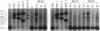Abstract
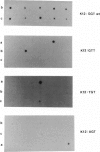
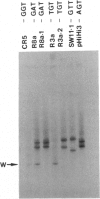
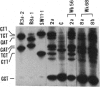
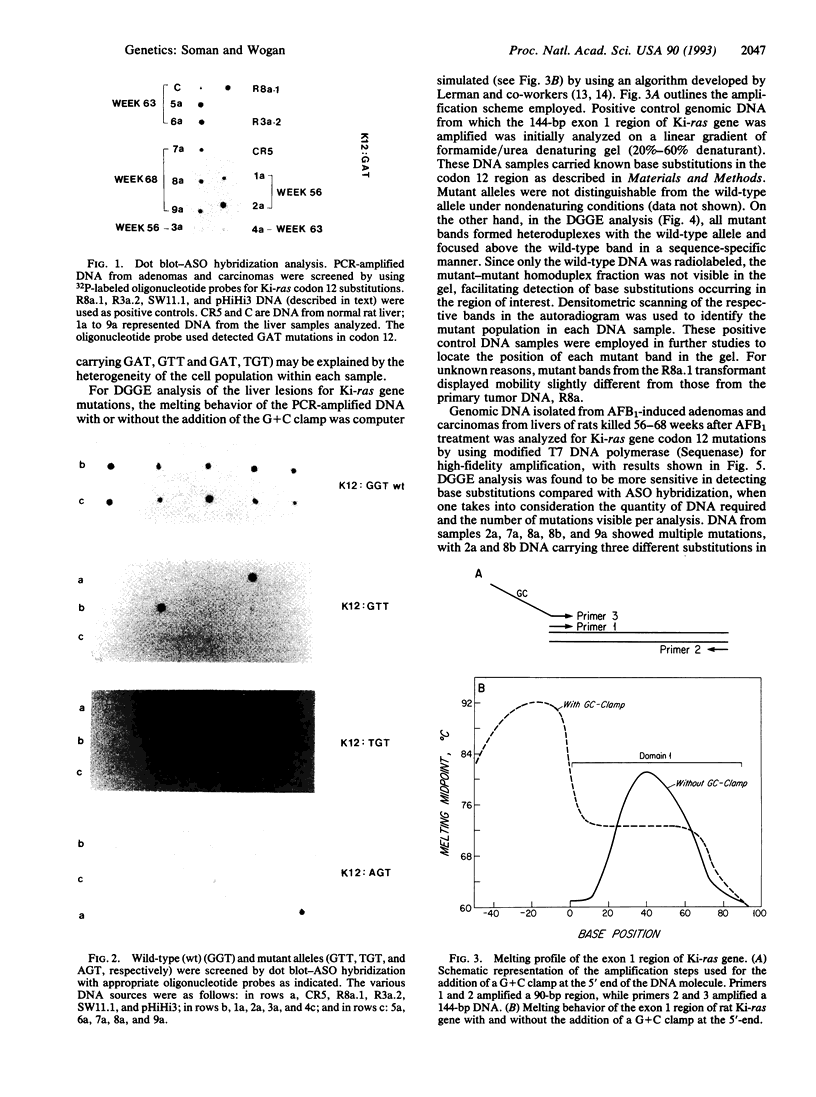
Sequence alterations in the exon 1 region of the rat c-Ki-ras gene were studied in DNA isolated from aflatoxin B1 (AFB1)-induced rat liver carcinomas and precursor lesions appearing 56 weeks after administration of the carcinogen. To detect the mutations with high sensitivity, DNA samples were analyzed by using polymerase chain reaction (PCR) amplification in conjunction with allele-specific oligonucleotide (ASO) hybridization together with a modified PCR-G+C clamp-denaturing gradient gel electrophoresis (DGGE) method. Mutations in the Ki-ras gene were present in all adenomas and carcinomas examined. The predominant mutation observed was a G.C-to-A.T base transition in codon 12 (GGT to GAT). Also present, but at low frequency, was a G.C-to-T.A base transversion in the same codon (GGT to TGT). In addition, 20% of the samples contained a G.C-to-T.A transversion in the second base position of codon 12 (GGT to GTT), a mutation not previously observed in AFB1-induced rat liver tumors. These results confirm and extend our previous findings that Ki-ras mutation is a prevalent event in hepato-cellular carcinogenesis induced in Fischer 344 rats by AFB1. The modified DGGE method described is applicable to the screening of multiple mutations in neoplastic lesions with high fidelity and sensitivity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Cariello N. F., Keohavong P., Sanderson B. J., Thilly W. G. DNA damage produced by ethidium bromide staining and exposure to ultraviolet light. Nucleic Acids Res. 1988 May 11;16(9):4157–4157. doi: 10.1093/nar/16.9.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariello N. F., Scott J. K., Kat A. G., Thilly W. G., Keohavong P. Resolution of a missense mutant in human genomic DNA by denaturing gradient gel electrophoresis and direct sequencing using in vitro DNA amplification: HPRT Munich. Am J Hum Genet. 1988 May;42(5):726–734. [PMC free article] [PubMed] [Google Scholar]
- Corominas M., Kamino H., Leon J., Pellicer A. Oncogene activation in human benign tumors of the skin (keratoacanthomas): is HRAS involved in differentiation as well as proliferation? Proc Natl Acad Sci U S A. 1989 Aug;86(16):6372–6376. doi: 10.1073/pnas.86.16.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy R. G., Essigmann J. M., Reinhold V. N., Wogan G. N. Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1745–1749. doi: 10.1073/pnas.75.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Wang C. C., Cha R. S., Thilly W. G. Enzymatic amplification and characterization of large DNA fragments from genomic DNA. Gene. 1988 Nov 15;71(1):211–216. doi: 10.1016/0378-1119(88)90094-7. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Fischer S. G., Hurley I., Silverstein K., Lumelsky N. Sequence-determined DNA separations. Annu Rev Biophys Bioeng. 1984;13:399–423. doi: 10.1146/annurev.bb.13.060184.002151. