Comment on: M Moulin et al (April 2012)
Initially classified as potent suppressors of programed cell death, members of the inhibitor of apoptosis (IAP) family have emerged as complex signaling proteins whose functions are not limited to apoptosis regulation, but extend to several signaling pathways involved in the regulation of immunity, inflammation, and cell migration (Gyrd-Hansen & Meier, 2010). Of the eight mammalian IAPs, X-linked IAP and cellular IAPs 1 and 2 (XIAP, cIAP1, cIAP2) show particularly strong structural and functional homology. Genetic approaches have been used to assign precise functions to XIAP, cIAP1, and cIAP2, and initial studies showed that the deletion of XIAP, cIAP1, or cIAP2 resulted in viable animals with relatively subtle phenotypes that may reflect functional compensation between these three family members (Harlin et al, 2001; Conze et al, 2005; Conte et al, 2006).
ciap1 and ciap2 are located only 15 kb apart on mouse chromosome 9. A conditional approach has been used to selectively delete ciap1 and ciap2, and these mice have been analyzed in wild-type or xiap null backgrounds (Moulin et al, 2012). xiap−/−ciap2−/− null mice were viable, fertile, and lacked an obvious phenotype, whereas xiap−/−ciap1−/− and ciap1−/−ciap2−/− mice died in utero at E12.5. This suggested that cIAP1 could compensate for combined deletion of cIAP2 and XIAP, whereas cIAP2 could not compensate for the combined deletion of cIAP1 and XIAP.
Here, we report that compound xiap:ciap1 null mice generated by simple breeding are viable and fertile and that cells derived from them have essentially normal tumour necrosis factor (TNF) signaling properties. Crosses of mice bearing germline mutations of xiap, ciap1, or ciap2 (Harlin et al, 2001; Conze et al, 2005; Conte et al, 2006) resulted in Mendelian distributions of expected genotypes at birth, with no evidence of embryonic or perinatal lethality. Notably, xiap−/−ciap1−/− and xiap−/−ciap2−/− compound nulls developed normally, were fertile, and lacked obvious phenotypes. Reverse-transcription PCR (RT–PCR) performed on mouse embryonic fibroblasts (MEFs) derived from each of the strains confirmed that gene disruptions were complete (Fig1A). Previous studies from several laboratories have shown that cIAP2 protein levels are dramatically increased in MEFs and tissues lacking cIAP1 (Mahoney et al, 2008; Enwere et al, 2012) and consistent with this, cIAP2 protein levels are strongly increased in several, but not all, tissues derived from xiap−/−ciap1−/− mice (Fig1B).
Figure 1.
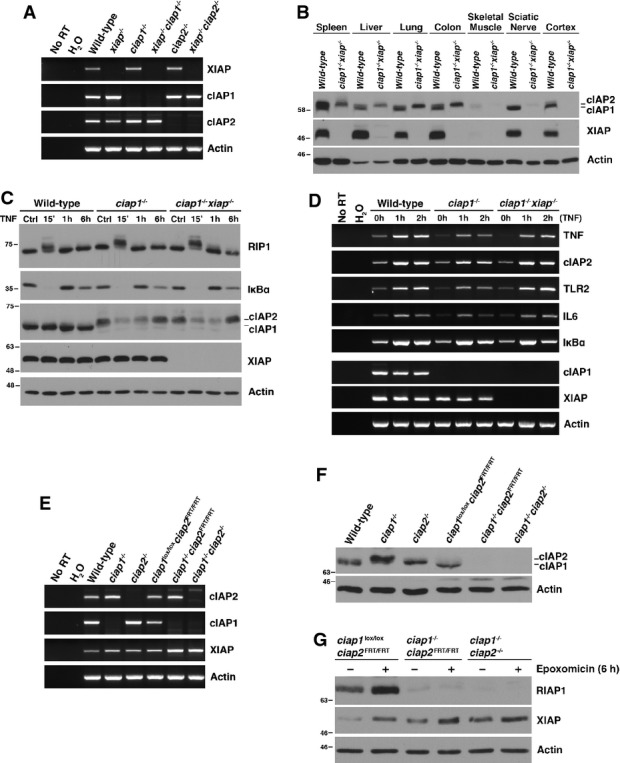
cIAP2 compensates for cIAP1 and XIAP deficiency in vivo
(A) Reverse-transcription PCR (RT–PCR) demonstrating the absence of mRNA transcript in primary MEFs generated from IAP null mice. (B) Immunoblot demonstrating the upregulation of cIAP2 in various xiap−/−ciap1−/− tissues. cIAP1 and cIAP2 protein were detected using a pan-cIAP antibody (RIAP1). (C, D) Western blot (C) and RT–PCR (D) of protein lysates and mRNA collected from MEFs incubated with or without 10 ng/ml recombinant mTNFα for the indicated times. (E, F) Comparison of IAP mRNA transcripts (E) and protein expression (F) in unstimulated MEFs derived from mice generated by classical (ciap1−/−, ciap2−/−) and conditional (ciap1lox/loxciap2FRT/FRT, ciap1−/−ciap2FRT/FRT, ciap1−/−ciap2−/−) knockout approaches. (G) MEFs were treated with proteasomal inhibitor epoxomicin (0.5 μM) for 6 h and harvested for protein detection by Western blot.
