Abstract
The default-mode network (DMN) is vital in the neurobiology of schizophrenia, and the cerebellum participates in the high-order cognitive network such as the DMN. However, the specific contribution of the cerebellum to the DMN abnormalities remains unclear in unaffected siblings of schizophrenia patients. Forty-six unaffected siblings of schizophrenia patients and 46 healthy controls were recruited for a resting-state scan. The images were analyzed using the functional connectivity (FC) method. The siblings showed significantly increased FCs between the left Crus I and the left superior medial prefrontal cortex (MPFC), as well as between the lobule IX and the bilateral MPFC (orbital part) and right superior MPFC compared with the controls. No significantly decreased FC was observed in the siblings relative to the controls. The analyses were replicated in 49 first-episode, drug-naive patients with schizophrenia, and the results showed that the siblings and the patients shared increased FCs between the left Crus I and the left superior MPFC, as well as between the lobule IX and the left MPFC (orbital part) compared with the controls. These findings suggest that increased cerebellar-DMN connectivities emerge earlier than illness onset, which highlight the contribution of the cerebellum to the DMN alterations in unaffected siblings. The shared increased cerebellar-DMN connectivities between the patients and the siblings may be used as candidate endophenotypes for schizophrenia.
Key words: unaffected siblings of schizophrenia patients, schizophrenia, cerebellum, functional connectivity, default-mode network
Introduction
As a complex psychiatric disorder, schizophrenia is interpreted as a “disconnection” disease.1 The “disconnection” hypothesis proposed that the underlying neurobiology of schizophrenia arises from disruptive integration of extensive brain regions, which comprise brain networks, such as the prefrontal-limbic-sensorimotor network,2–5 cortico-cerebellar-thalamo-cortical network,6 and default-mode network (DMN).7
Among these networks, the DMN, which is involved in self-referential and stream-of-consciousness processing,8 is one of the most examined networks. Abnormal DMN connectivities have been reported in schizophrenia during task-based activity and at rest.7,9 For example, increased DMN connectivities have been observed in schizophrenia patients7,10–13 and their siblings13 at rest. Meanwhile, a bulk of studies have found decreased DMN connectivities in schizophrenia.14–16 Interestingly, some researchers have reported both increased and decreased DMN connectivities in schizophrenia.17,18 Studies that used other parameters, such as regional homogeneity, amplitude of low-frequency fluctuation (ALFF), and fractional ALFF, have also identified DMN abnormalities in schizophrenia.19,20 Previously, we found abnormal network homogeneity within the DMN in the patients21 and their siblings22 using a network homogeneity method. These studies highlight the contribution of the DMN in the neurobiology of schizophrenia.
However, the findings of the above-mentioned studies are inconsistent, such as increased and decreased DMN connectivities in schizophrenia. In addition to such confounding factors as sample size, analysis methods, and scanners, sample heterogeneity may be attributed to the inconsistency in schizophrenia. First-episode and/or chronic schizophrenia patients are recruited as participants in the above-mentioned studies. Medication and long illness duration may have biased the results of these studies.23–25 For example, the duration of untreated psychosis (DUP) appears to have a neurotoxic effect on the brain structure and function,26,27 and prolonged DUP is associated with decreased gray matter volume and function28 in certain brain regions in first-episode schizophrenia. Antipsychotic drugs normalize brain anatomical and functional alterations in both first-episode and chronic schizophrenia.23,24 Therefore, recruiting alternative participants, such as unaffected siblings of schizophrenia patients, in neuroimaging studies is beneficial. Sharing similar genetic and environmental background with the patients, unaffected siblings have a higher risk to develop schizophrenia than individuals without a family history of schizophrenia.29 Unaffected siblings also exhibit similar but mild brain abnormalities as the patients.30,31 Hence, unaffected siblings provide a unique opportunity to examine functional connectivity (FC) abnormality associated with the neurobiology of schizophrenia.
