Abstract
Cognitive remediation training (CRT) for schizophrenia has been found to improve cognitive functioning and influence neural plasticity. However, with various training approaches and mixed findings, the mechanisms driving generalization of cognitive skills from CRT are unclear. In this meta-analysis of extant imaging studies examining CRT’s effects, we sought to clarify whether varying approaches to CRT suggest common neural changes and whether such mechanisms are restorative or compensatory. We conducted a literature search to identify studies appropriate for inclusion in an activation likelihood estimation (ALE) meta-analysis. Our criteria required studies to consist of training-based interventions designed to improve patients’ cognitive or social functioning, including generalization to untrained circumstances. Studies were also required to examine changes in pre- vs posttraining functional activation using functional magnetic resonance imaging or positron emission tomography. The literature search identified 162 articles, 9 of which were appropriate for inclusion. ALE analyses comparing pre- and posttraining brain activation showed increased activity in the lateral and medial prefrontal cortex (PFC), parietal cortex, insula, and the caudate and thalamus. Notably, activation associated with CRT in the left PFC and thalamus partially overlapped with previous meta-analytically identified areas associated with deficits in working memory, executive control, and facial emotion processing in schizophrenia. We conclude that CRT interventions from varying theoretic modalities elicit plasticity in areas that support cognitive and socioemotional processes in this early set of studies. While preliminary, these changes appear to be both restorative and compensatory, though thalamocortical areas previously associated with dysfunction may be common sources of plasticity for cognitive remediation in schizophrenia.
Key words: cognitive remediation, schizophrenia, activation likelihood estimate, prefrontal cortex, thalamus, plasticity
Introduction
Schizophrenia is characterized by broad and pervasive cognitive deficits1 affecting functional ability and contributing to poor outcomes in this population.2 Because these impairments respond only mildly to antipsychotic treatments,3 clinical researchers have examined psychological approaches to ameliorate these cognitive problems. Cognitive remediation training (CRT) is an increasingly viable strategy for treating the cognitive and functional deficits experienced by patients with schizophrenia. These interventions consist of clinician-led or computerized training that utilizes a mix of cognitive exercises and generalization strategies to improve the attention, problem solving, and memory skills that support daily functioning.
Meta-analytic findings spanning various CRT approaches and domains indicate that this treatment has modest effects on global cognition and functioning and that these changes may persist beyond the acute training period.4 Still, much remains unclear about the neurobiology underlying CRT, and whether increases, decreases, or functional reorganization of brain activity reflect improvements in cognition. Randomized trials studying CRT in schizophrenia have approached these questions by adding pre- and posttreatment neuroimaging protocols. Numerous studies have observed training-related neuroplasticity, which is broadly characterized in this context by functional activation changes associated with treatment. However, with small sample sizes, various CRT approaches, and heterogeneity among findings, it is useful at this point to integrate these studies and provide a set of modal findings to guide future hypothesis tests.
In the current study, we used a spatial meta-analytic approach known as activation likelihood estimation (ALE) to examine whether CRT across training modalities influences common neuroanatomical regions. This approach examines the cumulative evidence of activation for various brain locations across published studies. Though CRT treatments often differ, they share a common aim of generalization beyond their training domain to transfer cognitive and psychosocial abilities to untrained circumstances. In the context of the current study, we sought to understand generalization as it related to performance on untrained tasks to measure the transfer of trained skills. Identifying the neural substrates associated with generalization of cognitive skill transfer to untrained abilities will be crucial to an emerging understanding of neural plasticity in schizophrenia and may identify candidate neural targets supported by these mechanisms. This is of timely importance, as it will provide direction toward candidate brain areas of interest in ongoing investigations of cognitive training. Furthermore, this study hoped to clarify discrepancies in this field to determine whether CRT interventions invoke compensatory or rehabilitative neuroplastic changes.
