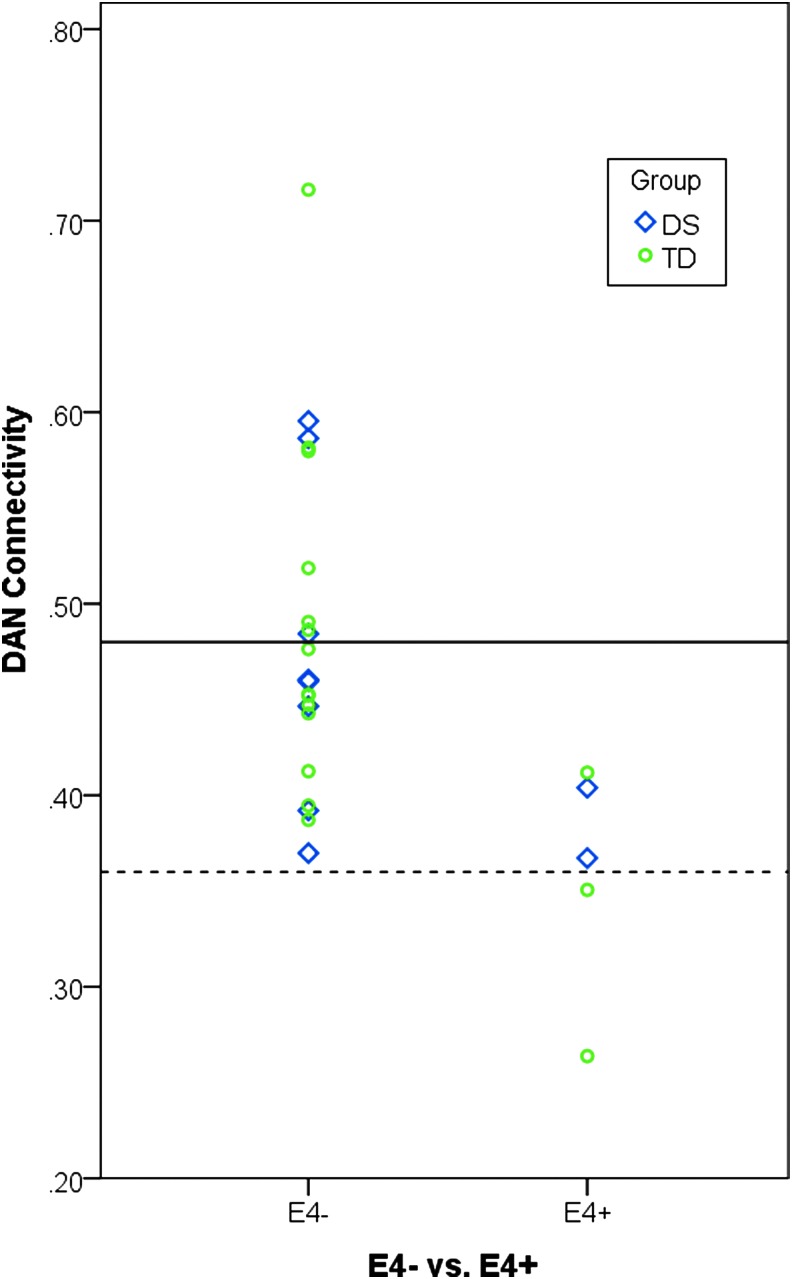
In the APOE ɛ4 post hoc analysis for between-network connectivity, no differences were observed in any of the network pairs that were shown to be statistically significant for the DS versus TD comparison (Supplementary Table S3). In the APOE ɛ4 post hoc analysis for within-network connectivity, ɛ4 carriers were shown to have decreased connectivity within the DAN compared with noncarriers (t(25)=3.10, p=0.005, Table 4 and Fig. 2).
Table 4.