Traditionally, the human cerebellum is thought to simply subserve motor learning and motor coordination. This issue has been challenged with the increase in recognition of the cerebellum participating in the high-order function. For example, patients with cerebellar damage have been observed to exhibit widespread cognitive deficits, including emotional and social behaviors and disrupted mental performance.32,33 Task-based neuroimaging investigations have indicated that the cerebellum is vital in various emotional and cognitive tasks.34 Cerebellar substrates have also been associated with some personality traits. For example, cerebellar volumes have been linked to novelty-seeking and harm-avoidance personality traits in healthy participants.35 Resting-state FC studies have also documented that the cerebellum has FCs with high-order brain networks, such as the DMN.36,37 Among the subregions of the cerebellum, Crus I and Lobule IX are believed to connect with the DMN.36–40 The cerebellum may play its role in emotional and cognitive processing through its anatomical links with the cerebrum.41 For example, point-to-point links between the cerebellum and the cerebrum have been demonstrated by Strick’s group,42,43 connected by the pons and thalamus. However, the exact neurobiology of the cerebellum that participates in high-order processing remains unclear.
Evidence that schizophrenia patients have cerebellar abnormalities has been accumulated for several decades.44 Anatomical studies have reported reduction in cerebellar size in schizophrenia,45,46 which may result from decreased linear density or size in Purkinje cells.47 Purkinje cells are important in modulating the output from the cerebellum to the cerebrum for providing input to the deep nuclei, such as the dentate nucleus. Functional imaging studies have revealed reduced cerebellar metabolism and dysfunction in schizophrenia (for reviews, see48,49). The above-mentioned studies have provided important evidence for cerebellar abnormalities in schizophrenia. However, the abnormalities of the cerebellar-cerebral connectivities in schizophrenia, especially the cerebellar-DMN connectivities, remain unclear.
Recently, Wang et al50 reported significantly decreased cerebellar FCs with the DMN, including the middle frontal gyrus, anterior cingulate cortex (ACC), supplementary motor area, and thalamus. The patients especially lacked the positive correlations between the strength of frontocerebellar and thalamocerebellar FCs found in the controls. However, this study is limited for their medicated patients with relatively long illness duration. As previously mentioned, medication use, and long illness duration could have biased their findings. Therefore, recruiting alternative participants, such as unaffected siblings of schizophrenia patients, is beneficial in establishing the potential contribution of the cerebellum to the DMN abnormalities in schizophrenia.
A relatively large sample of unaffected siblings was recruited in this study to examine the cerebellar FCs with the DMN. The differences in the cerebellar-DMN connectivities between the siblings and the controls were compared using cerebellar seeds involved in the DMN.36–40 Based on the general consideration that schizophrenia has overall reduced FCs51 and a previous study reporting decreased cerebellar-DMN connectivities in schizophrenia,50 we hypothesized that the unaffected siblings would show decreased FCs with the DMN. Meanwhile, an endophenotype (1) is heritable, (2) segregates with the disease within families and differs between the patients and the controls, and (3) may or may not be observed in unaffected relatives of the patient within families, but the patient have it.29 Thereafter, the analysis process was replicated in a group of first-episode, drug-naive schizophrenia patients to identify the candidate endophenotypes for schizophrenia. Given that unaffected siblings of schizophrenia patients exhibited similar but mild brain abnormalities as the patients,30,31 the findings of decreased cerebellar-DMN connectivities were expected to be present in the patients.
Methods and Materials
Participants
A total of 92 individuals were recruited for this study, including 46 unaffected siblings of schizophrenia patients and 46 healthy controls. All participants were right-handed and aged from 16 to 30 years with more than 9 years of normal education. The siblings and the controls were group-matched based on age, sex, and years of education. This study was approved by the local ethics committee of the First Affiliated Hospital, Guangxi Medical University. All participants provided their written informed consent to participate in this study.
The unaffected siblings were recruited from the Mental Health Center, the First Affiliated Hospital, Guangxi Medical University, China, while the healthy controls were recruited from the community. Unaffected siblings had brothers or sisters diagnosed with schizophrenia based on the Structured Clinical Interview of the Diagnostic and Statistical Manual of Mental Disorders-IV (SCID) criteria, patient edition.52 The siblings and the controls were unrelated to one another, and screened by SCID, nonpatient edition.52 Physical exam and routine laboratory examination, such as liver function and electrocardiogram, were done for all participants to exclude possible common medical problems. Healthy controls had no first-degree relatives with a psychiatric disorder. All participants shared the following exclusion criteria: neurological or psychiatric disorders (including past head injury based on self-reported healthy information), acute physical disease, substance abuse or dependence (including cannabis use or alcohol abuse), and contraindications for magnetic resonance imaging (MRI) scanning.
