Abstract
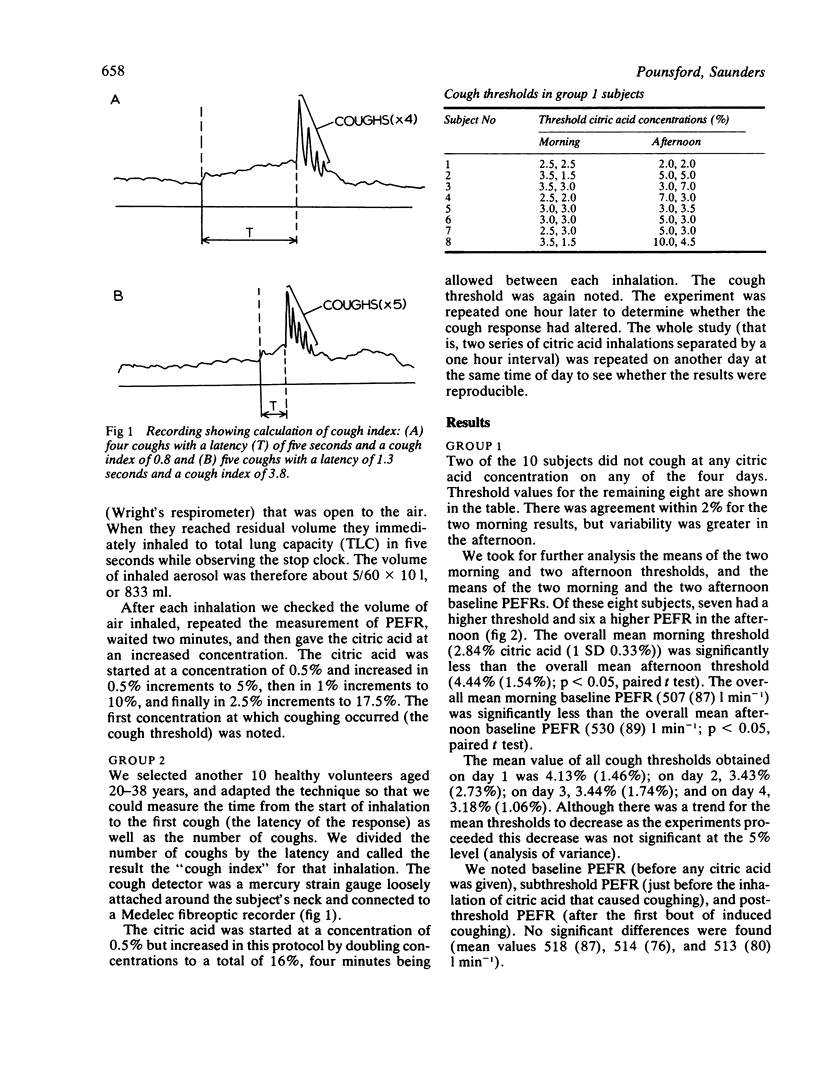
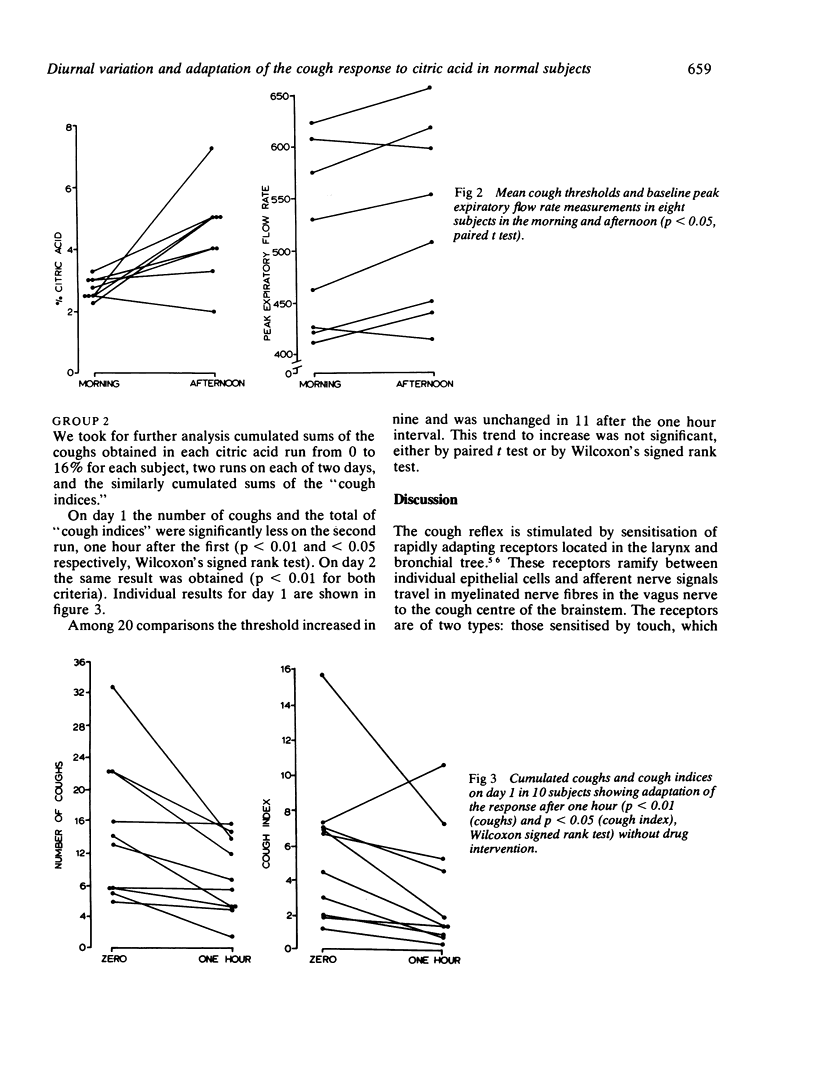
The lowest concentrations of citric acid were measured that caused coughing in 10 normal subjects who inhaled successively higher concentrations. Two subjects did not cough at any concentration. In the remaining eight the threshold concentration was significantly higher when measured in the afternoon than it was in the morning (p less than 0.05). The expected diurnal variation in peak expiratory flow rate (PEFR) was found (significant in the whole group--p less than 0.05); but PEFR did not change significantly when measured before and immediately after coughing caused by citric acid inhalations. In a second group of 10 normal subjects two series of citric acid inhalations were given, separated by one hour. The total number of coughs was significantly lower on the second run (p less than 0.05). Thus diurnal variation and adaptation of the cough response must be taken into account when antitussive drugs are tested.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BICKERMAN H. A., BARACH A. L. The experimental production of cough in human subjects induced by citric acid aerosols; preliminary studies on the evaluation of antitussive agents. Am J Med Sci. 1954 Aug;228(2):156–163. doi: 10.1097/00000441-195408000-00005. [DOI] [PubMed] [Google Scholar]
- BICKERMAN H. A., GERMAN E., COHEN B. M., ITKIN S. E. The cough response of healthy human subjects stimulated by citric acid aerosol. II. Evaluation of antitussive agents. Am J Med Sci. 1957 Aug;234(2):191–206. doi: 10.1097/00000441-195708000-00010. [DOI] [PubMed] [Google Scholar]
