Abstract
The effectiveness of cognitive remediation therapy (CRT) for the neuropsychological deficits seen in schizophrenia is supported by meta-analysis. However, a recent methodologically rigorous trial had negative findings. In this study, 130 chronic schizophrenic patients were randomly assigned to computerized CRT, an active computerized control condition (CC) or treatment as usual (TAU). Primary outcome measures were 2 ecologically valid batteries of executive function and memory, rated under blind conditions; other executive and memory tests and a measure of overall cognitive function were also employed. Carer ratings of executive and memory failures in daily life were obtained before and after treatment. Computerized CRT was found to produce improvement on the training tasks, but this did not transfer to gains on the primary outcome measures and most other neuropsychological tests in comparison to either CC or TAU conditions. Nor did the intervention result in benefits on carer ratings of daily life cognitive failures. According to this study, computerized CRT is not effective in schizophrenia. The use of both active and passive CCs suggests that nature of the control group is not an important factor influencing results.
Key words: schizophrenia, cognition, neuropsychology, cognitive remediation
Introduction
Cognitive impairment is now established as an important part of the clinical picture of schizophrenia, where it affects particularly though by no means exclusively executive function, long-term memory, and sustained attention.1,2 Although varying widely in severity from patient to patient, the degree of impairment is on average substantial,3 and there is increasing evidence that it accounts for a significant part of the social and occupational functioning disability seen in the disorder.4,5 A variety of pharmacological treatments aimed at improving schizophrenic cognitive impairment have so far demonstrated only marginal efficacy.6,7 An alternative, however, exists in cognitive remediation therapy (CRT),8,9 in which graded training is given on memory, executive, and sometimes other tasks. Improvement is expected to take place on the training tasks, and the aim is that this will generalize to other tasks in the same cognitive domains.
Over 40 trials of CRT in schizophrenia have now been carried out. Wykes et al10 meta-analyzed those carried out up to 2010 and found a pooled effect size of 0.45 in 38 studies which reported a measure of global cognition. Pooled effect sizes were also significant for verbal episodic memory (0.41, 23 studies), verbal working memory (0.35, 20 studies), reasoning/problem solving (0.57, 24 studies), and speed of processing (0.26, 24 studies). Against these encouraging findings, however, a recent large and methodologically rigorous trial has had negative findings. Dickinson et al11 randomly assigned 69 schizophrenic patients to either computerized CRT or a control intervention consisting of other computer activities. Both treatment conditions were manualized; the patients were not informed whether the one they were assigned to was intended to be therapeutic or not; outcome ratings were made under blind conditions; and all analyses were carried out intention-to-treat. It was found that the CRT group improved on most of the training exercises, but their performance on a range of cognitive and functional outcome measures was not significantly better than that of the controls at the end of the trial.
There are several possible reasons why this trial might have had negative findings. One is simply that it was methodologically superior to previous trials, which were not always randomized, and in several cases did not address other sources of bias, particularly incomplete outcome data.10 Another possible reason concerns the use by Dickinson et al11 of computerized activities as the control intervention. Wykes and Spaulding8 have made the point that in trials of CRT, if the control task employed is too similar to the intervention, it may itself have beneficial effects on cognition. For this and other reasons, Wykes et al10 have recommended that future studies should use a 3-group design with a control for nonspecific effects in addition to a no treatment control.
In 2009, the Schizophrenia Patient Outcomes Research Team12 noted that while CRT was an emerging area of therapeutic interest, rigorous clinical trials were still too few to permit its recommendation. We report here the results of a multicenter, parallel group, efficacy trial of the effects of computerized CRT on memory and executive function in chronic schizophrenic patients, that incorporated several of the design features referred to above, and which compared it to both active and passive control conditions. We also used neuropsychological measures designed to be sensitive to cognitive impairment in daily life and evaluated changes not only in test scores but also in carer ratings of the patients’ cognitive functioning.
Methods
The trial was a parallel group, efficacy trial of computerized CRT in chronic schizophrenic patients. Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed. The trial is registered at ClinicalTrials.gov, identifier NCT02201888.
Participants
Six sites from across Spain participated in the study. Four of the sites were inpatient rehabilitation services of hospitals and the other 2 were rehabilitation centers for community-dwelling patients. The patients were required to meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for schizophrenia or schizoaffective disorder based on interview and review of clinical history. Other inclusion criteria included age between 18 and 65 years, estimated premorbid IQ in the normal range (see below), chronic illness (ie, duration ≥2 years), and relative clinical stability. Exclusion criteria were history of brain trauma and alcohol or substance abuse/dependence within the previous 6 months. All patients were taking antipsychotic medication.
All sites’ research ethics committees approved the study and all participants signed written informant consent prior to enrollment. Participants did not receive any compensation for participation in the study.
Trial Design
Patients were randomized to a computer-assisted cognitive training condition (CRT) , a computerized active control condition (CC), or treatment as usual (TAU) using blocked randomization with minimization.13 Specifically, after all participants were recruited, they were grouped into blocks of 3 from the same site showing similar demographic characteristics (diagnosis, sex, age, and current and premorbid IQ). Each of the 3 patients in a block was then randomly assigned to a different arm. An extra block was created for 7 patients who could not be included in standard blocks due to sites’ sample sizes not being multiples of 3. All steps of the randomization process were automatically carried out in a central location using a computer program. The results of each block randomization were communicated to the patient group supervisors in each center by J.J.G. who had no other involvement in the assignment process or therapy.