To identify the candidate endophenotypes for schizophrenia, we recruited 49 right-handed schizophrenia patients from the Mental Health Center, the First Affiliated Hospital, Guangxi Medical University, China. Schizophrenia was diagnosed using the SCID, patient version.52 Positive and Negative Symptom Scale (PANSS) was used to assess symptom severity at the scan time. The patients were first-episode and drug-naive with the DUP of less than 3 years, and aged from 16 to 30 years with more than 9 years of education level. The exclusion criteria were as follows: neurological disorders, severe medical disorders, substance abuse, or any contraindications for MRI scan. Fourteen patients and 14 siblings were sib pairs, and the others came from different families. The patients also gave their written informed consent to participate in this study.
Scan Acquisition
Images were obtained in a Siemens 3T scanner. Participants were instructed to keep motionless with their eyes closed and remain awake. Soft earplugs and foam pads were used on the participants to minimize scanner noise and head motion. Resting-state functional images were acquired with the following parameters using a gradient-echo echo-planar imaging (EPI) sequence: repetition time/echo time = 2000ms/30ms, 30 slices, 64×64 matrix, 90° flip angle, 24cm field of view, 4mm slice thickness, 0.4mm gap, and 250 volumes (500 s).
Image Preprocessing
Functional images were preprocessed with Data Processing Assistant for Resting-State fMRI.53 After slice timing and head motion correction, no participants had more than 2mm of maximal translation and more than 2° of maximal rotation. The images were then normalized to the standard Montreal Neurological Institute (MNI) EPI space in SPM8 and resampled to 3×3 × 3mm3. The obtained images were subsequently smoothed (with a 4-mm full width at half maximum Gaussian kernel), bandpass filtered (0.01–0.08 Hz), and linearly detrended. We regressed out several spurious covariates, including 6 head motion parameters obtained by rigid body correction, signal from a ventricular region of interest (ROI), and signal from a region centered in the white matter. The global signal was not regressed out as suggested in processing the FC data.54
FC Analysis
A seed-based FC method was employed to detect the resting-state cerebellar FC patterns in the siblings and in the controls. Three seed ROIs were chosen in the right Crus I (MNI: 33, −76, −34), left Crus I (MNI: −33, −76, −34), and lobule IX (MNI: 0, −55, −49). These ROIs showed intrinsic connectivities with the DMN in patients with schizophrenia50 and healthy participants.36,37 Seed ROIs were defined as 6mm radius spheres for FC analysis using the software REST.55 Correlation maps were created for each seed and each participant by computing the Pearson correlation coefficients between the ROI and other voxels of the whole brain. The obtained correlation maps were z-transformed with Fisher’s r-to-z transformation to improve the normality of the distribution.
One-sample t-tests were used for each seed and each group to identify voxels that exhibited significant correlations with the ROI. The significance level was set at P < .01 corrected for multiple comparisons using the Gaussian Random Field (GRF) theory (min z > 2.5758, cluster significance: P < .01). Voxelwise two-sample t-tests were then performed to calculate group differences within the union mask of one-sample t-test results of both groups. The significance level for each group was set at P < .01 (GRF corrected). Given that head micromovement may affect the FC findings,56 we calculated the framewise displacement (FD) for each participant as described in a previous study,56 and used it as a covariate in the group comparisons.
Results
Participants
The characteristics of the participants are shown in table 1. The 2 groups have no significant differences in age, sex ratio, years of education, and FD values.
Table 1.
