Abstract
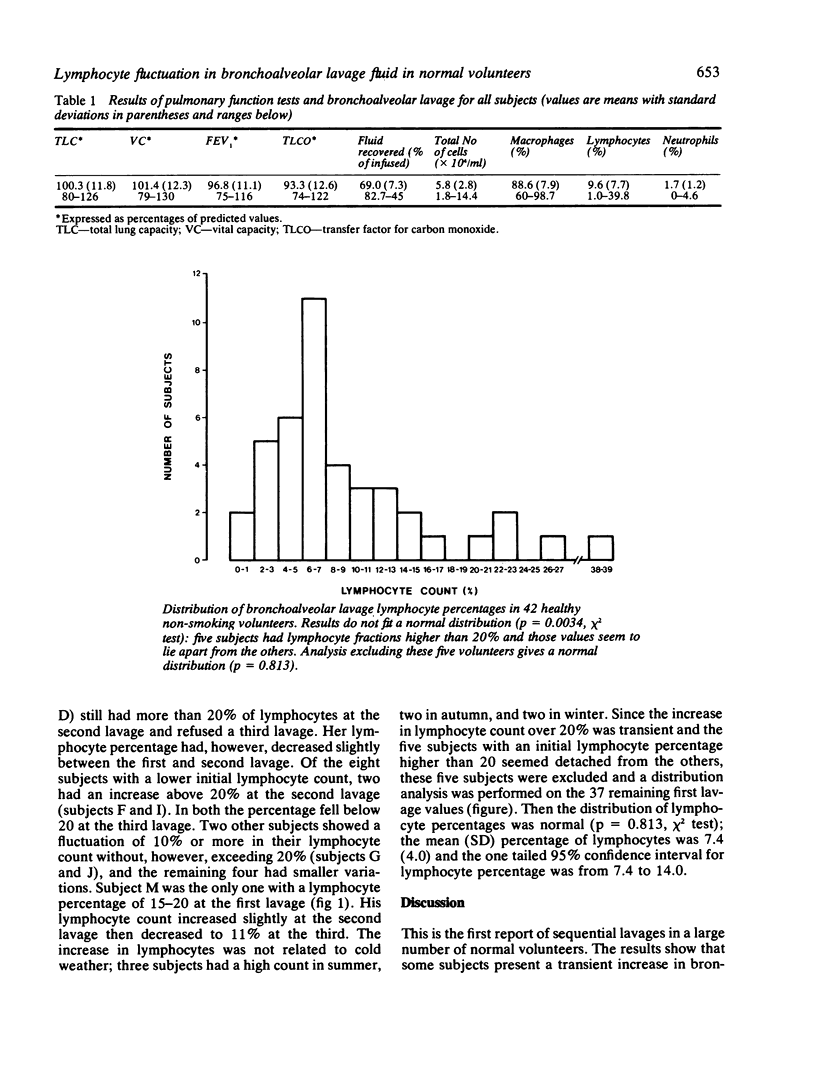
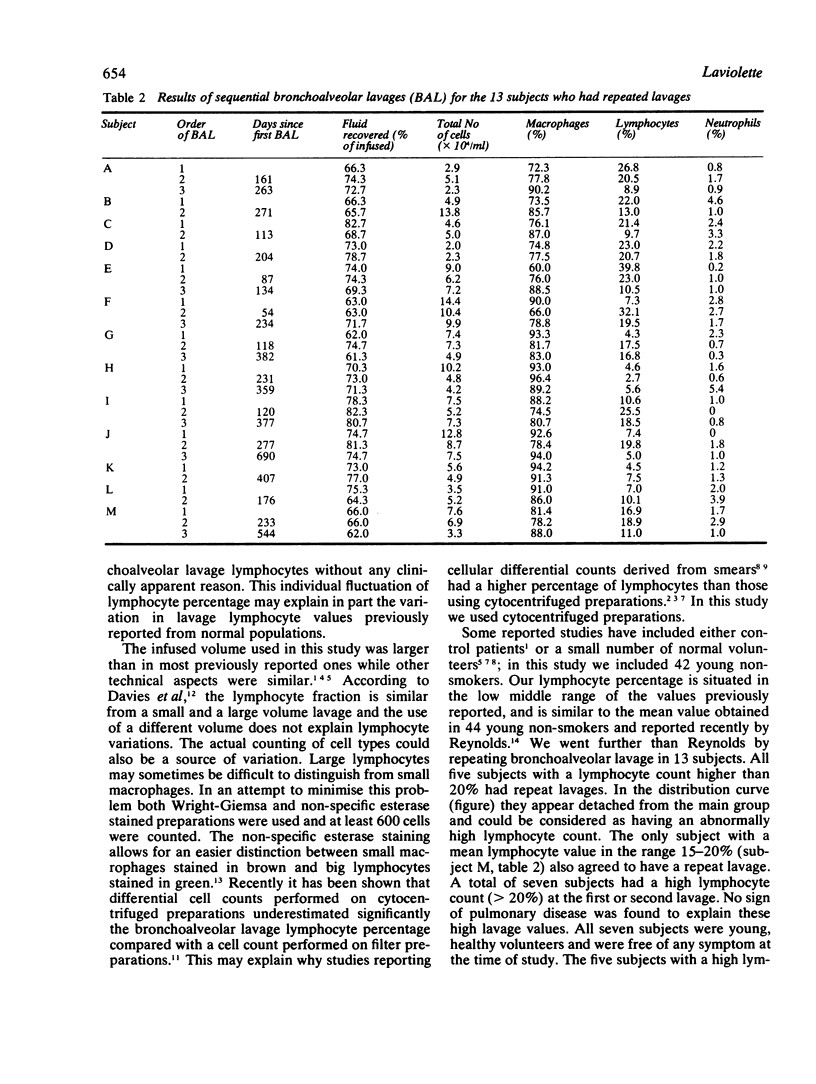
In an attempt to understand the widely varying bronchoalveolar lavage lymphocyte counts reported in normal subjects, we performed bronchoalveolar lavage in 42 healthy nonsmokers. The mean (SD) lymphocyte percentage in this first lavage was 9.6% (7.7%). The values did not fit a normal distribution. Five subjects had more than 20% of lymphocytes, and when they were excluded the distribution of lymphocyte counts was normal. Bronchoalveolar lavage was repeated once or twice in these five subjects 47 days or more after the previous lavage and the lymphocyte count decreased below 14% in four. Eight volunteers with an initial lymphocyte percentage less than 20% also had repeat lavages; two presented a transient increase of lymphocyte count above 20%. These data show that the percentage of lymphocytes in lavage fluid fluctuates significantly in normal subjects and suggest that lymphocyte counts counts higher than 14% should not be considered as normal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGLUND E., BIRATH G., BJURE J., GRIMBY G., KJELLMER I., SANDQVIST L., SODERHOLM B. Spirometric studies in normal subjects. I. Forced expirograms in subjects between 7 and 70 years of age. Acta Med Scand. 1963 Feb;173:185–192. [PubMed] [Google Scholar]
- Cormier Y., Bélanger J., Beaudoin J., Laviolette M., Beaudoin R., Hebert J. Abnormal bronchoalveolar lavage in asymptomatic dairy farmers. Study of lymphocytes. Am Rev Respir Dis. 1984 Dec;130(6):1046–1049. doi: 10.1164/arrd.1984.130.6.1046. [DOI] [PubMed] [Google Scholar]
- Dauber J. H., Rossman M. D., Daniele R. P. Bronchoalveolar cell populations in acute sarcoidosis. Observations in smoking and nonsmoking patients. J Lab Clin Med. 1979 Dec;94(6):862–871. [PubMed] [Google Scholar]
- Davis G. S., Giancola M. S., Costanza M. C., Low R. B. Analyses of sequential bronchoalveolar lavage samples from healthy human volunteers. Am Rev Respir Dis. 1982 Oct;126(4):611–616. doi: 10.1164/arrd.1982.126.4.611. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D. Bronchoalveolar lavage. Am Rev Respir Dis. 1982 Dec;126(6):961–963. doi: 10.1164/arrd.1982.126.6.961. [DOI] [PubMed] [Google Scholar]