- Beaupré A., Orehek J. Factors influencing the bronchodilator effect of a deep inspiration in asthmatic patients with provoked bronchoconstriction. Thorax. 1982 Feb;37(2):124–128. doi: 10.1136/thx.37.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushey H. A., Richardson P. S., Widdicombe J. G., Wise J. C. The response of laryngeal afferent fibres to mechanical and chemical stimuli. J Physiol. 1974 Jul;240(1):153–175. doi: 10.1113/jphysiol.1974.sp010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Young G. A., Bye C. E., Hughes D. T. Comparison of the antitussive effects of codeine phosphate 20 mg, dextromethorphan 30 mg and noscapine 30 mg using citric acid-induced cough in normal subjects. Eur J Clin Pharmacol. 1979;16(6):393–397. doi: 10.1007/BF00568199. [DOI] [PubMed] [Google Scholar]
- Hetzel M. R., Clark T. J. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. 1980 Oct;35(10):732–738. doi: 10.1136/thx.35.10.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. E., Sellick H., Widdicombe J. G. Activity of lung irritant receptors in pulmonary microembolism, anaphylaxis and drug-induced bronchoconstrictions. J Physiol. 1969 Aug;203(2):337–357. doi: 10.1113/jphysiol.1969.sp008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps R. J., Richardson P. S. The effects of irritation at various levels of the airway upon tracheal mucus secretion in the cat. J Physiol. 1976 Oct;261(3):563–581. doi: 10.1113/jphysiol.1976.sp011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):55–70. doi: 10.1113/jphysiol.1954.sp005033. [DOI] [PMC free article] [PubMed] [Google Scholar]