Characteristics of the Participants
| Demographic Data | Siblings (n = 46) | Controls (n = 46) | P value |
|---|---|---|---|
| Sex (male/female) | 29/17 | 23/23 | .21a |
| FD (mm) | 0.06±0.03 | 0.05±0.02 | .13b |
| Age (years) | 22.96±4.01 | 23.30±2.30 | .62b |
| Years of education (years) | 11.50±2.21 | 11.34±1.78 | .85b |
Note: FD, framewise displacement.
aThe P value for sex distribution was obtained by chi-square test.
bThe P values were obtained by two samples t-tests.
FC patterns of the cerebellar seed ROIs
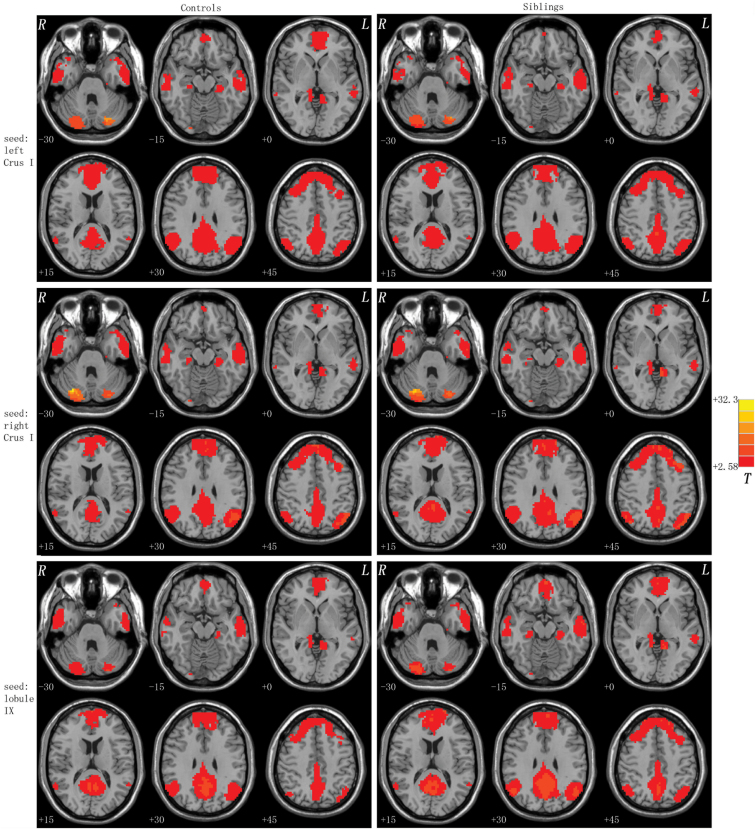
The resting-state FC patterns of the seed ROIs are shown in figure 1. For each group, each seed ROI exhibited distributed FCs with the DMN using one-sample t-tests. A union mask for each seed was made based on the results of the one-sample t-tests of both groups for the following group comparisons.
Fig. 1.
Brain regions with the cerebellar-DMN connectivities at rest. Correlation maps for the controls and the unaffected siblings of schizophrenia patients are displayed in the left and right columns. Red denotes higher connectivities and the color bar indicates the T values from one-sample t-tests. DMN, default-mode network.
Seed-Based FCs: Group Differences
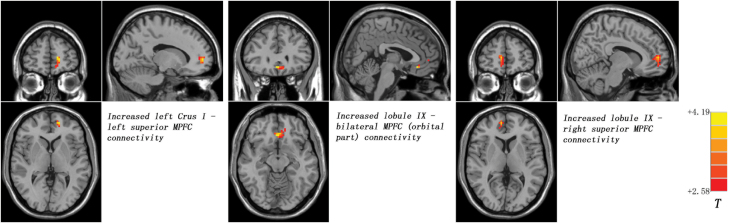
Compared with the controls, the siblings showed significantly increased FCs between the left Crus I and the left superior medial prefrontal cortex (MPFC), as well as between the lobule IX and the bilateral MPFC (orbital part) and right superior MPFC (figure 2; table 2). No significantly decreased FC was found in the siblings relative to the controls.
Fig. 2.
Statistical maps showing group differences of the cerebellar-DMN connectivities in the unaffected siblings of schizophrenia patients at rest. Red denotes higher connectivities in the siblings and the color bar indicates the T values from two-sample t-tests. DMN, default-mode network, MPFC, medial prefrontal cortex.