Wild-type, ciap1−/− and xiap−/−ciap1−/− MEFs were compared for their responses to tumour necrosis factor (Fig1C). In wild-type cells, cIAP2 protein was not detectable and cIAP1 levels were not altered by TNF (10 ng/ml) exposure. In ciap1−/− and xiap−/−ciap1−/− MEFs, cIAP2 protein was readily detected and, interestingly, was sharply reduced by TNF exposure by 15 min, with recovery to control levels only after 6 h of treatment. Otherwise, TNF responses appeared normal across each genotype, with a rapid and complete degradation of IκBα protein that returned to baseline levels within 1 h, and a characteristic molecular weight shift in RIP1 after 15 min of TNF exposure (Fig1C). TNF-dependent expression of several NF-κB target genes (cIAP2, TLR2, IL6, IκBα) was essentially normal, with identical responses in wild-type and xiap−/−ciap1−/− MEFs and a slightly attenuated response in ciap1−/− MEFs (Fig1D).
These results differ from those in Moulin et al (2012) who reported that TNF-dependent NF-κB activation was absent in xiap−/−ciap1−/− MEFs produced using a conditional gene knockout approach, suggesting that ciap2 could not compensate for their deletion. However, cIAP2 protein levels in these MEFs did not increase upon ciap1 deletion, which is at odds with the cIAP2 regulation seen here and by others.
We directly compared cIAP2 mRNA and protein levels in the ciap1 null MEFs produced by Conze et al (2005) to those in the ciap1lox/loxciap2FRT/FRT, ciap1−/−ciap2FRT/FRT, and ciap1−/−ciap2−/− MEFs produced by Moulin et al (2012). Figure1E shows that MEFs from all strains produced cIAP2 mRNA, but the ciap1−/−ciap2FRT/FRT MEFs produced by Moulin et al (2012) did not produce detectable levels of cIAP2 protein, in contrast to the ciap1−/− MEFS derived from the mice produced by Conze et al (2005) (Fig1F). To determine whether cIAP2 undergoes unusually rapid proteosomal turnover in ciap1−/−ciap2FRT/FRT cells, we exposed cells to epoxomicin, a proteasome inhibitor; this treatment significantly elevated cIAP1 levels in ciap1lox/loxciap2FRT/FRT MEFs but did not increase cIAP2 levels in the ciap1−/−ciap2FRT/FRT cells (Fig1G), indicating that cIAP2 production is significantly impaired in ciap1−/−ciap2FRT/FRT MEFs. Although we were unable to detect cIAP2 in the ciap1−/−ciap2FRT/FRT MEFs, these animals are not completely devoid of cIAP2 as Moulin et al (2012) were able to immunoprecipitate cIAP2 protein from whole embryo lysates using a biotinylated Smac mimetic compound. We conclude that the mice described in Moulin et al (2012) have an unanticipated defect in cIAP2 production and as a result, levels of cIAP2 in the xiap−/−ciap1−/−ciap2FRT/FRT animals are unable to compensate for the loss of cIAP1 and XIAP. Therefore, the divergence in phenotype between the xiap−/−ciap1−/−ciap2FRT/FRT mice (dead at E12.5) and the xiap−/−ciap1−/− mice produced by simple breeding (viable, fertile, apparently normal) is likely to reflect this difference in cIAP2 expression.