Cognitive Remediation Therapy.
Patients in this arm of the trial carried out computerized online training drawn from the FesKits program (www.feskits.com), focusing on components related to attention, memory, and executive function. The sessions included the following exercises: sustained attention (4min), attention/perception (5min), working memory (8min), auditory and visual memory (8min), executive function (10min), language (6min), and games (4min). The FesKits program is organized in a hierarchical fashion, ie, each session begins with lower cognitive demands and progressively advances to more complex exercises. The program guides progression through the exercises interactively and performance feedback is given at the end of each exercise. An algorithm that adjusts the complexity of the exercises to the individual’s level of performance is automatically applied. Participants work on all of the cognitive domains in each session and progress on each domain is independent from the others (for further details of FesKits, see online supplementary material 1).
Control Condition.
Patients allocated to this condition completed the same number of sessions as the CRT group but engaged in a computerized typing program (www.rapidtyping.com). This had similar design characteristics to the CRT condition in that it was hierarchically organized with level of difficulty of the exercises being adjusted to the individual’s level of performance and feedback being given at the end of each exercise. Additionally, patients in this condition played computerized games (crosswords, word puzzles, etc) and were taught basic internet navigation by a supervisor. Exposure to the computer was of equivalent duration to the CRT condition.
Treatment as Usual.
Patients in this condition participated in their (individually variable) daily rehabilitative activities. Patients allocated to the other 2 conditions also participated in these activities.
Treatment lasted 6 months. The CRT and CC conditions consisted of biweekly sessions of 45min. Patients in both conditions trained in groups of up to 8, supervised by a single person. The supervisor’s role was to respond to patients’ queries and help when there were technical problems with the computer. If patients had difficulties understanding the program, they were prompted to consult the program’s online tutor in the first instance. The CRT and CC groups were scheduled at different times. The same personnel supervised both treatment conditions. All supervisors were trained by J.J.G. in the technical management of both conditions.
Assessments
Cognitive, clinical, and functional assessments were performed at baseline and after treatment was completed. Post-treatment assessment was carried out as close to the end of the treatment period as possible (mean 2.5 weeks, range 0–4).
Cognitive Measures.
The primary outcome measures were the Spanish versions of 2 executive and memory tests, the Behavioral Assessment of the Dysexecutive Syndrome (BADS),14,15 and the Rivermead Behavioral Memory Test (RBMT).16,17 Both tests are designed to be “ecologically valid,” that is to capture a broad range of aspects of executive and memory function required in real-life settings.
The BADS consists of 6 subtests covering cognitive estimation, rule shifting, planning, problem solving, and decision making under multiple task demands (the Modified Six Elements Test). Subtest scores of 0–4 are summed to give a profile score. The BADS has been standardized on groups of healthy subjects and patients with head injury.18 It has also been used in a recent trial of CRT.19 The RBMT consists of 12 subtests examining verbal recall, recognition, orientation, remembering a route, and 3 measures of prospective memory, the ability to remember to do things. Each subtest is scored 0–2 and scores are summed to give a “profile” score. The RBMT has been normed against large samples of healthy adults and patients with head injury. It has not previously been used as an outcome measure in trials of CRT, but it has been used in a number of studies of schizophrenia, including those carried out with chronic patients.20,21
Secondary outcome measures included (1) a performance-based daily living skills test of general cognitive competence, the University of California Performance Skills Assessment (UPSA),22,23 and (2) a range of standard neuropsychological tests designed to probe specific aspects of memory and executive function. These included subtests of the Wechsler Memory Test24: digit span (forwards and backwards), logical memory immediate, immediate memory for faces, and letter-number sequencing; and 3 executive tests, the Stroop Test,25 the Trail Making Test,26 and the FAS test.27
All neuropsychological tests were administered by graduate psychologists working in each of the centers. In most cases, there were between 1 and 2 assessors per center (1 center used 3). J.J.G. visited each center and trained the assessors in the use of each test. The assessors were blind to the treatment assignments and were not otherwise involved in the study. At the beginning of the post-therapy assessment, the assessors also instructed the patients not to indicate which group they belonged to.
Carer Measures.
Memory and executive failures in daily life were assessed using informant-rated versions of questionnaires developed for use with the BADS and the RBMT, the Dysexecutive Questionnaire (DEX),14 and the Memory Checklist (MCL).16,28 The DEX consists of 20 questions directed to areas such as impulsiveness (eg, “She/he acts without thinking, doing the first thing that comes to mind”), planning problems (eg, “She/he has difficulty thinking ahead or planning for the future”), and perseveration (eg, “She/he finds it hard to stop repeating saying or doing things once they’ve started”). Failures are rated over the preceding month on a 5-point rating scale ranging from “never” to “very often.” The MCL consists of 19 questions such as “Did he/she forget where things are normally kept or look for things in the wrong places?” “Did he/she get details of what someone had said confused?” Failures are rated on a 5-point scale ranging from “never” to “very often.” We used a version of the scale modified by Ornstein et al21 to make the rating period the previous month, rather than the preceding 24h as in the original version.16,28
For hospitalized patients, information to score the carer scales was obtained in weekly meetings with staff who had daily interactions with the patients, eg, in self-care and disease management groups, occupational therapy, and other activities. For patients in community settings, therapists who supervised the patients in daily function and occupational activities scored the scales.