Impairments in executive functioning, cognitive control, working memory, and emotional processing are believed to be core cognitive deficits in schizophrenia, and a number of studies have identified neural disruptions associated with these disabilities.5–8 Meta-analyses indicate that though patients and healthy individuals recruit the same neural networks in response to executive and working memory tasks, patients have disrupted activity in these areas.7,9 Using an ALE approach, Minzenberg and colleagues9 demonstrated that both patient and control groups activated prefrontal regions including dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, and anterior cingulate cortex (ACC) as well as lateral temporal areas, parietal areas, and motor areas in response to cognitive tasks. However, differences were observed between groups, wherein patients showed less activation in areas including the lateral prefrontal cortex (PFC), ACC, and thalamus. Some of these same regions have shown abnormal responses during working memory tasks in patients’ healthy siblings, suggesting they may also mark an unexpressed genetic liability for schizophrenia.10
ALE meta-analyses have also demonstrated that patients with schizophrenia show patterns of impairment associated with facial affect processing.11 Frontal areas including the medial PFC and precentral gyrus, limbic areas such as the amygdala and insula, and midbrain areas including the caudate and thalamus were shown to activate less in response to emotional faces in patients compared with controls. As such, these aberrant patterns of prefrontal, limbic, and midbrain neural activations are thought to underlie the cognitive and socioemotional deficits observed in schizophrenia, making them critical targets for cognitive interventions in schizophrenia.
Efforts to develop treatments for cognitive dysfunction in schizophrenia have not only looked toward biological markers of pathology but have also built on a literature demonstrating the brain’s ability to change. Research over the last 3 decades has indicated that the brain is “plastic” well into adulthood, and studies examining synaptic and cortical map plasticity have demonstrated that long-term potentiation may underlie the implicit reorganization of the brain when it learns new material.12 Plasticity and potential skill transfer are thought to occur as the result of practice-driven coordination at multiple processing levels. Like in the case of working memory, engagement of both perceptual processes and top-down modulation of frontal brain areas are thought to impact cognitive processing.13 Rewarded behavior is also known to promote plasticity and is associated with neural activation in midbrain areas such as the ventral tegmental area, nucleus accumbens, and hippocampus.14
Though evidence for plasticity is strong, the argument that cognitive training can promote meaningful plasticity in healthy adults remains controversial. Cognitive training studies have been critiqued for their inability to demonstrate transfer to untrained tasks, their lack of applicability to real-world skills, and a dearth of placebo-controlled trials to examine their efficacy.15 However, working memory training has been reported to increase activity in the lateral PFC and parietal cortices16 along with other patterns of change.17 Increases in activation may indicate strengthened cortical engagement resulting from training,18 while decreases may be interpreted as improved neural efficiency, shifting neural processes from being effortful to more automatic.19 Redistribution and reorganization of neural activity in response to cognitive training may reflect both increases and decreases in activity, but also evokes activity in new areas as cognitive processes are learned or developed.19 Gray matter and other structural anomalies, which have been widely observed in schizophrenia,20 are also worth considering in regard to functional changes, because compensatory vs rehabilitative activation changes may be related to the status of baseline atrophy and dysfunction. It is yet unclear which specific factors of cognitive training reflect changes in activity via improved cognition in healthy adults. An emerging picture suggests that areas susceptible to training-induced plasticity in healthy adults largely overlap with those implicated in cognitive deficits in schizophrenia.9
Emerging from findings in healthy adults, hypotheses about the neural systems influenced by cognitive training for schizophrenia largely implicate similar prefrontal mechanisms to support improved cognitive function. Individual studies examining CRT in schizophrenia have primarily demonstrated increases in activation in cognitive control areas such as the lateral PFC.21 However, other trainings with both cognitive and social training components have demonstrated more nuanced patterns of activation change, with both increases and decreases in prefrontal and subcortical limbic areas.22 Though these various approaches to CRT may have similar influences on cognition and psychosocial functioning, it is currently unclear whether they affect similar brain areas. A clearer understanding of the neural systems impacted by these interventions will be crucial as novel psychiatric treatments aim to intervene at the level of spatial and temporal neural dynamics.
The goal of the current study was to clarify the emerging understanding of CRT-induced plasticity in patients with schizophrenia and characterize the observed functional activation changes evoked by training. Using an ALE approach, we examined whether CRT across training modalities change common neuroanatomical regions associated with interventions that influence the generalization of cognitive skill transfer. Additionally, we aimed to clarify whether CRT interventions invoke brain plasticity that is rehabilitative (change in areas previously shown to be disrupted) or compensatory (activation in new areas hypothesized to support cognitive functioning). In doing so, we examined “target engagement” by comparing the current findings to previous meta-analytic results to determine whether areas supporting CRT were similar to areas known to be deficient in this population. We hypothesized that CRT would show a restorative effect, increasing activation in areas previously shown to be dysfunctional, with maximal impact on areas of the brain that support cognition and social functioning. Predicted brain regions included the lateral PFC supporting cognitive and executive control and socioemotional brain areas including the insula and medial PFC. To clarify whether specific aspects of the varying CRT approaches also support these hypotheses, we performed exploratory analyses to examine differences between extant studies based on the modality of training, intensity of the intervention, whether trainings were computerized, whether studies were placebo controlled, and differences in the task used to measure generalization of functioning and neural plasticity.