Table 2.
Brain Regions With Increased Cerebellar Connectivity in Unaffected Siblings of Schizophrenia Patients
| Cluster Location | Peak (MNI) | Number of Voxels | T Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Seed: Left Crus I | |||||
| Left superior MPFC | −15 | 54 | 3 | 24 | 3.5101 |
| Seed: Right Crus I | |||||
| None | |||||
| Seed: lobule IX | |||||
| Bilateral MPFC (orbital part) | 3 | 30 | −12 | 36 | 4.1881 |
| Right superior MPFC | 9 | 54 | 6 | 43 | 3.6804 |
Note: MPFC, medial prefrontal cortex; MNI, Montreal Neurological Institute.
Identifying Candidate Endophenotypes for Schizophrenia
To identify candidate endophenotypes for schizophrenia, the analyses were repeated in the patients from our previous study.3 The characteristics of the patients are exhibited in supplementary table 1. Supplementary figure 1 shows that both the patients and the controls exhibited widespread FCs with the DMN for each cerebellar seed using one-sample t-tests. For each seed, analyses of covariance (ANCOVA) were performed at each voxel within the union mask of one-sample t-test results of 3 groups with the mean FD as a covariate (supplementary figure 2), followed by post hoc t-tests to identify differences between each pair of groups. Compared with the controls, the patients exhibited significantly increased FCs between the left Crus I and the left superior MPFC and left angular gyrus (AG), as well as between the lobule IX and the left MPFC (orbital part) and left middle cingulate cortex (Supplementary figure 3; Supplementary table 2). By contrast, decreased FC was observed between the left Crus I and the bilateral ACC in the patients relative to the controls (Supplementary figure 3; Supplementary table 2). The post hoc t-test results between the siblings and the controls (supplementary table 2) were similar to the results in table 2.
The mean FC values were extracted from the clusters with abnormal cerebellar-DMN connectivities in the patients. After assessing the normality of the data, Pearson correlations were conducted between the mean FC values and the clinical variables (such as DUP and symptom severity). A significantly negative correlation was found between the PANSS negative scores and the mean FC values of the left Crus I-left AG connectivity in the patient group (r = −.365, P = .010, supplementary figure 4).
Discussion
Cerebellar seeds that were linked to the DMN were used in this study to examine the cerebellar FC patterns with the DMN in unaffected siblings of schizophrenia. The main results showed that the siblings exhibited increased cerebellar-DMN connectivities compared with the controls.
Combined with the results from the patients, the siblings and the patients shared increased FCs between the left Crus I and the left superior MPFC, as well as between the lobule IX and the left MPFC (orbital part), which could serve as candidate endophenotypes for schizophrenia. As mentioned in the Introduction, the endophenotype concept refers to the trait intermediate clinical symptoms and underlying gene-based pathophysiology of psychiatric disorders.57 From the definition, candidate endophenotype can be biochemical, neurophysiological, neuroanatomical, endocrine, or cognitive measures. The patients and the siblings in this study shared increased cerebellar-DMN connectivities, which may serve as candidate endophenotypes based on its concept.
In contrast to the traditional sib pair approach (using the patient-sibling pairs from the same families), only 14 patients and 14 siblings were sib pairs, whereas the other participants were from different families and unrelated to one another. The inclusion criterion is a novel approach as previously mentioned.58 First, the traditional sib pair approach indicates the heritability given the approximately 50% genetic similarity between the patients and the siblings. The shared genes include the susceptibility genes for schizophrenia and genes unrelated to schizophrenia.59 Thus, the specificity of the findings from the traditional sib pair approach as candidate endophenotypes is low (supplementary figure 5). In contrast, the inclusion of the patients and the siblings unrelated to one another can minimize the effect from genes unrelated to schizophrenia, and thus enhance the specificity of increased cerebellar-MPFC connectivities as candidate endophenotypes for schizophrenia. Second, the patients and unrelated siblings were from different early life and family environment. The present recruitment criterion can also minimize the effect from the shared environment background as observed in the traditional sib pair studies. Finally, no correlations were found between the mean FC values of the cerebellar-MPFC connectivities and clinical variables (such as DUP and symptom severity) in the patients (only a significantly negative correlation was found between the PANSS negative scores and the mean FC values of the left Crus I-left AG connectivity in the patients), indicating that the increased cerebellar-MPFC connectivities may be trait alterations for schizophrenia independent of symptom severity and illness duration. Previously, increased prefrontal hyperconnectivity has been reported to correlate positively with treatment improvement,60 and increased negative symptom scores are generally considered to be with poor treatment outcome. Hence, it is not surprised that a significantly negative correlation was observed between the PANSS negative scores and the mean FC values of the left Crus I-left AG connectivity in the patients. Based on the above-mentioned reasons, the specificity of the increased cerebellar-MPFC connectivities serving as candidate endophenotypes is high.