Other Measures Recorded at Baseline.
Premorbid IQ was estimated using the Word Accentuation Test (Test de Acentuación de Palabras).29,30 This is conceptually similar to the National Adult Reading Test31 used in the United Kingdom and the Wide Range of Achievement Test32 in the United States. Subjects are required to pronounce low-frequency Spanish words whose accents have been removed. Current IQ was measured using 4 subtests of the Wechsler Adult Intelligence Scale III (WAIS-III; vocabulary, similarities, block design, and matrix reasoning). Symptoms at baseline were rated using the Positive and Negative Syndrome Scale (PANSS).
Statistical Analysis
In order to detect treatment effects, we modeled each measure of interest (eg BADS and RBMT scores) as a function of group (CRT, CC or TAU), time point (pre vs post), and their interaction, with the site and subject as nested random factors. The model also included baseline score on the relevant neuropsychological test to control for the regression to the mean effect,33 though we also carried out an analysis without including this covariable. Variance was not assumed to be equal across groups and was separately estimated for each group. (Although use of randomization implies that variance should be equal at baseline, this might not hold true after treatment.) The effects of group, time point, and their interaction were statistically tested using an analysis of deviance and χ2 statistics for mixed models analysis (analogous to analysis of variance and F statistics for simpler linear models). When interactions were statistically significant, post hoc z-tests were used to determine which pairs of groups showed significant differences. Results are reported both uncorrected and corrected for multiple comparisons using false discovery rate (FDR).
Analyses of treatment effects were by intention-to-treat (ITT), including all the participants who provided pre-treatment assessments irrespective of whether they completed the training. Missing observations were multiply imputed with the R package “mi”34; this generates multiple imputations for incomplete data using iterative regression imputation. For completeness, we also report findings for participants who provided end of study data.
Results
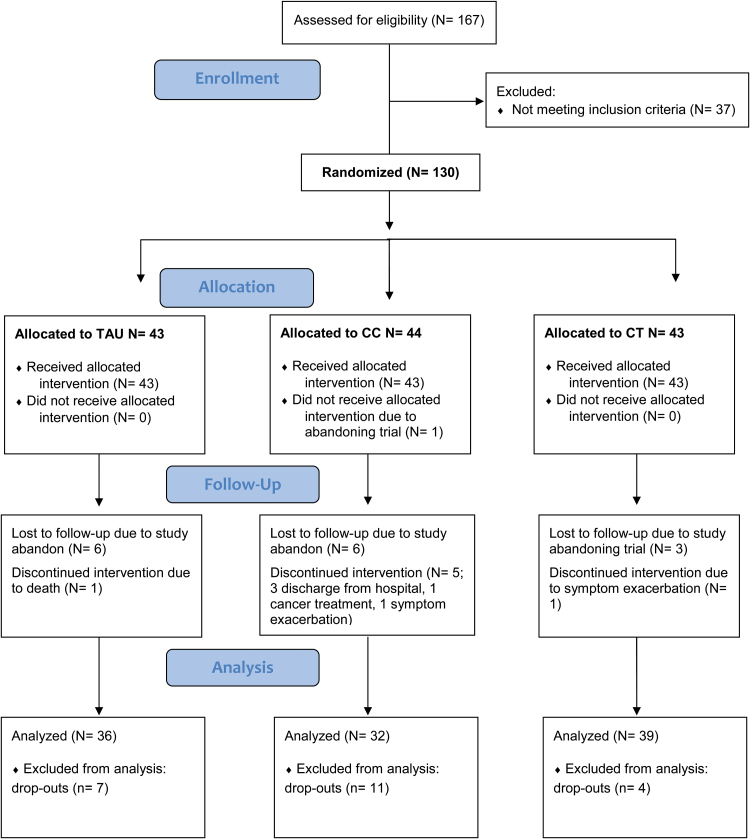
Recruitment took place from September 2010 and continued to December 2010 in all but one of the centers; this center started the groups slightly later and finished in February 2011. The CONSORT flow diagram for the recruitment and participation in the trial is shown in figure 1. From 167 patients who were assessed for eligibility, 130 met the inclusion criteria and entered the randomization procedure (2 sites enrolled 22 patients, 2 sites 21 patients, and the other 2 sites 20 and 24 patients, respectively). The age range was 20–65. Of the 130 patients randomized, 108 (84%) were assessed at post-treatment, 39 (91% of those randomized) in the CRT arm (mean sessions completed 36.69, SD 9.68), 33 (77% of those randomized) in the CC arm (mean sessions completed 36.36, SD 10.26), and 36 (84% of those randomized) in the TAU arm. One of the patients allocated to CC did not start treatment. Details of the dropouts are given in figure 1, and comparisons with those who remained in the study are given in online supplementary material 2.