Difference in Mean Within-Network Connectivity by APOE Carrier Status
| Independent samples t-test results for within-network connectivity and APOEɛ4 carrier status | |||||||
|---|---|---|---|---|---|---|---|
| t | df | Sig. (two-tailed) | APOEɛ4 carrier status | Mean | Std. deviation | Direction | |
| DMN | −0.853 | 25 | 0.402 | + | 0.46 | 0.10 | |
| − | 0.50 | 0.05 | |||||
| Executive network | 1.335 | 25 | 0.194 | + | 0.46 | 0.07 | |
| − | 0.41 | 0.06 | |||||
| Limbic network | 1.478 | 25 | 0.152 | + | 0.36 | 0.09 | |
| − | 0.30 | 0.04 | |||||
| VAN | 0.873 | 25 | 0.391 | + | 0.48 | 0.09 | |
| − | 0.45 | 0.09 | |||||
| DAN | 3.101 | 25 | 0.005** | + | 0.48 | 0.08 | ɛ4−>ɛ4+ |
| − | 0.36 | 0.06 | |||||
| Somatomotor network | −0.272 | 25 | 0.788 | + | 0.54 | 0.15 | |
| − | 0.56 | 0.09 | |||||
| Visual network | 0.325 | 25 | 0.748 | + | 0.55 | 0.14 | |
| − | 0.53 | 0.13 | |||||
Correlation is significant at the 0.05 level (Bonferroni corrected).
FIG. 2.
For the purpose of visualization, the presence (ɛ4+) or absence (ɛ4−) of APOE ɛ4 alleles is plotted in relationship to within-DAN connectivity across the DS (blue) and TD (green) groups. The solid line indicates the mean for within-DAN connectivity value for the ɛ4− group, and the dashed line indicates the mean within-DAN connectivity value for the ɛ4+ group. A two-sample t-test showed APOE ɛ4 carrier status to be associated with connectivity within the DAN; however, a larger sample size will be needed to accurately estimate this effect.
Discussion
Between-network connectivity
The first aim of the current study was to confirm previous findings of increased connectivity in DS between most brain regions compared with TD. As in Anderson et al. (2013), we evaluated connectivity between pairs of the following resting-state networks: (1) Visual network, (2) Somatomotor network, (3) DAN, (4) VAN, (5) Limbic network, (6) Frontoparietal, and (7) DMN (for a total of 21 network pairs). DS participants showed greater functional connectivity in 12 out of 21 between-network pairs compared in our analyses, which is quite comparable to the 14 out of 21 between-network pair differences previously observed (Anderson et al., 2013). Six of these comparisons were statistically significant after correction for multiple testing. The greater connectivity disturbances observed in frontoparietal network pairs in DS are consistent with results from the Arizona Cognitive Test Battery, specifically designed for DS, in which neuropsychological measures that probe frontal lobe function demonstrate the most prominent cognitive deficits in DS (Edgin et al., 2010). However, unlike the previous work comparing DS with autism (Anderson et al., 2013), our results also suggest that while the magnitude of between-network connectivity is slightly higher in DS compared with WS, the overall profile of the two developmental disabilities is quite similar. Both groups show trend level or statistically significant differences in many of the between-network combinations when compared with TD controls. Therefore, between-network connectivity alterations, particularly involving the DMN and/or frontoparietal networks, may be a more general feature of neurodevelopmental disorders. Such a possibility is further supported by previous findings of default mode dysfunction in autism (Assaf et al., 2010; Maximo et al., 2013; Starck et al., 2013) and ADHD (Choi et al., 2013; Uddin et al., 2008) comparable to the observed alterations in WS outlined next. However, it should be noted that Anderson et al. (2013) did not observe this atypical pattern of greater between-network connectivity pattern in their cohort of individuals with autism with normal IQ (Anderson et al., 2013). More research on the between-network connectivity in developmental disabilities is warranted to clarify which alterations (if any) are specific to the pathogenesis of a given neurodevelopmental disorder.
Although there appears to be a global difference in between-network connectivity in individuals with neurodevelopmental disorders compared with controls, our results also replicate a preferential abnormality across many brain regions in DS. As highlighted by Anderson et al. (2013), this may be due to a lack of global network differentiation, or dedifferentiation, in DS due to alterations in inhibitory circuitry, although our data cannot evaluate such a model directly.
Within-network connectivity
The second aim of this article was to characterize how the within-network connectivity profiles differ between groups. Interestingly, when we looked at the within-network connectivity in DS compared with TD controls, most regions showed similar within-network connectivity patterns; only the limbic network showed a nominally significant difference between the two groups. That is, although the inter-relationships between these distributed brain networks are abnormal in DS, such an abnormality must be interpreted within the context of seemingly intact within-network synchronization. When comparing WS with TD, however, the WS group showed elevated between-network connectivity, particularly in the DMN-frontoparietal network pair, as well as lower levels of within-network connectivity in the DMN, VAN, and somatomotor network. That is, in WS, the high levels of between-network connectivity appear in the context of reduced within-network connectivity, suggesting that a lack of differentiation between the DMN and other networks may be driving both observations. However, in DS, the within-network connectivity findings suggest that the increased synchrony between networks is not being driven by global dedifferentiation per se, as regions within each network appear to be co-activating in a typical way.