Methods
Literature Search
For this study, we characterized cognitive remediation broadly, aiming to identify studies that examined any training-based intervention designed to improve schizophrenia patients’ cognitive or social functioning, using data on generalization to untrained tasks, abilities, or circumstances. A literature search of English language–speaking journals was conducted in PubMed using the following combinations of keywords: “Cognitive Remediation,” or “Cognitive Training,” or “Cognitive Rehabilitation,” or “Psychiatric Rehabilitation,” or “Working Memory Training,” with “Schizophrenia,” or “Psychosis,” and with “fMRI,” or “Imaging,” or “Neural Activation.” Inclusion criteria required studies to (a) be part of a clinical trial examining cognitive remediation in schizophrenia, (b) examine change in hemodynamic response on a fMRI or PET task both before and after training using a general linear model (GLM), (c) rely on a generalization task that was not used for cognitive training, and (d) report findings in either Montreal Neurologic Institute (MNI) or Talairach space.
ALE Analysis
ALE analysis was conducted in GingerALE v2.323–25 in the BrainMap environment. Study coordinates were entered into the database in Talairach space. Coordinates reported in MNI space were transformed to Talairach space based on the icbm2tal algorithm.26 GingerALE calculations are carried out in multiple steps: First, modeled activation (MA) maps are created for each experiment by modeling individual foci within experiments as Gaussians, with widths calculated based on the group Ns.23 Next, an unthresholded ALE map is calculated based on the union of these MA maps, with the probabilities of finding a given value within a map combined across studies to build a 3D P-value image. The P-value image is then thresholded using a false discovery rate (FDR) correction and then cluster-thresholded by simulating random data given the characteristics of the entered data set. In the cluster-level inference, contiguous voxels of the simulated data that exceed the set FDR threshold form a final cluster-corrected image. In the current analysis, we employed a FDR correction of P < .05, 1000 permutations of simulated data, and a cluster-level inference threshold of P < .05. To control for within-subject effects, separate investigations that contained the same subjects were treated as single studies in the ALE model, with foci from both experiments pooled into 1 study entry. Therefore, interventions with multiple studies or imaging protocols were treated as 1 experiment in this investigation.
Results
Literature Search Results
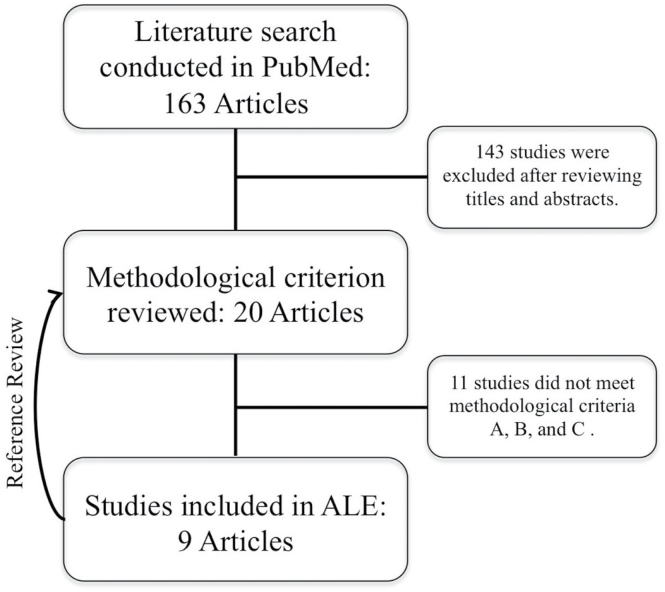
Search results identified 163 unique articles, 19 of which were studies that used neuroimaging measures associated with CRT (figure 1). Of these, 9 met the inclusion criteria, with a total of 128 subjects and 74 foci. Two pairs of included studies were conducted on the same subjects using a different fMRI task and were treated as random effects in the ALE. As such, the final ALE contained 7 experiments modeled as random effects.
Fig. 1.
Consort diagram of literature search. Note: No new articles were identified as the result of the reference review.