Fig. 1.
CONSORT flow diagram of the clinical trial.
The 3 treatment groups had similar demographic characteristics (table 1). There were no group differences in antipsychotic dose in chlorpromazine equivalents35 or in number of patients receiving anticholinergic treatment. Mean baseline scores on the primary and secondary outcome measures were also similar (see table 2). Logistic regression of dropout status with center, sex, age, antipsychotic chlorpromazine equivalent dose, and also PANSS negative subscale score at baseline revealed only antipsychotic dose and sex as predictors of dropout (see online supplementary material 2).
Table 1.
Demographic Features and Clinical Scores at Baseline (SDs in Brackets)
| TAU (N = 43) | CC (N = 44) | CRT (N = 43) | Comparison | P-value | |
|---|---|---|---|---|---|
| Gender, M/F | 32/11 | 28/16 | 29/14 | χ2 = 1.20 | .55 |
| Age | 45.40 (9.77) | 46.13 (10.11) | 46.68 (9.97) | F = 0.18 | .84 |
| Years of education | 10.33 (2.65) | 9.53 (3.08) | 9.30 (2.86) | F = 1.50 | .23 |
| Length of illness | 23.38 (8.63) | 22.58 (9.10) | 24.30 (8.52) | F = 0.40 | .67 |
| Estimated premorbid IQ (Test de Acentuación de Palabras) | 100.70 (9.36) | 99.59 (9.90) | 98.76 (9.97) | F = 0.42 | .66 |
| Current IQ (Wechsler Adult Intelligence Scale) | 87.49 (15.48) | 86.70 (16.12) | 84.23 (16.05) | F = 0.49 | .61 |
| PANSS total | 76.85 (19.12) | 75.22 (20.75) | 75.60 (18.49) | F = 0.08 | .92 |
| PANSS positive | 17.63 (5.72) | 17.18 (6.38) | 17.26 (6.04) | F = 0.07 | .94 |
| PANSS negative | 20.24 (7.57) | 21.08 (7.11) | 20.64 (8.06) | F = 0.12 | .89 |
| Antipsychotic dosea | 675.92 (518.80) | 667.14 (537.80) | 557.21 (333.19) | F = 0.77 | .47 |
| No. of patients receiving anticholinergics | 6 | 8 | 9 | χ2 = 1.24 | .54 |
Note: TAU, Treatment as Usual; CC, Control Condition; CRT, Cognitive Remediation Therapy.
aCalculated as chlorpromazine equivalents.
Table 2.
Baseline and Post-treatment Neuropsychological Test Scores in the 3 Groups (SDs in brackets)
| Measure | Baseline Score | Post-treatment Score | Group × Time Interaction (χ2) | P-value (False Discovery Rate-corrected) | ||||
|---|---|---|---|---|---|---|---|---|
| TAU (N = 43) | CC (N = 44) | CRT (N = 43) | TAU (N = 36) | CC (N = 32) | CRT (N = 39) | |||
| BADS | 11.70 (3.89) | 11.77 (4.25) | 11.14 (4.30) | 13.65 (3.74) | 13.36 (4.33) | 12.05 (4.04) | 1.57 | .46 (.67) |
| RMBT | 16.02 (4.77) | 15.17 (4.66) | 15.10 (4.10) | 16.39 (4.98) | 16.39 (4.85) | 14.87 (4.53) | 0.96 | .62 (.67) |
| UPSA | 70.83 (12.56) | 67.16 (13.10) | 66.08 (17.31) | 72.69 (13.56) | 66.39 (14.87) | 68.11 (13.70) | 1.32 | .52 (.67) |
| Stroop interference | 49.38 (10.73) | 46.79 (7.90) | 48.56 (8.76) | 50.49 (9.85) | 49.25 (8.34) | 50.78 (7.21) | 0.84 | .66 (.67) |
| Trails B-Aa | 71.08 (63.02) | 86.05 (62.77) | 96.43 (77.59) | 115.94 (86.72) | 132.79 (100.46) | 126.46 (118.88) | 2.76 | .25 (.63) |
| FAS | 27.07(9.78) | 25.84 (10.70) | 27.60 (13.37) | 26.64 (8.94) | 25.58 (9.39) | 28.56 (13.58) | 0.80 | .67 (.67) |
| WMS-III logical memory immediate | 6.28 (3.38) | 5.52 (2.83) | 5.17 (3.38) | 5.86 (3.35) | 5.85 (2.79) | 5.62 (3.72) | 2.87 | .24 (.63) |
| WMS-III faces immediate | 9.08 (3.02) | 8.28 (3.51) | 7.68 (3.00) | 8.69 (3.64) | 8.58 (2.97) | 9.00 (3.34) | 7.30 | .03 (.13) |
| WMS-III digit span | 8.03 (2.58) | 7.07 (2.61) | 7.32 (2.49) | 7.60 (2.46) | 8.00 (2.83) | 8.10 (2.76) | 14.79 | .0006 (.006) |
| WMS-III letter/number sequencing | 6.46 (2.61) | 6.21 (3.10) | 6.05 (3.03) | 6.32 (2.64) | 7.00 (3.27) | 6.41 (3.01) | 1.36 | .51 (.67) |
Note: TAU, Treatment as Usual; CC, Control Condition; CRT, Cognitive Remediation Therapy; BADS, Behavioral Assessment of the Dysexecutive Syndrome; RMBT, Rivermead Behavioral Memory Test; UPSA, University of California San Diego Performance-Based Skills Assessment, WMS-III: Wechsler Memory Scale III.