WS participants showed decreased connectivity in the DMN and the visual attention network, relative to controls and DS participants. Previous studies have shown that, compared with controls, people with WS have a higher ratio of frontal to posterior (parietal and occipital) lobe volume and cortical thickness, which specifically includes the PCC and inferior parietal cortex (Meda et al., 2012; Reiss et al., 2000, 2004). Abnormal corpus callosum morphology and shape have been implicated in the WS cognitive and behavioral phenotype (Sampaio et al., 2013). Such differences in integrity may influence inter-hemispheric connectivity in WS, driving our findings of reduced within network connectivity (i.e., across left and right parts of a single network) in this group compared with DS or TD. Decreased connectivity in the visual orientation network in WS may be related to decreased WM integrity previously observed in the inferior fronto-occipital fasciculus (Arlinghaus et al., 2011), which ipsilaterally connects the parietal, temporal, and occipital lobes to the frontal lobe. Decreased WM integrity in WS also has been identified in prefrontal-amygdala tracts and the uncinate fasciculus, the latter of which connects the amygdala to temporal gyri and the subcallosal region (Arlinghaus et al., 2011; Avery et al., 2012). These reports, while seemingly paradoxical to our findings of typical (or perhaps greater) connectivity within the limbic network, may together present a possibility for compensatory mechanisms. Our findings in the context of these previous reports of WM tract differences warrant future investigation, possibly with the use of a task-based neuroimaging approach that would disentangle the relationship between brain function and cognitive performance in WS.
One possible mechanism driving the increased connectivity observed between networks in DS, in the presence of typical within-network connections, is the unique dendritic arborization that has been characterized in DS. Becker et al. (1986) observed that infants with DS show increased dendritic arborization early in life, with more interconnections and greater average dendritic length when compared with controls (Becker et al., 1986). However, this increased arborization showed a steady decrease during early neural development, ultimately resulting in fewer connections and shorter dendrites. Similar findings have been observed in parietal and motor cortices (Kaufmann, 2000). One possible outcome of such abnormal neural development and pruning is that some of the longer, between-network connections may remain intact resulting in abnormal between-network co-activation after the networks have fully developed (Kaufmann, 2000). Less work on dendritic density has been performed in WS; however, other neurodevelopmental disorders, such as autism (Kelleher and Bear, 2008; Pardo and Eberhart, 2007; Walsh et al., 2008) and Rett syndrome (Ramocki and Zoghbi, 2008), are also characterized by altered dendritic arborization in early development (Jan and Jan, 2010).
A second possibility is that the increased network co-activation is due to some type of compensatory mechanism that is exhibited to some degree in all neurodevelopmental disorders but is perhaps particularly salient in DS where the neurodegenerative process associated with amyloid deposition may compound any neuronal injury. In support of such a possibility, DS subjects appear to show increases in glucose metabolism in regions vulnerable to AD pathology before the onset of clinical symptoms of dementia, and some of the genes over-expressed in DS may play a role in such a compensatory pathway (Head et al., 2007). However, it is difficult to know exactly how such compensatory brain activity would affect connectivity patterns. In addition, a relationship between the amount of between-network connectivity and cognitive performance was not observed in our DS cohort. Longitudinal studies tracking alterations in brain network architecture and cognitive decline will be needed to clarify whether the observed abnormal connectivity patterns are compensatory or a state characteristic of DS.
Exploratory analyses in DS
Our post hoc analyses focused on cognitive and genetic markers of dementia, because adults with DS account for approximately 60% of individuals with intellectual disabilities who exhibit signs of AD (Zigman et al., 2004). Postmortem studies have revealed that by 40 years of age nearly all individuals with DS have lesions present in the brain that meet the pathological criteria for AD (Mann, 1988) Therefore, we wanted to explore the relationship between rsFC patterns specific to DS and cognitive and genetic markers of AD, even though our effective sample size is clearly too low to draw conclusions at this point.