The theoretic approach to cognitive remediation varied among the 9 included studies (table 1). Two relied on an auditory training approach27,28 (which utilizes errorless-learning strategies to improve the speed and accuracy of auditory information processing), while 2 others relied on an auditory training plus social cognition training program.22,29 These 4 studies used a computer game active placebo control condition. Wykes and colleagues30 used an approach that relied on paper and pencil tasks to practice information processing strategies in areas of executive functioning and memory. Their study used an occupational therapy as the control condition. Two studies used computerized cognitive training to target broad areas of cognitive functioning including attention, verbal working memory, logical reasoning, and executive functioning and were compared with treatment as usual (TAU) as the control condition.31,32 One study used a computerized working memory–focused cognitive training program with a cognitive behavioral social skills training control group.21 Finally, 1 study trained affective and social cognition more specifically using various facial emotion and affect recognition tasks and used TAU as a control.33
Table 1.
Studies Included in Activation Likelihood Estimation (ALE)
| Source | Active N | Control N | Treatment | Control Condition | Duration/ Dose | Intensity | Task | Direction of Activity |
|---|---|---|---|---|---|---|---|---|
| Wykes et al30 | 6 | 6 | Individualized CRT | Occupational therapy | 12/40 | 3.333 | n-Back | ↑ |
| Haut et al21 | 9 | 9 | Working memory training | Cognitive behavioral social skills training | 4–6/25 | 5 | n-Back | ↑ |
| Habel et al33 | 10 | 10 | Training of affect recognition | TAU | 6/9 | 1.5 | Facial Emotion and Age Recognition Task | ↑ |
| Bor et al31 | 8 | 9 | Rehacom-CRT | TAU | 7/28 | 4 | n-Back | ↑ |
| Hooker et al29 | 11 | 11 | Auditory training + social cognition training | Computer game placebo | 10/50 | 5 | Emotion Recognition Task | ↑↓ |
| Subramaniam et al27 | 15 | 14 | Auditory and visual training | Computer game placebo | 16/90 | 5.625 | Reality Monitoring Task | ↑ |
| Hooker et al22 | 11 | 11 | Auditory training + social cognition training | Computer game placebo | 10/50 | 5 | Facial Emotion Recognition Task | ↑↓ |
| Vianin et al32 | 8 | 8 | RECOS CRT | TAU | 14/42 | 3 | Verbal Fluency Task | ↑ |
| Subramaniam et al28 | 16 | 15 | Auditory training and visual training | Computer game placebo | 16/90 | 5.625 | n-Back | ↑ |
Note: N, subject number; TAU, treatment as usual; ↑, increase; ↓, decrease; CRT, cognitive remediation training. The ALE included a total of 128 subjects (active = 68; control = 60). Participants underwent an average of 40 sessions (dose), an average of 10 weeks of training, and an average treatment intensity of 3.92 (calculated based on “dose” divided by “duration”). The 2 studies each by Hooker and colleagues and Subramaniam and colleagues were counted only once because they constituted the same patient groups. We used the higher N from Subramaniam et al.28 The study by Vianin and colleagues measured time 2 > time 1 in active treatment group only and therefore only active treatment N was included; all others studies measured a Group × Task interaction. Intensity calculated based on dose/duration.
Of the 9 studies summarized in table 1, 4 relied on an n-back task to assess activation associated with cognitive functioning.21,28,30,31 Three studies used facial emotion assessment tasks to examine brain activation.22,29,33 One study used a verbal fluency task,32 and the last used a reality monitoring task.28 All 9 studies observed increases in activation as a result of training, but only 2 of these studies also reported decreases in activation as a result of training.22,29 All studies reported a group by time interaction except for Vianin and colleagues,32 which reported comparisons of time 2 vs time 1 in the CRT condition and CRT vs TAU during time 2. For the purposes of this meta-analysis, only coordinates and the subject N from the time 2 vs time 1 in the active CRT condition were included in the ALE.