Values are means (SD).
aHigher scores indicate worse performance.
Cognitive Outcomes
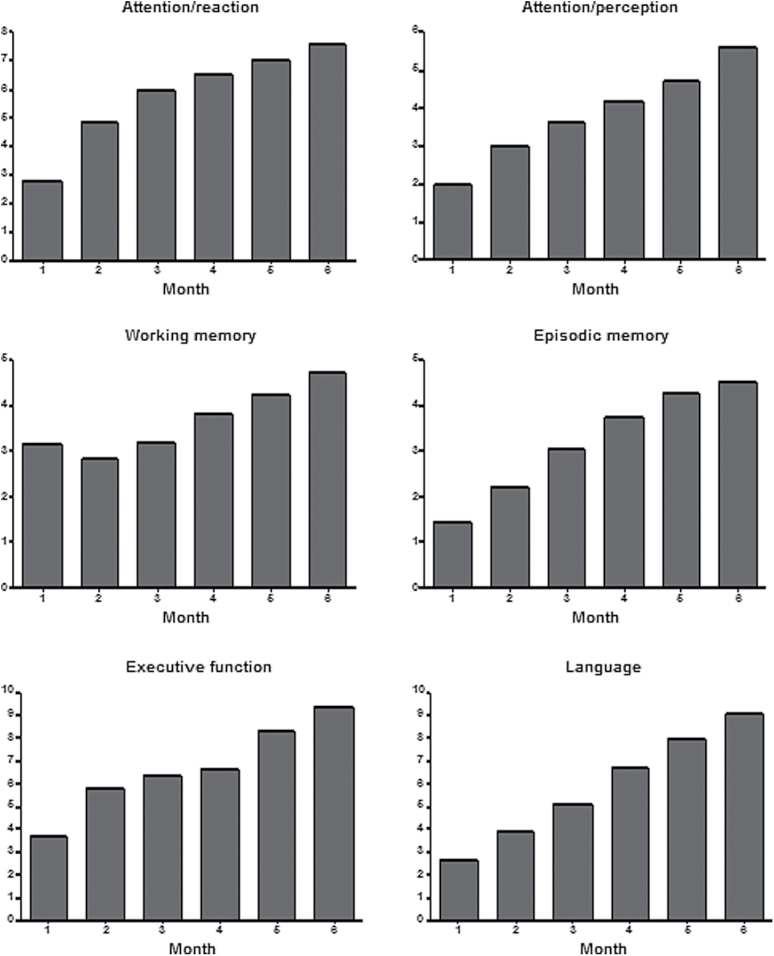
As shown in figure 2, the patients randomized to receive CRT showed progressive improvement on all the tasks they trained on.
Fig. 2.
Changes in performance over time in the cognitive remediation therapy group on the different components of the FesKits program. Means for each time point (month) represent level of difficulty reached on the exercises of every component of the program.
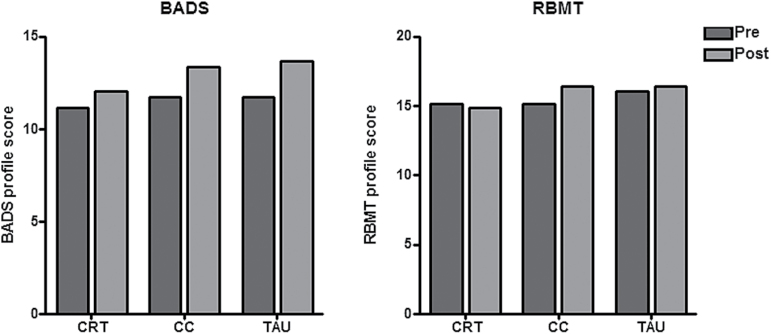
Findings for the 2 primary outcome measures are shown graphically in figure 3, and the results for these and the secondary outcome measures are given in table 2. All groups showed improvement on the BADS from the beginning to the end of the trial, with a nonstatistically significant trend towards greater improvement in the CC and TAU groups than in the CRT group. The CC group showed the greatest improvement over time on the RBMT, with the CRT and TAU groups showing minimal changes, though differences were not significant. There was no significant group × time interaction for either RBMT or BADS scores.
Fig. 3.
Overall scores on the executive function battery (Behavioral Assessment of the Dysexecutive Syndrome) and memory the battery (Rivermead Behavioral Memory Test) in the 3 groups at the beginning and end of the trial.
Group × time interactions were nonsignificant for all but two of the other neuropsychological measures. One was digit span, where both the CRT and CC groups improved more than the TAU group (χ2 = 15.70, P = .004; post hoc CRT vs TAU P = .05; CC vs TAU P = .03). An advantage for CRT over the other 2 groups on immediate memory for faces (χ2 = 8.37, P = .01) was reduced to trend-level (P = .08) after FDR correction. Effect sizes for all measures are given in online supplementary material 2.