The DLD is often used to aid in the diagnosis of dementia in people with DS (Prasher, 1997; Shultz et al., 2004). No relationship between DLD-SOS score and any of the significant seven between-network pairs or for within-network connectivity was observed; however, there was a trend toward higher DLD-SOS scores (greater impairment) being associated with lower VAN and DAN within-network connectivity. While between-network connectivity differences are pervasive in DS, these were not significantly correlated with cognitive status or intellectual ability. However, more sensitive measures of cognitive function and a larger, more heterogeneous sample across the spectrum of cognitive impairment may be necessary to evaluate the association between connectivity and cognitive impairment in DS.
Finally, we evaluated the association between connectivity and APOE genotype. The APOE ɛ4 allele has been shown to increase risk for developing AD in both the DS (Coppus et al., 2008; Prasher et al., 2008; Rohn et al., 2014) and TD populations (Bertram and Tanzi, 2008; Corder et al., 1993; Liu et al., 2015). The observed ɛ4 carrier frequency was 5 out of 54 alleles, or 9%, which is similar to the frequency observed in the general population (13%) (Bertram and Tanzi, 2008; Corder et al., 1993; Liu et al., 2015). No significant difference between ɛ4 carriers and noncarriers was observed for any between-network pairs tested. However, ɛ4 carriers were shown to have decreased connectivity within the DAN compared with noncarriers. The validity and size of this effect would need to be confirmed in a larger sample. In summary, we did not find evidence that AD-related factors were responsible for the large differences in between-network connectivity observed in DS, compared with the WS and TD groups.
Strengths and weaknesses
The strengths of this study include our replication and extension of previous findings, showing between-network connectivity alterations in DS compared with TD controls (Anderson et al., 2013). Further, our results dissociate patterns in connectivity between and within established resting-state brain networks in DS. This study design included two informative contrast groups and allowed us to interpret our results within the larger framework of intellectual and development disabilities.
Many of the comparisons reported here have not been previously published, and it must be noted that, because the sample size for the DS group is small (although not dissimilar to that of other neuroimaging studies of neurodevelopmental disorders), it is possible that observed effects of the DS group in comparison to the WS and TD groups will not be replicated in an independent sample. In addition, while we used the very conservative Bonferroni approach to correct for the 21 different networks and network pairs tested, we did not also correct for having conducted these analyses for each pair of groups. For these reasons, many of the reported effects should be considered preliminary.
Other potential concerns are related to the quality and reliability of neuroimaging data and analysis methods. For example, noise from non-neuronal sources can drive spurious associations in studies of rsFC, particularly when recruiting participant groups with developmental disabilities or other cognitive or affective disorders. Artifacts related to head motion during data acquisition have been shown to significantly influence intrinsic functional connectivity measurements (Satterthwaite et al., 2012; Van Dijk et al., 2012); therefore, we took care to account for such issues by performing rigorous corrections for subject motion, including initial motion correction during preprocessing and additional artifact and motion regression using the ART toolbox. However, even with such corrections in place, it remains possible that subtle differences in motion or other confounding factors such as respiration may have had some contribution to the observed differences in functional connectivity. That we replicated previous results that were performed using different corrections for motion would suggest that any such confounds are at least consistent across connectivity studies in DS patients.
It has been consistently reported that the brains of DS individuals show reduced intracranial volume relative to TD individuals (Aylward et al., 1999; Kesslak et al., 1994; Raz et al., 1995). Therefore, it is possible that volumetric differences could be driving our findings. However, there is no reason to expect that such differences would have an effect on between-network connectivity without having any effect on within-network connectivity; thus, the dissociation in this article would suggest that such volumetric differences are not driving the observed result.
Finally, there was a large age difference in our WS group compared with our DS and TD groups, in that our WS group was much younger. Given the known associations between age and functional connectivity, particularly in the DMN (Damoiseaux et al., 2008), it is possible that such age differences could have biased our results. We aimed at addressing this question by repeating our between-network and within-network analyses after restricting the TD age range to more closely match that of both the DS and WS groups and also by conducting regression analyses while controlling for age as a covariate. Our results suggest that while age is certainly related to connectivity, this relationship was similar enough between groups such that it could not be deemed responsible for the majority of between-group effects observed in this study. Future work should expand recruitment to adults across the life span for all groups to confirm these results.
Conclusions
This study sought to characterize the pattern of rsFC in individuals with DS relative to WS and TD. Our results replicate previous findings (Anderson et al., 2013) of higher levels of between-network connectivity in DS compared with TD and also provide evidence for preserved within-network connectivity in DS. In summary, these findings suggest that altered between-network connectivity, particularly in the DMN, may be characteristic of a number of neurodevelopmental disorders involving intellectual disability, including DS and WS, and that perhaps within-network connectivity is a feature that shows more variable patterns across different neurodevelopmental disorders.