The increased cerebellar-DMN connectivities in the siblings appear inconsistent with our hypothesis and general consideration that schizophrenia patients had overall reduced FCs.51 The prevailing perspective of overall reduced FCs in schizophrenia is based on studies with chronic and/or medicated schizophrenia patients. In contrast, some studies have observed increased frontal FCs in early-course, drug-naive patients.3,60 Increasing glutamate concentrations in the frontal regions were found in early-course schizophrenia patients,61,62 which may lead to frontal hyperconnectivities. When the findings from chronic and early-course schizophrenia are combined, progressive reduction of frontal FCs with illness duration is portrayed, with increased frontal FCs in early-course patients and decreased frontal FCs in chronic patients. The patients in the present study are first-episode, drug-naive individuals with a relatively short DUP (mean DUP: 22.45 months). Therefore, patients who show increased cerebellar-DMN connectivities are not surprising. Decreased FC was observed between the left Crus I and the bilateral ACC in the patients; however, whether reduced cerebellar-DMN connectivity is present before or after the emergence of increased cerebellar-DMN connectivities remains unclear. By contrast, only increased cerebellar-DMN connectivities were found in the siblings. Although the siblings were unaffected at the scan time, they were individuals with higher risk than the general population to develop schizophrenia. Together with the findings from the patients and the siblings, this study suggests that increased cerebellar-DMN connectivities appear earlier than illness onset,63 and decreased cerebellar-DMN connectivities emerge after illness onset.
The results offer supporting evidence for the neurodevelopmental model of schizophrenia. Previously, a seed-based FC analysis was conducted to examine the FC differences of the prefrontal-thalamic-cerebellar circuit in healthy participants (children, adolescents, and adults),64 and the results showed that the connectivities of this circuit present an inverted U-curve with maximal point in adolescents. In particular, healthy children showed absent prefrontal-thalamic connectivity. However, the exact time points of developmental alterations are difficult to decide because of limited evidence from a single cross-sectional study.64 Considering that the patients in the present study are aged from 16 to 30 years, which is the developmental stage from adolescents to adults, the increased cerebellar-DMN connectivities are supposed to be disrupted by schizophrenia, and thereby remain at a relatively high position of the inverted U-curve. As previously mentioned, hyperconnectivities emerged before illness onset,63 and the siblings were at a high risk to develop schizophrenia. Therefore, the siblings who exhibit increased cerebellar-DMN connectivities, which may serve as candidate endophenotypes for schizophrenia, are not surprising.
The increased cerebellar-DMN connectivities are also informative from the physiological meaning of FC. Increased connectivity is often interpreted as compensatory reallocation or dedifferentiation.65–68 The compensatory effort may be modulated by inflammation in the early course of schizophrenia.60 In the early course of the disease, the astrocytes can be activated by proinflammatory cytokines (such as interleukin-6) and exhibit hyperfunction (increased metabolism and blood flow).69 Regional hyperfunction can lead to increased regional activity and FC. As supporting information, hyperconnectivities across the DMN regions have been reported in early-course schizophrenia.60 The siblings also exhibited increased cerebellar-DMN connectivities, indicating that the inflammatory effect may be present before illness onset.63 Although this study speculated the increased cerebellar-DMN connectivities were compensatory effort associated with the inflammatory effect in the patients and the siblings, the exact neurobiology underlying the compensatory effort requires further evidence.