ALE Results
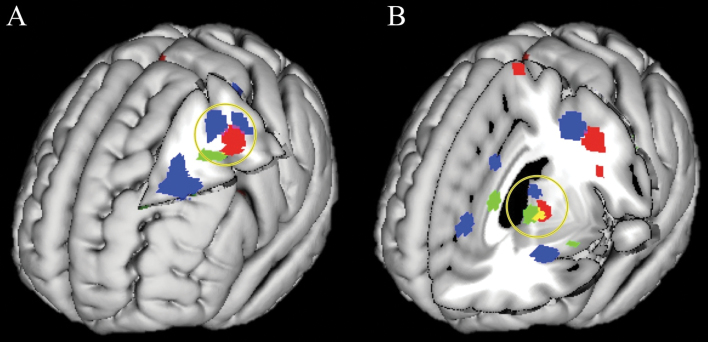
Cluster-thresholded ALE results identified 8 distinct brain area clusters that significantly increased in response to cognitive remediation (see table 2 and supplementary figure 1). Increases from time 1 to time 2 occurred in the left middle frontal gyrus (MFG), left inferior frontal gyrus (IFG), left superior frontal gyrus, pre- and postcentral gyrus, bilateral insula, parietal lobe, and medial frontal gyrus. Target engagement was examined by comparing this pattern to previous ALE meta-analyses contrasting healthy controls vs schizophrenia patients on tasks of executive control and working memory9 and facial affect recognition.11 Areas that increased in activation in response to CRT in the left MFG and precentral gyrus were shown to partially overlap with similar areas previously found to be impaired during both cognitive and emotion recognition tasks (figure 2A). A similar pattern was found in the thalamus and caudate nucleus, with substantial overlap between areas associated with CRT and deficits in emotional processing and a nonoverlapping but adjacent region associated with deficits in cognition (figure 2B). These a priori comparisons suggested that CRT increased task-related activity in areas of the brain previously shown to exhibit impairments in schizophrenia. These findings were consistent with the hypothesis that cognitive remediation works toward normalization of brain functioning among patients with schizophrenia.
Table 2.
Brain Areas Associated With Change From Cognitive Remediation Training
| Brain Area | Brodmann Area | Volume (mm3) | Maximum ALE Value | x | y | z |
|---|---|---|---|---|---|---|
| Left middle frontal gyrus, left precentral gyrus | 6 | 624 | 0.015 | −40 | −8 | 40 |
| Left inferior frontal gyrus, left insular cortex, left precentral gyrus | 9 | 496 | 0.014 | −44 | 6 | 24 |
| Right superior parietal lobe | 7 | 448 | 0.012 | 32 | −66 | 50 |
| Right postcentral gyrus | 2 | 440 | 0.017 | 38 | −24 | 42 |
| Thalamus, lentiform nucleus, caudate | NA | 312 | 0.013 | −10 | −2 | 0 |
| Right insular cortex | 13 | 264 | 0.013 | 38 | 16 | 4 |
| Left superior frontal gyrus, left middle frontal gyrus | 10 | 264 | 0.012 | −28 | 52 | 6 |
| Left medial frontal gyrus | 6 | 248 | 0.012 | −6 | −8 | 68 |
Note: All areas reported in Talairach space. Brodmann areas are defined by the Brain Map Talairach atlas. ALE = activation likelihood estimation; NA = not applicable.
Fig. 2.
Cognitive remediation training (CRT) supports restorative functioning in prefrontal and thalamic areas. Note: Areas in red depict brain regions that showed significant change as a result of CRT in the current activation likelihood estimation (ALE). Blue areas depict previously published results comparing controls > patients with schizophrenia (HC > SZ) on tasks measuring working memory (WM) and executive functioning. Green areas depict previously published results comparing HC > SZ on tasks measuring affective processing. Purple indicates overlap between CRT and the WM ALE, and yellow indicates overlap between CRT and the affective processing ALE. (A) Increased functional activation as the result of CRT in the left prefrontal cortex overlaps with areas shown to have dysfunctional processing in previous cognition and affective processing ALE meta-analyses. (B) Increased functional activation as the result of CRT in the thalamus and caudate nucleus overlaps with an area showing deficits in affective processing and is adjacent to a thalamic area showing deficits in the WM ALE. Results from previous ALE studies are displayed here for comparison purposes with the kind permission of Minzenberg and colleagues9 and Delvecchio and colleagues.11
To further clarify the relationship between increased task-based activity resulting from training and the characteristics of the interventions themselves, we contrasted ALE maps on the basis of training intensity, theoretic CRT approach used, whether or not the approach was computerized, whether it was placebo controlled, and the fMRI task used to measure change. Based on these comparisons, we were unable to show any group differences. However, power for these analyses was low, and continued study will be required to clarify more subtle differences.