Results were closely similar in the analysis of completers (see online supplementary material 2). Results were also little changed when baseline score was not included as a covariate (data not shown, available on request).
We also computed correlation coefficients between improvement scores (end of study—baseline) in the CRT subjects and age and antipsychotic dosage in chlorpromazine equivalents. For age, none of the correlations reached FDR-corrected significance. For antipsychotic dose, there was a significant correlation only with improvement on the UPSA (r = .49, P = .006, FDR-corrected), which was in the direction of greater improvement with higher dosage. Inspection of the scatter plot revealed that this result was due to the presence of an outlier; after removing this subject, correlations were no longer significant (r = .23, P = .24). Detailed results are given in online supplementary material 2.
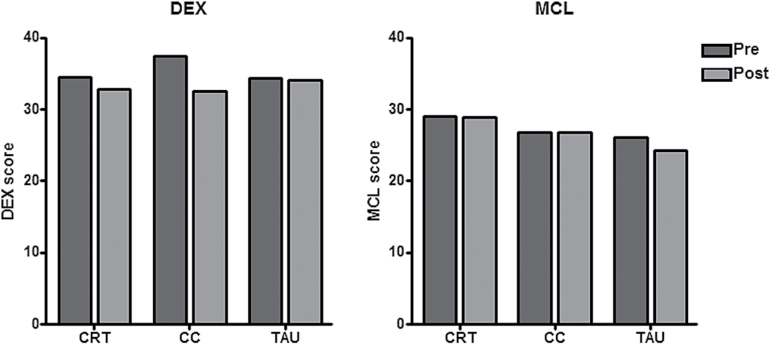
Functional Outcomes
The findings are summarized in figure 4; lower scores reflect better functioning. The group × time interaction was significant for the DEX, with the CC group showing more improvement than the other 2 groups; however, no pairwise differences were found in the post hoc analysis (F = 6.81, P = .03; all post hoc P-values > .05). The interaction was not significant for the MCL (F = 2.07, P = .36).
Fig. 4.
Carer ratings of executive functioning (Dysexecutive Questionnaire) and memory (Memory Checklist) in the 3 groups at the beginning and end of the trial.
Discussion
This multicenter trial of 130 chronic schizophrenic patients found that while computerized CRT led to improvement on the training tasks used, this did not transfer to gains on the primary outcome measures or most of a range of other cognitive measures. Nor was there any evidence of benefit on carer-rated cognitive functioning in daily life. Along with Dickinson et al11 (N = 69), therefore, 2 relatively large, well-controlled trials of CRT for schizophrenia have now had negative findings. Possible reasons for the discrepancy between these findings and the mainly positive results of other trials, as reflected in the meta-analysis of Wykes et al10, are considered below.
One factor that needs to be considered is trial quality—it is known that effect sizes in meta-analyses can be inflated by failure to consider bias due to factors such as inadequate randomization, incomplete outcome data or, lack of blinding.36,37 These sources of bias were examined by Wykes et al10, who rated each study on a quality scale and carried out a meta-regression; no significant moderating effects were found. However, the use of quality scales is no longer considered acceptable as a way of testing for bias in meta-analyses; this is because such scales often rate aspects of a study that bear little relationship to known sources of bias38, and also because different scales have been found to give different results.39 Wykes et al10 additionally examined adequacy of randomization and blinding separately, and again found that neither of these variables significantly moderated effect size. However, they did not examine bias due to incompleteness of outcome data, even though they noted that the dropout rate was higher than 15% in 12 studies, a level that they considered would make the findings statistically questionable.
Another factor that theoretically has the potential to influence whether CRT is found to be effective or not is choice of the control intervention. Bearing in mind that CRT targets cognitive function rather than symptoms or distress, there is little reason to suspect it would be susceptible to the nonspecific or “shared” effects of psychotherapy.40,41 On the other hand, CRT may not be immune to a related phenomenon, the so-called Hawthorne effect,42 the tendency of people singled out for a study of any kind to improve their performance or behavior simply because they are receiving special attention. Whether this actually occurs with CRT remains an open question: There was no indication in our study of a greater effect of CRT compared with TAU than CC. On the other hand, however, a Cochrane review of CRT in healthy elderly people and in patients with mild cognitive impairment43 found that the effect sizes in several of the domains of memory examined were substantially larger in the comparison between CRT and TAU than between CRT and active control (although the authors did not conclude that choice of control was an important factor).
Although use of an active control may be desirable in trials of CRT, it is important to ensure, as Wykes and Spaulding8 have noted, that the control task is sufficiently different from therapy to avoid it itself having positive effects on cognition. The computerized control intervention we employed consisted principally of a motor skill task, learning to type. Learning of motor skills makes at most minimal demands on executive function, and while it does require memory, this is procedural memory, which is universally considered to be dissociable from episodic memory as trained in CRT. Also arguing against the possibility that our control intervention was therapeutic is the fact that we did not find significant differences at the end of the trial between the CC and the TAU groups on any neuropsychological measure.