At first glance, the results of this study are inconsistent with a previous study in schizophrenia patients,50 which reported decreased cerebellar-DMN connectivities in schizophrenia patients. However, the findings of Wang et al are consistent with the results of this study when their results are conceived from the neurodevelopmental perspective. The patients in the study of Wang et al were chronic and medicated, with a mean age of 38 years old. Long illness duration and medication use, in addition to the age effect, might have influenced their decreased cerebellar-DMN connectivities. Their patients are supposed to have decreased cerebellar-DMN connectivities based on the neurodevelopmental model. Indeed, their patients showed reduced cerebellar-DMN connectivities without increased cerebellar-DMN connectivities. Therefore, the study of Wang et al supports our view that increased cerebellar-DMN appears earlier than illness onset (as seen in the siblings) and decreases with illness duration.
Increased cerebellar-DMN connectivities are observed in the siblings using cerebellar seeds that have been proven to connect with the DMN. This selection method enhances the specificity of the results that focus on the DMN. The MPFC is a key node of the DMN and is essential in self-referential processing and emotional regulation.70–72 Increased cerebellar-MPFC connectivities might affect the function integration of the DMN, and they contributed to the disturbances of cognition and enhanced risk for the disease in the siblings.7
This study has certain limitations. First, this study is cross-sectional, and whether part of the unaffected siblings would subsequently develop schizophrenia is unknown. Conducting a longitudinal study to compare the differences between unaffected siblings who would develop schizophrenia later and those who would not would be interesting. Second, psychological assessments were not performed in this study, and the relationship between increased cerebellar-DMN connectivities and psychological parameters in the siblings is unknown. Finally, cerebellar seeds that have been proven to link to the DMN were used. This method enhances the specificity of the results that focus on the DMN. For the same reason, other brain connectivities have been excluded from the results.
Despite the limitations, increased cerebellar-DMN connectivities in unaffected siblings of schizophrenia patients are first reported in this study. The results indicate that increased cerebellar-DMN connectivities appear earlier than illness onset, and highlight the cerebellar contribution to the DMN abnormalities in unaffected siblings. The siblings and the patients share increased cerebellar-left MPFC connectivities, which may serve as candidate endophenotypes for schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81260210), the Natural Science Foundation of Guangxi Province for Distinguished Young Scientists (2014GXNSFGA118010), and the Natural Science Foundation of Guangxi Province (2013GXNSFAA019107).
Conflict of Interest
All authors announce that they have no conflict of interest.
Supplementary Material
References
- 1. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 2. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo W, Liu F, Liu J, et al. Abnormal causal connectivity by structural deficits in first-episode, drug-naive schizophrenia at rest. Schizophr Bull. 2015;41:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 7. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. [DOI] [PubMed] [Google Scholar]
- 10. Zhou Y, Liang M, Tian L, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. [DOI] [PubMed] [Google Scholar]
- 11. Mannell MV, Franco AR, Calhoun VD, Cañive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31:424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skudlarski P, Jagannathan K, Anderson K, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Repovš G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. [DOI] [PubMed] [Google Scholar]
- 17. Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mingoia G, Wagner G, Langbein K, et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138:143–149. [DOI] [PubMed] [Google Scholar]
- 19. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. [DOI] [PubMed] [Google Scholar]
- 20. Hoptman MJ, Zuo XN, Butler PD, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo W, Yao D, Jiang J, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:16–20. [DOI] [PubMed] [Google Scholar]
- 22. Guo W, Liu F, Yao D, et al. Decreased default-mode network homogeneity in unaffected siblings of schizophrenia patients at rest. Psychiatry Res. 2014;224:218–224. [DOI] [PubMed] [Google Scholar]
- 23. Lieberman JA, Tollefson GD, Charles C, et al. ; HGDH Study Group. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. [DOI] [PubMed] [Google Scholar]
- 24. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 25. Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, et al. Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res. 2007;91:87–96. [DOI] [PubMed] [Google Scholar]