Supplementary Material
Acknowledgments
The authors would like to thank all the study volunteers for their time and willingness to participate in this research. They are grateful to the sponsors of the Williams Syndrome Music Camp, including the Vanderbilt Kennedy Center for Research on Human Development, the Academy of Country Music's Lifting Lives program, and the Vanderbilt Blair School of Music. They would also like to thank and acknowledge the research staff of the Williams Syndrome Music Camp, including Elizabeth Roof, Research Coordinator and the MRI technologists in the Vanderbilt University Institute of Imaging Science, including Dave Pennell, Leslie McIntosh, Kristen George-Durrett, and Donna Butler, for their expertise, professionalism, and wonderful manner of dealing with participants.
This research was supported in large part by a Vanderbilt Kennedy Center Hobbs Discovery Grant (to T.A.T.W.), the Recruitment for Genetic Aging Research (P30 AG036445 to T.A.T.W.), the Training Program on Genetic Variation and Human Phenotypes Training Grant (5T32 GM080178-06 to J.N.V.), the Vanderbilt Brain Institute Clinical Neuroscience Scholars Program (to J.N.V.), the Vanderbilt/National Institute of Mental Health Neurogenomics Training grant (T32 MH65215 to T.J.H.), a PhRMA Foundation Postdoctoral fellowship in Translational Medicine and Therapeutics (to T.J.H.), and a National Center for Advancing Translational Sciences award (Vanderbilt CTSA Grant 2UL1TR000445 VR#5003.1 to J.R.P.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this article. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson JS, Nielsen JA, Ferguson MA, Burback MC, Cox ET, Dai L, Korenberg JR. 2013. Abnormal brain synchrony in Down syndrome. Neuroimage Clin 2:703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlinghaus LR, Thornton-Wells TA, Dykens EM, Anderson AW. 2011. Alterations in diffusion properties of white matter in Williams syndrome. Magn Reson Imaging 29:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 53:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Thornton-Wells TA, Anderson AW, Blackford JU. 2012. White matter integrity deficits in prefrontal-amygdala pathways in Williams syndrome. Neuroimage 59:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Li Q, Honeycutt NA, Warren AC, Pulsifer MB, Barta PE, et al. 1999. MRI volumes of the hippocampus and amygdala in adults with Down's syndrome with and without dementia. Am J Psychiatry 156:564–568 [DOI] [PubMed] [Google Scholar]
- Beacher F, Daly E, Simmons A, Prasher V, Morris R, Robinson C, et al. 2010. Brain anatomy and ageing in non-demented adults with Down's syndrome: an in vivo MRI study. Psychol Med 40:611–619 [DOI] [PubMed] [Google Scholar]
- Becker LE, Armstrong DL, Chan F. 1986. Dendritic atrophy in children with Down's syndrome. Ann Neurol 20:520–526 [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. 2008. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9:768–778 [DOI] [PubMed] [Google Scholar]
- Brown JH, Johnson MH, Paterson SJ, Gilmore R, Longhi E, Karmiloff-Smith A. 2003. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia 41:1037–1046 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. 2005. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Lee SW, Go H-J. 2013. Aberrant development of functional connectivity among resting state-related functional networks in medication-naïve ADHD children. PLoS One 8:e83516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Wilson GN. 2003. Behavioral assessment of children with Down syndrome using the Reiss psychopathology scale. Am J Med Genet A 118A:210–216 [DOI] [PubMed] [Google Scholar]
- Conners FA, Moore MS, Loveall SJ, Merrill EC. 2011. Memory profiles of Down, Williams, and fragile X syndromes: implications for reading development. J Dev Behav Pediatr 32:405–417 [DOI] [PubMed] [Google Scholar]
- Coppus AMW, Evenhuis HM, Verberne G-J, Visser FE, Arias-Vasquez A, Sayed-Tabatabaei FA, et al. 2008. The impact of apolipoprotein E on dementia in persons with Down's syndrome. Neurobiol Aging 29:828–835 [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261:921–923 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, et al. 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864 [DOI] [PubMed] [Google Scholar]
- Dykens EM. 2003. Anxiety, fears, and phobias in persons with Williams syndrome. Dev Neuropsychol 23:291–316 [DOI] [PubMed] [Google Scholar]
- Dykens EM, Rosner BA, Ly T, Sagun J. 2005. Music and anxiety in Williams syndrome: a harmonious or discordant relationship? Am J Ment Retard 110:346–358 [DOI] [PubMed] [Google Scholar]
- Edgin JO, Mason GM, Allman MJ, Capone GT, Deleon I, Maslen C, et al. 2010. Development and validation of the Arizona Cognitive Test Battery for Down syndrome. J Neurodev Disord 2:149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenhuis HM. 1990. The natural history of dementia in Down's syndrome. Arch Neurol 47:263–267 [DOI] [PubMed] [Google Scholar]
