Abstract
The primary cilium provides a hub for reception of extracellular chemical and mechanical cues that influence differentiation, proliferation, and polarity, and contributes to cell cycle control. Ciliary length impacts the cilium’s ability to coordinate these processes, and length control defects are linked to a number of clinically important developmental disorders. An exciting new study identifies a new mechanism of ciliary regulation based on interactions of CDK5 and the FBW7 tumor suppressor in regulating the degradation of the centrosomal protein NDE1 (Maskey et al, 2015).
See also: D Maskey et al (October 2015)
Protruding from the surface of most cells organized in solid tissues in vertebrates, the primary cilium serves as a sensor for a broad variety of environmental molecular stimuli. This common expression reflects the fact that the majority of cells are quiescent, as the expression of the primary cilium is precisely coordinated with cell cycle progression (Fig1A). Typically, cilia shorten rapidly as cells progress from G1 to S phase of cell cycle, and ciliary disassembly is required for cells to enter mitotic division. Cilia reassembly occurs after a cell completes mitosis and enters G0/G1. In part, because the ciliary basal body competes with the centrosome for the use of centrioles, cilia have been proposed to contribute to the negative regulation of cell cycle control. In further growth regulatory roles, the cilium provides an essential platform for receptors for Hedgehog, Notch, and other signaling systems that govern cell growth. Most (although not all) cancer cells have significantly downregulated expression of cilia (Plotnikova et al, 2008); conversely, genetic defects causing abnormalities in ciliary structure or function are typically associated with abnormal growth of tissues. For these reasons, there has been intense interest in identifying factors that govern not only ciliary assembly and disassembly but also ciliary length, which provides a rheostat tuning the activity of cilia-associated signaling systems (Avasthi & Marshall, 2012; McMurray et al, 2013; He et al, 2014). A number of proteins have been identified as contributing to ciliary length control, but to date their mechanistic relationship remains confusing, and the connection between ciliary length and cell cycle is still not clearly defined.
Figure 1.
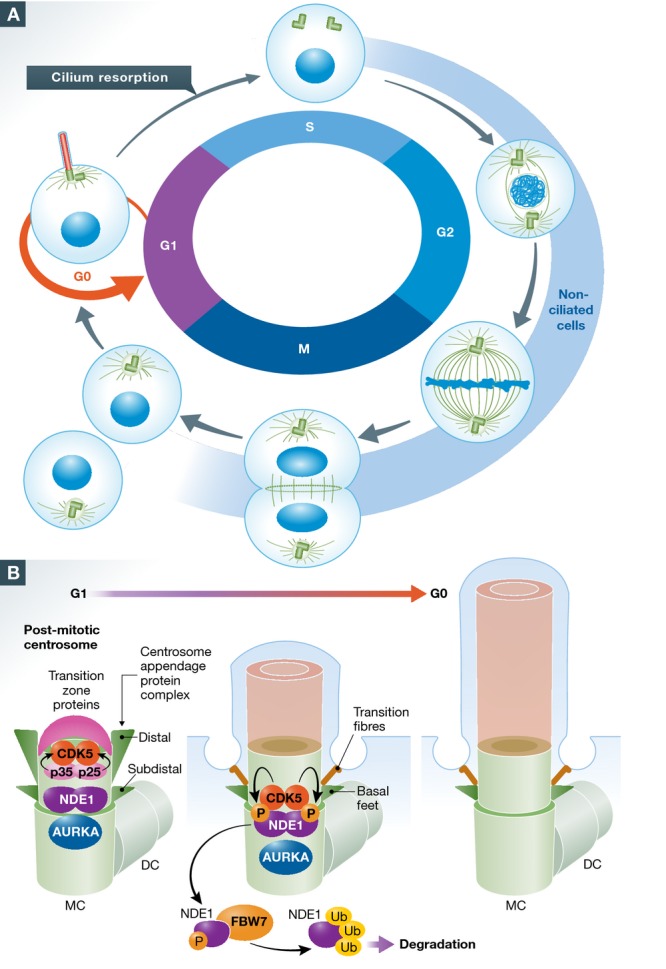
Primary cilium dynamics during the cell cycle
(A) During G1/S transition, vertebrate cells undergo ciliary resorption to allow centrosome duplication and centrosome function as a microtubule organizing center in M phase. The primary cilium is re-established in post-mitotic G1/G0 cells, with the basal body of the primary cilium formed from the mother centriole (MC). (B) Formation of primary cilium requires prior assembly of a centrosome appendage protein complex and transition zone proteins at the MC. Ciliogenesis is controlled by both negative (including AURKA) and positive regulatory factors, some of which also influence ciliary length. In this context, levels of CDK5 increase in the centrosome in post-mitotic cells, and activation of CDK5 by p25 and p35 leads to NDE1 phosphorylation. NDE1 phosphorylation allows recruitment of FBW7, which targets NDE1 for ubiquitin-dependent proteasomal degradation.