Rather than the control intervention being therapeutic, is it possible that the CRT we used was not efficacious, by virtue of being different in nature to other forms of computerized CRT? This seems unlikely, as the FesKits program is quite similar to existing comprehensive computerized CRT batteries and was developed with the aim of building on their strengths rather than introducing novel methodologies. The main differences concern the incorporation of a “virtual tutor” that accompanies the patient during the session, and an improved algorithm for adjusting the level of difficulty of each module independently of the others. A comparison between the features of FesKits and other comprehensive CRT packages is given in online supplementary material 3.
A final reason why we and Dickinson et al11 failed to find an effect of CRT concerns the use of computerized CRT. Although this method of administration has been considered to have advantages over paper and pencil tasks in terms of flexibility and adjustment of the learning program to each participant’s level,44 providing CRT this way usually means that there is a lack of one-to-one interaction with a therapist who can explicitly encourage “bridging” strategies, as well as provide nonspecific support. Nor can motivational coaching, which has been employed in some CRT trials,19,45 form a meaningful component of the therapy. So far, there appears to be little to this potential objection: Use of computerized vs noncomputerized CRT was not found to moderate effect size in the meta-analysis of Wykes et al10, and another meta-analysis that included only computerized trials found an effect size of 0.38 for general cognition, quite close to the value of 0.45 found by Wykes et al.44
Some limitations of this study need to be acknowledged. These include particularly the 17% dropout rate over the 6-month study period. Although this is below the threshold usually considered to adversely impact on trial findings,46 the dropout rate for the CC condition was higher at 25%. In any case, any distortions this might have produced were compensated for by the use of ITT analysis. It is also important to make the point that our negative findings apply to computerized CRT; it is quite possible that positive effects will continue to be found in larger and better controlled trials of pencil-and-paper CRT, as one recent study suggests.47 Finally, our findings also do not disallow the possibility that CRT combined with other cognitive interventions, for instance as part of a cognitive “package” or embedded within a comprehensive rehabilitation program, might be efficacious.
Supplementary Material
Supplementary materials 1, 2 and 3 are available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Catalonian Government (2009SGR211 to the Research Unit of FIDMAG) and several grants from the Plan Nacional de I+D+i and cofunded by the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación and the European Regional Development Fund (FEDER): Miguel Servet Research Contracts (CP07/00048 to R.S. and CP10/00596 to E.P.-C.) and Rio Hortega Research Contract (CM11/00024 to J.R.); Intensification grant (INT10/231 to S.S. and INT12/325 to P.M.); Research Project Grant (PI09/90483 to P.M.). The funding organizations played no role in the study design, data collection and analysis, or manuscript approval.
Supplementary Material
Acknowledgments
The authors thank Hermanas Hospitalarias, Spain for providing the setting to carry out this clinical trial, and the following centers that took part in the study: Centros de Rehabilitacion Picosocial de Aranjuez and Vallecas, Clinica San Miguel Madrid, Complejo Asistencial Benito Menni Ciempozuelos, Hospital Benito Menni C.A.S.M. Sant Boi de Llobregat, Hospital Sagrat Cor Martorell, Hospital de Sant Luis Palencia, and Psicoclínica Mare de Déu de la Mercè Barcelona. We also thank the staff from these centers, who made possible the implementation and development of this trial. The Cognitive rehabilitation study group consists of: Bàrbara Segura, Salvador Sarró, Raymond Salvador, Rosa Rodríguez-Jareño, José A. Larraz, Tomás Rodero, Pedro Roy, Urbano Barrientos, Eva Ojalvo, Verónica Robles, Fernando Cobo, Leticia Ramos, Miriam Benavides, Carlos Martín-Lorenzo, Yolanda García-Alonso, Lucia Sicilia, Rubén Mosquera, Violeta Guarido(†), Luis A. Flores-Pérez, Manuel Martin-Carrasco, Núria Descalzo, María Fajardo-Vera, Maite Lorente-Ballesteros, José M. Cebamanos, Josep Treserra and Matilde Porras. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19:365–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reichenberg A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin Neurosci. 2010;12:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 4. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 5. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 6. Harvey PD. Pharmacological cognitive enhancement in schizophrenia. Neuropsychol Rev. 2009;19:324–335. [DOI] [PubMed] [Google Scholar]
- 7. Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. Br J Psychiatry. 2013;203:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy–what works and could we do better? Schizophr Bull. 2011;37:S80–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harvey PD, Bowie CR. Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am. 2012;35:683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 11. Dickinson D, Tenhula W, Morris S, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry. 2010;167:170–180. [DOI] [PubMed] [Google Scholar]