- Faresjö T, Faresjö A. 2010. To match or not to match in epidemiological studies—same outcome but less power. Int J Environ Res Public Health 7:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 106:7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB. 2009. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage 47:1678–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103:10046–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. 2006. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci 18:227–241 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. 2004. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101:4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. 2001. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694 [DOI] [PubMed] [Google Scholar]
- Head E, Lott IT, Patterson D, Doran E, Haier RJ. 2007. Possible compensatory events in adult Down syndrome brain prior to the development of Alzheimer disease neuropathology: targets for nonpharmacological intervention. J Alzheimers Dis 11:61–76 [DOI] [PubMed] [Google Scholar]
- Hocking DR, Rinehart NJ, McGinley JL, Moss SA, Bradshaw JL. 2011. A kinematic analysis of visually-guided movement in Williams syndrome. J Neurol Sci 301:51–58 [DOI] [PubMed] [Google Scholar]
- Hodapp RM, Dykens EM. 2005. Measuring behavior in genetic disorders of mental retardation. Ment Retard Dev Disabil Res Rev 11:340–346 [DOI] [PubMed] [Google Scholar]
- Jacola LM, Byars AW, Chalfonte-Evans M, Schmithorst VJ, Hickey F, Patterson B, et al. 2011. Functional magnetic resonance imaging of cognitive processing in young adults with Down syndrome. Am J Intellect Dev Disabil 116:344–359 [DOI] [PubMed] [Google Scholar]
- Jan Y-N, Jan LY. 2010. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11:316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. 2004. Kaufman Brief Intelligence Test, Second Edition. Pearson, Bloomington, MN [Google Scholar]
- Kaufmann WE. 2000. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10:981–991 [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Bear MF. 2008. The autistic neuron: troubled translation? Cell 135:401–406 [DOI] [PubMed] [Google Scholar]
- Kesler SR, Gugel M, Pritchard-Berman M, Lee C, Kutner E, Hosseini SMH, et al. 2014. Altered resting state functional connectivity in young survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer 61:1295–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesslak JP, Nagata SF, Lott I, Nalcioglu O. 1994. Magnetic resonance imaging analysis of age-related changes in the brains of individuals with Down's syndrome. Neurology 44:1039–1045 [DOI] [PubMed] [Google Scholar]
- Koran ME, Hohman TJ, Edwards CM, Vega JN, Pryweller JR, Slosky LE, et al. 2014. Differences in age-related effects on brain volume in Down syndrome as compared to Williams syndrome and typical development. J Neurodev Disord 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg JR, Chen XN, Hirota H, Lai Z, Bellugi U, Burian D, et al. 2000. VI. Genome structure and cognitive map of Williams syndrome. J Cogn Neurosci 12 Suppl 1:89–107 [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu J-T, Wang H-F, Han P-R, Tan C-C, Wang C, et al. 2015. APOE genotype and neuroimaging markers of Alzheimer's disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 86:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, Dierssen M. 2010. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol 9:623–633 [DOI] [PubMed] [Google Scholar]
- Mann DM. 1988. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev 43:99–136 [DOI] [PubMed] [Google Scholar]
- Martens MA, Wilson SJ, Reutens DC. 2008. Research review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. J Child Psychol Psychiatry 49:576–608 [DOI] [PubMed] [Google Scholar]
- Maximo JO, Keown CL, Nair A, Müller R-A. 2013. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci 7:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Pryweller JR, Thornton-Wells TA. 2012. Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with williams syndrome. PLoS One 7:e31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégarbané A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethoré M-O, et al. 2009. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med 11:611–616 [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE. 2010. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. Am J Med Genet C Semin Med Genet 154C:229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Kistler DJ, John AE, Morris CA. 2012. Longitudinal assessment of intellectual abilities of children with Williams syndrome: multilevel modeling of performance on the Kaufman Brief Intelligence Test-Second Edition. Am J Intellect Dev Disabil 117:134–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA. 2010. The behavioral phenotype of Williams syndrome: A recognizable pattern of neurodevelopment. Am J Med Genet C Semin Med Genet 154C:427–431 [DOI] [PubMed] [Google Scholar]