Discussion
Nine studies were identified for the current ALE meta-analysis, with a total of 128 subjects and 74 foci. We observed that across studies, schizophrenia patients who undergo CRT interventions generally increased neural activation in areas of the lateral and medial PFC, parietal cortex, the insula, and the caudate and thalamus. In spite of heterogeneous treatment approaches, this review demonstrated that broadly speaking, CRT influences brain regions known to support working memory, cognitive control, and socioemotional functioning in healthy individuals. Critically, these observed functional changes are in response to previously untrained tasks, showing that improved cognition may generalize not only to untrained tasks but also to the brain areas that support those tasks. Of note, CRT was shown to increase task-based activity in areas of the left MFG and thalamus/caudate that were previously found to show impairment on working memory, executive functioning, and emotion recognition processing tasks. This is preliminary, but suggestive evidence to indicate that CRT restores activation in the thalamocortical circuits that potentially support improved cognition and psychosocial functioning. While it is important to note that the areas associated with pathology only partially overlapped with the current ALE results, it underscores the need to examine specifically the engagement of these neural targets in future CRT studies. Also, no relationships were observed when comparing studies on the basis of training intensity, training approach, and computerization or on the basis of the task used to measure neural activity. However, the small number of available studies meant we were sensitive to only large effects if they were present.
Critically, the current findings indicate that among studies using various CRT approaches, neural activity increased in prefrontal, insular, and thalamic areas previously demonstrated to be disrupted in patients with schizophrenia.9,11 Increased activity in the left MFG as well as posterior cortex in response to CRT may support improvements in working memory and executive functioning, while increases in insular activation may support improved socioemotional processing. Furthermore, despite the links among some of these brain areas and genetic liability for schizophrenia, the current findings support the notion that there remains residual neural plasticity to support functional recovery among these patients. Though increased activity in these areas suggests that patients who undergo cognitive training normalize neural activity to reflect that of healthy individuals, increases in other brain areas may also reflect compensatory brain activation as a result of training. Robust increases in the left IFG may support compensatory integration of cognitive and socioemotional information, as this area was not shown to be impaired in either of the previous meta-analyses. Other areas exhibiting change include the pre- and postcentral gyrus, where increased activation could be indicative of improved motoric functioning in response to cognitive training as well as strengthened somatosensory representations of task goals. This may be the result of improved confidence or practice associated with prolonged training on a computer or other laboratory tools.
Restorative activity observed in the left PFC and thalamus/caudate may be particularly relevant in light of recent observations demonstrating that patients with schizophrenia show both structural and functional irregularities in the thalamocortical circuit.34,35 We propose that increased activity in these connected areas as a result of cognitive training could be an underlying mechanism that supports the efficacy of CRT in schizophrenia. Future studies should investigate this functional circuit to determine whether improved coengagement of these areas supports specific cognitive and psychosocial improvements from CRT. It will also be critical for these studies to examine these neural targets both at baseline and over the course of training because individual differences in various neural systems may be predictive of positive outcomes.
Of the included studies, only 2 studies22,29 reported deactivations as the result of training, identifying areas of the bilateral thalamus, MFG, ACC, and superior frontal gyrus. Decreased engagement of these areas may reflect neural efficiency,19 especially in areas crucial to attention, as well as both cognitive and emotional control.36 It is unclear whether other studies included in this ALE analysis did not evoke deactivations as a result of training, observed but did not report these negative deflections, or simply did not examine nonhypothesized contrasts. Future studies should examine time 1 vs time 2 contrasts more closely to further clarify the relationships between neural deactivation in response to CRT in schizophrenia. This is relevant because a combination of increases and decreases in neural activity in response to a task may characterize the brain patterns associated with functional improvement.
While the current study has examined change in functional activation measured by a GLM, other studies have examined neural responses to cognitive training with different imaging measures. One study that used single-photon emission computed tomography found increases in prefrontal activity in response to CRT with this method,37 while another showed mixed results, with decreased ACC activity in one subject and increased temporal activity in another in response to a verbal fluency task following training.38 More recently, near infrared spectroscopy has been used to examine CRT, with 1 study showing an increase in prefrontal activity on an n-back task and improved verbal fluency and memory following 6 months of CRT.39 Studies using these alternative methods largely support the current results, demonstrating that prefrontal areas may be particularly amenable to neuroplastic changes in response to CRT.