It is important to note that we have previously shown that ciap1−/− mice contain an inactivating mutation in the casp4 gene, due to a 5-bp deletion which originated in the 129-derived ES cell line used to create the cIAP1 knockout strain (Kenneth et al, 2012) and that this passenger mutation is also present in the xiap:ciap1 null strain described here (data not shown). The casp4 allele produces caspase 11, which has recently been shown to function as a direct innate immune receptor for intracellular lipopolysaccharide and to promote pyroptosis (Shi et al, 2014). Although there is no evidence indicating that caspase 11 plays an essential role in development, it is conceivable that the normal survival of the xiap:ciap1 mice described here may in part reflect absence of caspase 11 activity.
Our data indicate that cIAP2 protein levels are dramatically upregulated in mice lacking cIAP1 and XIAP and that TNF signaling events proceed almost normally in MEFs lacking cIAP1 and XIAP. The xiap: ciap1 and xiap:ciap2 compound nulls described here, and cells derived from them, will be useful for isolating cell type- and pathway-specific signaling properties of cIAP1 and cIAP2.
Materials and Methods
Cell culture
Primary mouse embryonic fibroblasts (MEFs) were derived from E12.5 timed pregnant mice and generated in accordance to standard procedures. All MEFs were maintained in 10% fetal bovine serum, 2 mM l-glutamine, and 100 mg/ml penicillin/streptomycin in 5% CO2 at 37°C.
Antibodies and reagents
The polyclonal XIAP and pan-cIAP antibodies were generous gifts from Dr. Robert G. Korneluk (University of Ottawa, ON). Mouse monoclonal antibodies for β-actin and RIP1 were purchased from MP Biomedicals and BD Biosciences, respectively. Rabbit polyclonal anti-IkBα was purchased from Santa Cruz. Recombinant mTNFα was purchased from R&D Systems. Epoxomicin was purchased from VWR.
Reverse-transcription PCR (RT–PCR)
mRNA was isolated using Qiagen’s RNeasy Mini kit as per the manufacturer’s instructions. cDNA was produced using the Omniscript RT kit (Qiagen) with random hexamers (GE Healthcare) as primers. PCR primer sequences available upon request.
Sample preparation for SDS–PAGE and immunoblotting
Cells were washed once with PBS then lysed in either 2× Laemmli sample buffer (2% SDS, 50 mM DTT, 60 mM Tris (pH 6.8), 5% glycerol, 0.01% (w/v) bromophenol blue) or NP-40 lysis buffer (1.0% NP-40, 10 mM Tris pH 8.0, 150 mM NaCl, 10% glycerol) supplemented with Complete Mini Protease Inhibitor Cocktail tablets (Roche; Laval, QC). Tissues were dissected from adult mice and homogenized in NP-40 lysis buffer.
Acknowledgments
We thank Kathleen M. Dickson for supervising mouse husbandry and providing technical assistance. This project was supported by a grant (#38942) to PAB from the Canadian Institutes of Health Research. MB has a tenure track position within the Multidisciplinary Research Program of Ghent University (GROUP-ID).
Author contributions
KNH performed the genomic PCR, immunoblots, and RT–PCR shown in Figure 1. MJMB set up initial mouse crosses and performed preliminary analyses of TNF signaling in resulting primary MEFs. KNH compiled the figures and KNH and PAB wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Conte D, Holcik M, Lefebvre CA, Lacasse E, Picketts DJ, Wright KE, Korneluk RG. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 2006;26:699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T, Ashwell JD. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere EK, Holbrook J, Lejmi-Mrad R, Vineham J, Timusk K, Sivaraj B, Isaac M, Uehling D, Al-awar R, LaCasse E, Korneluk RG. TWEAK and cIAP1 regulate myoblast fusion through the noncanonical NF-kappaB signaling pathway. Sci Signal. 2012;5:ra75. doi: 10.1126/scisignal.2003086. [DOI] [PubMed] [Google Scholar]
- Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth NS, Younger JM, Hughes ED, Marcotte D, Barker PA, Saunders TL, Duckett CS. An inactivating caspase 11 passenger mutation originating from the 129 murine strain in mice targeted for c-IAP1. Biochem J. 2012;443:355–359. doi: 10.1042/BJ20120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, Vaux DL. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]