- 12. Dixon LB, Dickerson F, Bellack AS, et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lachin JM, Matts JP, Wei LJ. Randomization in clinical trials: conclusions and recommendations. Control Clin Trials. 1988;9:365–374. [DOI] [PubMed] [Google Scholar]
- 14. Wilson BA, Burgess PW, Emslie H, Evans JJ. Behavioural Assessment of the Dysexecutive Syndrome. Bury St Edmunds, UK: Thames Valley Test Company/Pearson Assessment; 1996. [Google Scholar]
- 15. Vargas ML, Sanz JC, Marín JJ. Behavioral assessment of the dysexecutive syndrome battery (BADS) in schizophrenia: a pilot study in the Spanish population. Cogn Behav Neurol. 2009;22:95–100. [DOI] [PubMed] [Google Scholar]
- 16. Wilson B, Cockburn J, Baddeley A, Hiorns R. The Rivermead Behavioural Memory Test. Bury St Edmunds, UK: Thames Valley Test Company/Pearson Assessment; 1985. [Google Scholar]
- 17. Mozaz T. Test conductual de memoria de Rivermead. Madrid, Spain: TEA; 1991. [Google Scholar]
- 18. Wilson BA, Evans JJ, Emslie H, Alderman N, Burgess P. The development of an ecologically valid test for assessing patients with a dysexecutive syndrome. Neuropsychol Rehabil. 1998;8:213–228. [Google Scholar]
- 19. Franck N, Duboc C, Sundby C, et al. Specific vs general cognitive remediation for executive functioning in schizophrenia: a multicenter randomized trial. Schizophr Res. 2013;147:68–74. [DOI] [PubMed] [Google Scholar]
- 20. McKenna PJ, Tamlyn D, Lund CE, Mortimer AM, Hammond S, Baddeley AD. Amnesic syndrome in schizophrenia. Psychol Med. 1990;20:967–972. [DOI] [PubMed] [Google Scholar]
- 21. Ornstein TJ, Sahakian BJ, McKenna PJ. Memory and executive impairment in schizophrenia: comparison with frontal and temporal brain damage. Psychol Med. 2008;38:833–842. [DOI] [PubMed] [Google Scholar]
- 22. Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. [DOI] [PubMed] [Google Scholar]
- 23. Garcia-Portilla MP, Gomar JJ, Bobes-Bascaran MT, et al. Validation of a European Spanish-version of the University of California performance Skills Assessment (Sp-UPSA) in patients with schizophrenia and bipolar disorder. Schizophr Res. 2013;150:421–426. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler D, eds. Wechsler Memory Scale III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 25. Golden CJ, Freshwater SM, eds. Stroop Color and Word Test: Revised 2002 Adult Manual for Clinical and Experimental Uses. Fort Lauderdale, FL: Nova Southeastern University; 2002. [Google Scholar]
- 26. Reitan RM, Waltson D, eds. The Halstead-Reitan Neuropsychological Test Battery. Mesa, AZ: Reitan Neuropsychology; 1985. [Google Scholar]
- 27. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 2nd ed New York, NY: Oxford University Press; 1998. [Google Scholar]
- 28. Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. J Clin Exp Neuropsychol. 1989;11:855–870. [DOI] [PubMed] [Google Scholar]
- 29. Del Ser T, González-Montalvo JI, Martínez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn. 1997;33:343–356. [DOI] [PubMed] [Google Scholar]
- 30. Gomar JJ, Ortiz-Gil J, McKenna PJ, et al. Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr Res. 2011;128:175–176. [DOI] [PubMed] [Google Scholar]
- 31. Nelson HE, Willison JR. The Revised National Adult Reading Test. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- 32. Jastak S, Wilkinson GS. The Wide Range Achievement Test–Revised Administration Manual. Wilmington, DE: Jastak Associates; 1984. [Google Scholar]
- 33. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. [DOI] [PubMed] [Google Scholar]
- 34. Su YS, Gelman A, Hill J, Yajima M. Multiple imputation with diagnostics (mi) in R: opening windows into the black box. J Stat Softw. 2009;45:1–31. [Google Scholar]
- 35. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 2011. The Cochrane Collaboration; www.cochrane-handbook.org. Updated March 2011. Accessed 4 May 2015. [Google Scholar]
- 38. Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. [DOI] [PubMed] [Google Scholar]
- 39. Herbison P, Hay-Smith J, Gillespie WJ. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J Clin Epidemiol. 2006;59:1249–1256. [DOI] [PubMed] [Google Scholar]
- 40. Chambless DL, Hollon SD. Defining empirically supported therapies. J Consult Clin Psychol. 1998;66:7–18. [DOI] [PubMed] [Google Scholar]
- 41. Jensen PS, Weersing R, Hoagwood KE, Goldman E. What is the evidence for evidence-based treatments? A hard look at our soft underbelly. Ment Health Serv Res. 2005;7:53–74. [DOI] [PubMed] [Google Scholar]
- 42. Gillespie R. Manufacturing Knowledge: A History of the Hawthorne Experiments. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- 43. Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011:CD006220. [DOI] [PubMed] [Google Scholar]
- 44. Grynszpan O, Perbal S, Pelissolo A, et al. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. 2011;41:163–173. [DOI] [PubMed] [Google Scholar]
- 45. Medalia A, Revheim N, Casey M. Remediation of problem-solving skills in schizophrenia: evidence of a persistent effect. Schizophr Res. 2002;57:165–171. [DOI] [PubMed] [Google Scholar]
- 46. Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002;359:781–785. [DOI] [PubMed] [Google Scholar]
- 47. Sánchez P, Peña J, Bengoetxea E, et al. Improvements in negative symptoms and functional outcome after a new generation cognitive remediation program: a randomized controlled trial. Schizophr Bull. 2014;40:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.