Emerging findings indicate that in addition to functional disruptions in specific brain regions, schizophrenia may also be characterized by disruptions in neural connectivity,40,41 reflecting aberrant connections both between and within brain areas. One study used independent components analysis to assess whether functional connectivity changed as a result of CRT.42 After patients underwent 40 hours of training, they showed functional connectivity patterns in a network composed of prefrontal areas that looked more like that of healthy controls. They propose that this change in functional connectivity, which coincided with improvement in global cognition, represents enhanced neural efficiency. By examining functional connectivity, their conclusions offer insights that may be useful for understanding dysconnectivity in the context of assessing cognitive deficits in schizophrenia, especially as network-based approaches to understanding the brain are becoming increasingly germane to our understanding of CRT, related interventions, and serious mental illness more broadly.43,44 This puts the current findings into perspective because the present ALE may only be elucidating functional hubs associated with neural changes and that connections to and from these regions may also support these functional and psychosocial changes. It also highlights that we are currently only able to examine a very limited aspect of “plasticity” and cannot further resolve its source, which may be structural as well as functional, gross, or molecular. Future investigations will be required to examine plasticity more broadly to further our understanding of the mechanisms supporting CRT.
A limitation of the current investigation is that we were constrained by the number of studies that met criteria for inclusion in this kind of analysis. This also limited our ability to examine differences between approaches to training, intensity of training, whether the approach was computerized, and the task used to measure neural change. Though we anticipated potential differences between intervention types, it is also possible that the specific CRT approach is less important than patients participating in prolonged engagement in challenging activities over time more generally. Additionally, the small sample size limited our ability to examine the relationship between treatment-induced changes in cognition, symptoms, and functioning and regional activation changes across studies. We also note that the control conditions in the included studies ranged in terms of engagement, which may bias the current results. More placebo-controlled studies measuring pre- and post-CRT neural activation will be necessary to draw reliable conclusions about the nature of neural plasticity in response to training, though the current findings represent an important first step. A second and related limitation is that in addition to examining heterogeneous CRT approaches, there was heterogeneity among the fMRI tasks used to measure cognitive change, with some measuring various aspects of working memory (ie, n-back) and others measuring socioemotional cognition (facial recognition). Differences in evoked activity are obviously important to consider in the context of these findings. Despite this heterogeneity, we still observed coherent activity in both traditionally cognitive and limbic areas in our ALE analysis.
It will also be important to understand these findings in the context of motivational and meta-cognitive factors that influence response to cognitive remediation and learning more broadly.45 This is because cognitive improvement associated with training depends to some degree on factors related to intrinsic motivation.46 Additionally, controlling the role of extrinsic motivation, eg, by having control groups paid as much as CRT groups, is important for interpretation. Future studies will be called upon to disentangle the relationship between neural changes associated with cognition vs the other psychological factors involved in performance. Last, this investigation of the neural systems associated with plasticity from CRT was limited in that it can only speak to cognitive changes that immediately follow an intervention and those that relate to near transfer generalization effects. It will be useful to understand both long-term changes from CRT in schizophrenia, as well as distal impacts of training, such as improved psychosocial functioning, when they are observed.
Despite these limitations, the current findings come at an important time for understanding how to direct future development and examinations of CRT. They may also have important implications in the context of other psychiatric populations, as successful CRT interventions in schizophrenia are being applied to other mental disorders.47 To conclude, the current meta-analysis demonstrates that CRT for schizophrenia, irrespective of theoretical approach, shows target engagement for neural functions in brain regions crucial to cognitive and socioemotional functions. These coherent patterns of increased activity indicate that the neural mechanisms supporting CRT are both restorative and compensatory in nature and generalize to untrained circumstances. Notably, increases in activation associated with CRT partially overlap with prefrontal and thalamic regions previously shown to be impaired in schizophrenia, establishing this as a potential restorative mechanism. This also establishes the thalamocortical circuit as a specific target for CRT and other cognitive enhancing interventions in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institutes of Mental Health F31 National Research Service Award (1F31MH105080-01 to I.S.R.).