In this issue of The EMBO Journal, Maskey et al (2015) define a new signaling axis that places ciliary length regulation under control of the kinase CDK5 and the FBW7 tumor suppressor (Maskey et al, 2015). In earlier work, the Tsiokas group had established the centrosomal protein NDE1 as a controller of cilium length during cell cycle and a negative regulator of ciliogenesis (Kim et al, 2011). Expression of NDE1 [also known as NDE or LIS4, and best defined as a dynein-associated regulator of microtubule organization, mitosis, and neuronal migration (Bradshaw et al, 2013)] peaks in M phase of the cell cycle but is significantly reduced in G1 phase, thus correlating with the absence of cilia. The authors now show that NDE1 colocalizes to the centrosome and interacts directly with the FBW7 ubiquitin ligase (Welcker & Clurman, 2008). This interaction requires an intact phosphorylation site (Thr191) on NDE1, as well as the Arg465 residue within the WD40 interaction domain of FBW7.
Using a candidate approach, Maskey et al (2015) then evaluated a number of kinases known to target consensus sequences similar to the one surrounding NDE1 Thr191. Out of several candidates tested, only CDK5 with its cofactor p25/CDK5R1, again best studied as a neuronal kinase involved in brain development (Lew et al, 1994), was able to phosphorylate Thr191 and to induce NDE1 downregulation via the ubiquitin–proteasome system. Furthermore, depletion of either FBW7 or CDK5 significantly reduces cilia length, accompanied by augmented NDE1 levels. Finally, the authors probed for a functional role of these interactions in Hedgehog signaling: In control cells, processing of the Hedgehog effector GLI2 was triggered by SAG, an agonist of the ciliary Smoothened receptor, while in NDE1-depleted cells bearing elongated cilia, basal GLI2 levels were already elevated and furthermore not responsive to SAG treatment. Interestingly, in FBW7-depleted cells with significantly shortened cilia, basal GLI2 levels were notably depressed, and also not responsive to SAG. Taken together, these results suggest that CDK5 phosphorylation of NDE1 leads to recruitment of FBW7 as a negative regulator of NDE1 expression, resulting in increased ciliary length and ensuring appropriate signaling by ciliary receptors.
These findings raise questions for future investigation. For example, NDE1 shares 60% amino acid identity, 80% similarity, and many functions with the paralogous neuronal protein NDEL1/LIS1 (Bradshaw et al, 2013). CDK5 has previously been shown to bind and regulate NDEL1 in control of dynein-dependent organelle transport (Pandey & Smith, 2011). Whether NDEL1 and NDE1 have partially redundant function in control of ciliary length is currently not known, nor is the degree to which NDE1-dependent signaling mechanisms prevail in non-neuronal tissues, given the specificity of the p25 activator. Mutation of either NDE1 or NDEL1 is associated with defects in brain development; whether the disease phenotypes are related to functions of these proteins at cilia is not known. Finally, it is also intriguing that AURKA (Aurora A kinase), a regulator of ciliary shortening and disassembly, both phosphorylates and regulates NDEL1 function, and at the same time has been defined as an FBW7 target (reviewed in Nikonova et al, 2013); whether and how these activities link up to those now identified by Maskey and colleagues for NDE1 bears further scrutiny.
References
- Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation. 2012;83:S30–S42. doi: 10.1016/j.diff.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw NJ, Hennah W, Soares DC. NDE1 and NDEL1: twin neurodevelopmental proteins with similar ‘nature’ but different ‘nurture’. Biomol Concepts. 2013;4:447–464. doi: 10.1515/bmc-2013-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Jr, Kapoor TM, Anderson KV. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16:663–672. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Maskey D, Marlin MC, Kim S, Ong EC, Li G, Tsiokas L. Cell cycle-dependent ubiquitylation and destruction of NDE1 by CDK5-FBW7 regulates ciliary length. EMBO J. 2015;34:2424–2440. doi: 10.15252/embj.201490831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;3:3545. doi: 10.1038/srep03545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack RL, Jr, Golemis EA. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70:661–687. doi: 10.1007/s00018-012-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey JP, Smith DS. A Cdk5-dependent switch regulates Lis1/Ndel1/dynein-driven organelle transport in adult axons. J Neurosci. 2011;31:17207–17219. doi: 10.1523/JNEUROSCI.4108-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]