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- McCoy M. S., Bargmann C. I., Weinberg R. A. Human colon carcinoma Ki-ras2 oncogene and its corresponding proto-oncogene. Mol Cell Biol. 1984 Aug;4(8):1577–1582. doi: 10.1128/mcb.4.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Davis E. F., Huber L. J., Kim Y., Wogan G. N. Characterization of c-Ki-ras and N-ras oncogenes in aflatoxin B1-induced rat liver tumors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1104–1108. doi: 10.1073/pnas.87.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Davis E., Wogan G. N. Characterization of c-Ki-ras oncogene alleles by direct sequencing of enzymatically amplified DNA from carcinogen-induced tumors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4974–4978. doi: 10.1073/pnas.84.14.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Hanson L., Lee J. J., Wogan G. N. Identification of an activated c-Ki-ras oncogene in rat liver tumors induced by aflatoxin B1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9418–9422. doi: 10.1073/pnas.83.24.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Huber L. J., Moore M. J., Stegeman J. J., Wogan G. N. Mutations in c-Ki-ras oncogenes in diseased livers of winter flounder from Boston Harbor. Proc Natl Acad Sci U S A. 1990 Jan;87(2):841–845. doi: 10.1073/pnas.87.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Newberne P. M., Wogan G. N. Sequential morphologic changes in aflatoxin B carcinogenesis in the rat. Cancer Res. 1968 Apr;28(4):770–781. [PubMed] [Google Scholar]
- Noll W. W., Collins M. Detection of human DNA polymorphisms with a simplified denaturing gradient gel electrophoresis technique. Proc Natl Acad Sci U S A. 1987 May;84(10):3339–3343. doi: 10.1073/pnas.84.10.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Patterson R. M., Maronpot R. R., Aaronson S. A., Anderson M. W. Activated oncogenes in B6C3F1 mouse liver tumors: implications for risk assessment. Science. 1987 Sep 11;237(4820):1309–1316. doi: 10.1126/science.3629242. [DOI] [PubMed] [Google Scholar]
- Ross R. K., Yuan J. M., Yu M. C., Wogan G. N., Qian G. S., Tu J. T., Groopman J. D., Gao Y. T., Henderson B. E. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992 Apr 18;339(8799):943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Webber C., Marshall C. J., Knowles M. A., Proctor A., Barrass N. C., Neal G. E. Activation of ras oncogene in aflatoxin-induced rat liver carcinogenesis. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3673–3677. doi: 10.1073/pnas.85.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers S. J., Glover P. L., Reynolds S. H., Boone L. R., Maronpot R. R., Anderson M. W. Activation of the K-ras protooncogene in lung tumors from rats and mice chronically exposed to tetranitromethane. Cancer Res. 1987 Jun 15;47(12):3212–3219. [PubMed] [Google Scholar]
- Swenson D. H., Miller E. C., Miller J. A. Aflatoxin B1-2,3-oxide: evidence for its formation in rat liver in vivo and by human liver microsomes in vitro. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1036–1043. doi: 10.1016/0006-291x(74)90417-3. [DOI] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]