- Godard P., Chaintreuil J., Damon M., Coupe M., Flandre O., Crastes de Paulet A., Michel F. B. Functional assessment of alveolar macrophages: comparison of cells from asthmatics and normal subjects. J Allergy Clin Immunol. 1982 Aug;70(2):88–93. doi: 10.1016/0091-6749(82)90234-2. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Low R. B., Davis G. S., Giancola M. S. Biochemical analyses of bronchoalveolar lavage fluids of healthy human volunteer smokers and nonsmokers. Am Rev Respir Dis. 1978 Nov;118(5):863–875. doi: 10.1164/arrd.1978.118.5.863. [DOI] [PubMed] [Google Scholar]
- Moore V. L., Pedersen G. M., Hauser W. C., Fink J. N. A study of lung lavage materials in patients with hypersensitivity pneumonitis: in vitro response to mitogen and antigen in pigeon breeders' disease. J Allergy Clin Immunol. 1980 May;65(5):365–370. doi: 10.1016/0091-6749(80)90214-6. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. Lung inflammation: role of endogenous chemotactic factors in attracting polymorphonuclear granulocytes. Am Rev Respir Dis. 1983 Feb;127(2):S16–S25. doi: 10.1164/arrd.1983.127.2P2.S16. [DOI] [PubMed] [Google Scholar]
- Saltini C., Hance A. J., Ferrans V. J., Basset F., Bitterman P. B., Crystal R. G. Accurate quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Oct;130(4):650–658. doi: 10.1164/arrd.1984.130.4.650. [DOI] [PubMed] [Google Scholar]
- Territo M. C., Golde D. W. The function of human alveolar macrophages. J Reticuloendothel Soc. 1979 Jan;25(1):111–120. [PubMed] [Google Scholar]
- Warr G. A., Martin R. R. Immune receptors of human alveolar macrophages: comparison between cigarette smokers and nonsmokers. J Reticuloendothel Soc. 1977 Sep;22(3):181–187. [PubMed] [Google Scholar]
- Weinberger S. E., Kelman J. A., Elson N. A., Young R. C., Jr, Reynolds H. Y., Fulmer J. D., Crystal R. G. Bronchoalveolar lavage in interstitial lung disease. Ann Intern Med. 1978 Oct;89(4):459–466. doi: 10.7326/0003-4819-89-4-459. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]