- Nadel L. 2003. Down's syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav 2:156–166 [DOI] [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. 2007. The neurobiology of autism. Brain Pathol 17:434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. 2010. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 88:1008–1016 [DOI] [PubMed] [Google Scholar]
- Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. 2001. Amygdala and hippocampal volumes in children with Down syndrome: a high-resolution MRI study. Neurology 56:972–974 [DOI] [PubMed] [Google Scholar]
- Prasher VP. 1997. Dementia questionnaire for persons with mental retardation (DMR): modified criteria for adults with Down's syndrome. J Appl Res Intellect Disabil 10:54–60 [Google Scholar]
- Prasher VP, Schupf N, Sajith SG, Zigman WB, Rees S, Tewari S. 2008. Significant effect of APOE Epsilon 4 genotype on the risk of Alzheimer's disease and mortality In persons with Down syndrome. Int J Geriatr Psychiatry 23:1134–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryweller JR. 2013. A Neural Basis for Atypical Auditory Processing: A Williams Syndrome Model (Doctoral Dissertation). Vanderbilt University, Nashville, TN [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. 2007. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY. 2008. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 455:912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, et al. 1995. Selective neuroanatomic abnormalities in Down's syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology 45:356–366 [DOI] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JDE, Whitfield-Gabrieli S. 2013. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci 7:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, et al. 2004. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci 24:5009–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, Bellugi U. 2000. IV. Neuroanatomy of Williams syndrome: a high-resolution MRI study. J Cogn Neurosci 12 Suppl 1:65–73 [DOI] [PubMed] [Google Scholar]
- Reynolds Losin EA, Rivera SM, O'Hare ED, Sowell ER, Pinter JD. 2009. Abnormal fMRI activation pattern during story listening in individuals with down syndrome. Am J Intellect Dev Disabil 114:369–380 [DOI] [PubMed] [Google Scholar]
- Rohn TT, McCarty KL, Love JE, Head E. 2014. Is apolipoprotein E4 an important risk factor for dementia in persons with Down syndrome? J Parkinsons Dis Alzheimers Dis 1:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio A, Bouix S, Sousa N, Vasconcelos C, Férnandez M, Shenton ME, Gonçalves ÓF. 2013. Morphometry of corpus callosum in Williams syndrome: shape as an index of neural development. Brain Struct Funct 218:711–720 [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 60:623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz J, Aman M, Kelbley T, LeClear Wallace C, Burt DB, Primeaux-Hart S, et al. 2004. Evaluation of screening tools for dementia in older adults with mental retardation. Am J Ment Retard 109:98–110 [DOI] [PubMed] [Google Scholar]
- Starck T, Nikkinen J, Rahko J, Remes J, Hurtig T, Haapsamo H, et al. 2013. Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. Front Hum Neurosci 7:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Alexander GE, Schapiro MB, Möller H-J, Rapoport SI, Hampel H. 2004. Age-related cortical grey matter reductions in non-demented Down's syndrome adults determined by MRI with voxel-based morphometry. Brain 127:811–824 [DOI] [PubMed] [Google Scholar]
- Tsai S-W, Wu S-K, Liou Y-M, Shu S-G. 2008. Early development in Williams syndrome. Pediatr Int 50:221–224 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Milham MP. 2008. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods 169:249–254 [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CA, Morrow EM, Rubenstein JLR. 2008. Autism and brain development. Cell 135:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NS, Alkire MT, Haier RJ. 2003. A voxel-based morphometric study of nondemented adults with Down syndrome. Neuroimage 20:393–403 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. 2010. Conn. Retrieved from http://web.mit.edu/swg/software.htm Last accessed June26, 2014
- Whitfield-Gabrieli S, Ford JM. 2012. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141 [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Buckner RL. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Devenny DA, Miezejeski C, Ryan R, Urv TK, et al. 2004. Incidence and prevalence of dementia in elderly adults with mental retardation without down syndrome. Am J Ment Retard 109:126–141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.