- 26. Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162:1785–1804. [DOI] [PubMed] [Google Scholar]
- 27. Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62:975–983. [DOI] [PubMed] [Google Scholar]
- 28. Guo X, Li J, Wei Q, Fan X, Kennedy DN, Shen Y, Chen H, Zhao J. Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naive schizophrenia. PLoS One 2013;8:e83679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 30. Jang JH, Jung WH, Choi JS, et al. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res. 2011;127:58–65. [DOI] [PubMed] [Google Scholar]
- 31. van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012;142:237–243. [DOI] [PubMed] [Google Scholar]
- 32. Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708–1719. [DOI] [PubMed] [Google Scholar]
- 33. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121 (pt 4):561–579. [DOI] [PubMed] [Google Scholar]
- 34. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. [DOI] [PubMed] [Google Scholar]
- 35. Laricchiuta D, Petrosini L, Piras F, et al. Linking novelty seeking and harm avoidance personality traits to cerebellar volumes. Hum Brain Mapp. 2014;35:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bernard JA, Seidler RD, Hassevoort KM, et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alalade E, Denny K, Potter G, Steffens D, Wang L. Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS One. 2011;6:e20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. [DOI] [PubMed] [Google Scholar]
- 43. Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heath RG, Franklin DE, Shraberg D. Gross pathology of the cerebellum in patients diagnosed and treated as functional psychiatric disorders. J Nerv Ment Dis. 1979;167:585–592. [DOI] [PubMed] [Google Scholar]
- 45. James AC, James S, Smith DM, Javaloyes A. Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am J Psychiatry. 2004;161:1023–1029. [DOI] [PubMed] [Google Scholar]
- 46. Szeszko PR, Gunning-Dixon F, Goldman RS, et al. Lack of normal association between cerebellar volume and neuropsychological functions in first-episode schizophrenia. Am J Psychiatry. 2003;160:1884–1887. [DOI] [PubMed] [Google Scholar]
- 47. Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155:1288–1290. [DOI] [PubMed] [Google Scholar]
- 48. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernard JA, Mittal VA. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 2014;5:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang L, Zou F, Shao Y, et al. Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr Res. 2014;160:67–72. [DOI] [PubMed] [Google Scholar]
- 51. Karlsgodt KH, Sun D, Jimenez AM, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008;20:1297–1327. [DOI] [PubMed] [Google Scholar]
- 52. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 53. Yan C, Zang Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hahamy A, Calhoun V, Pearlson G, et al. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 2014;4:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Greenwood TA, Braff DL, Light GA, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo W, Hu M, Fan X, et al. Decreased gray matter volume in the left middle temporal gyrus as a candidate biomarker for schizophrenia: a study of drug naive, first-episode schizophrenia patients and unaffected siblings. Schizophr Res. 2014;159:43–50. [DOI] [PubMed] [Google Scholar]
- 59. Allen AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD. Endophenotypes in schizophrenia: a selective review. Schizophr Res. 2009;109:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anticevic A, Hu X, Xiao Y, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Natsubori T, Inoue H, Abe O, et al. Reduced frontal glutamate + glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull. 2014;40:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fair DA, Bathula D, Mills KL, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. [DOI] [PubMed] [Google Scholar]
- 66. Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. [DOI] [PubMed] [Google Scholar]
- 67. Guo W, Liu F, Liu J, Yu L, Zhang Z, Zhang J, Chen H, Xiao C. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry 2013;46:13–18. [DOI] [PubMed] [Google Scholar]
- 68. Su Q, Yao D, Jiang M, et al. Increased functional connectivity strength of right inferior temporal gyrus in first-episode, drug-naive somatization disorder. Aust N Z J Psychiatry. 2015;49:74–81. [DOI] [PubMed] [Google Scholar]
- 69. Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. [DOI] [PubMed] [Google Scholar]
- 70. Yu R, Chien YL, Wang HL, et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp. 2012. doi: 10.1002/hbm.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu F, Guo W, Yu D, et al. Classification of different therapeutic responses of major depressive disorder with multivariate pattern analysis method based on structural MR scans. PLoS One. 2012;7:e40968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu F, Guo W, Liu L, et al. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. 2013;146:401–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.