Supplementary Material
Acknowledgments
We thank the time and effort of Connie Du, Tasha Nienow, Krista Wisner, Andrew Poppe, Samantha Abram, Craig Moodie, and Merav Silverman in the development, analysis, and revision of this manuscript. A.W.M. has been a consultant for Astellas Pharma. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 2. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 3. Harvey PD. Pharmacological cognitive enhancement in schizophrenia. Neuropsychol Rev. 2009;19:324–335. [DOI] [PubMed] [Google Scholar]
- 4. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 5. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Callicott JH, Egan MF, Mattay VS, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. [DOI] [PubMed] [Google Scholar]
- 11. Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2013;43:553–569. [DOI] [PubMed] [Google Scholar]
- 12. Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. [DOI] [PubMed] [Google Scholar]
- 13. Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49:1410–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. [DOI] [PubMed] [Google Scholar]
- 15. Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychol Bull. 2012;138:628–654. [DOI] [PubMed] [Google Scholar]
- 16. Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. [DOI] [PubMed] [Google Scholar]
- 17. Dahlin E, Nyberg L, Bäckman L, Neely AS. Plasticity of executive functioning in young and older adults: immediate training gains, transfer, and long-term maintenance. Psychol Aging. 2008;23:720–730. [DOI] [PubMed] [Google Scholar]
- 18. Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Dev Cogn Neurosci. 2012;2(suppl 1):S167–S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. [DOI] [PubMed] [Google Scholar]
- 20. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haut KM, Lim KO, MacDonald A. Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35:1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hooker CI, Bruce L, Fisher M, et al. The influence of combined cognitive plus social-cognitive training on amygdala response during face emotion recognition in schizophrenia. Psychiatry Res. 2013;213:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramaniam K, Luks TL, Garrett C, et al. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage. 2014;99:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hooker CI, Bruce L, Fisher M, Verosky SC, Miyakawa A, Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophr Res. 2012;139:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wykes T, Brammer M, Mellers J, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy: functional magnetic resonance imaging in schizophrenia. Br J Psychiatry. 2002;181:144–152. [DOI] [PubMed] [Google Scholar]
- 31. Bor J, Brunelin J, d’Amato T, et al. How can cognitive remediation therapy modulate brain activations in schizophrenia? An fMRI study. Psychiatry Res. 2011;192:160–166. [DOI] [PubMed] [Google Scholar]
- 32. Vianin P, Urben S, Magistretti P, Marquet P, Fornari E, Jaugey L. Increased activation in Broca’s area after cognitive remediation in schizophrenia. Psychiatry Res. 2014;221:204–209. [DOI] [PubMed] [Google Scholar]
- 33. Habel U, Koch K, Kellermann T, et al. Training of affect recognition in schizophrenia: Neurobiological correlates. Soc Neurosci. 2010;5:92–104. [DOI] [PubMed] [Google Scholar]
- 34. Marenco S, Stein JL, Savostyanova AA, et al. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology. 2012;37:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atluri G, Steinbach M, Lim KO, Kumar V, MacDonald A. Connectivity cluster analysis for discovering discriminative subnetworks in schizophrenia. Hum Brain Mapp. 2015;36:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. [DOI] [PubMed] [Google Scholar]
- 37. Penadés R, Boget T, Catalán R, Bernardo M, Gastó C, Salamero M. Cognitive mechanisms, psychosocial functioning, and neurocognitive rehabilitation in schizophrenia. Schizophr Res. 2003;63:219–227. [DOI] [PubMed] [Google Scholar]
- 38. Wykes T. What are we changing with neurocognitive rehabilitation? Illustrations from two single cases of changes in neuropsychological performance and brain systems as measured by SPECT. Schizophr Res. 1998;34:77–86. [DOI] [PubMed] [Google Scholar]
- 39. Pu S, Nakagome K, Yamada T, et al. A pilot study on the effects of cognitive remediation on hemodynamic responses in the prefrontal cortices of patients with schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. 2014;153:87–95. [DOI] [PubMed] [Google Scholar]
- 40. Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Penadés R, Pujol N, Catalán R, et al. Brain effects of cognitive remediation therapy in schizophrenia: a structural and functional neuroimaging study. Biol Psychiatry. 2013;73:1015–1023. [DOI] [PubMed] [Google Scholar]
- 43. Kelly C, Castellanos FX. Strengthening connections: functional connectivity and brain plasticity. Neuropsychol Rev. 2014;24:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frangou S. A systems neuroscience perspective of schizophrenia and bipolar disorder. Schizophr Bull. 2014;40:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tas C, Brown EC, Esen-Danaci A, Lysaker PH, Brüne M. Intrinsic motivation and metacognition as predictors of learning potential in patients with remitted schizophrenia. J Psychiatr Res. 2012;46:1086–1092. [DOI] [PubMed] [Google Scholar]
- 46. Medalia A, Richardson R. What predicts a good response to cognitive remediation interventions? Schizophr Bull. 2005;31:942–953. [DOI] [PubMed] [Google Scholar]
- 47. Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.