Abstract
Dysfunctional patterns of activation in brain reward networks have been suggested as a core element in the pathophysiology of schizophrenia. However, it remains unclear whether this dysfunction is specific to schizophrenia or can be continuously observed across persons with different levels of nonclinical and clinical symptom expression. Therefore, we sought to investigate whether the pattern of reward system dysfunction is consistent with a dimensional or categorical model of psychosis-like symptom expression. 23 patients with schizophrenia and 37 healthy control participants with varying levels of psychosis-like symptoms, separated into 3 groups of low, medium, and high symptom expression underwent event-related functional magnetic resonance imaging while performing a Cued Reinforcement Reaction Time task. We observed lower activation in the ventral striatum during the expectation of high vs no reward to be associated with higher symptom expression across all participants. No significant difference between patients with schizophrenia and healthy participants with high symptom expression was found. However, connectivity between the ventral striatum and the medial orbitofrontal cortex was specifically reduced in patients with schizophrenia. Dysfunctional local activation of the ventral striatum depends less on diagnostic category than on the degree of symptom expression, therefore showing a pattern consistent with a psychosis continuum. In contrast, aberrant connectivity in the reward system is specific to patients with schizophrenia, thereby supporting a categorical view. Thus, the results of the present study provide evidence for both continuous and discontinuous neural substrates of symptom expression across patients with schizophrenia and the general population.
Key words: dimensional symptom expression, psychosis-like symptom expression, functional magnetic resonance imaging, cued reinforcement reaction time task, apathy, ventral striatum
Introduction
A growing number of studies suggest that symptoms of schizophrenia cannot only be observed in patients suffering from schizophrenia but also in subclinical form in persons without a psychotic disorder.1–3 Dimensional models of schizophrenia postulate a continuous symptom expression in the general population, where patients with schizophrenia lie at the extreme end of the population distribution.4,5 However, more recent publications have begun to integrate categorical and dimensional approaches.6,7
Recent neuroimaging studies have begun to investigate the psychosis continuum on a neural level.8–10 These studies have focused on samples defined by subclinical psychotic experiences or schizotypal personality traits. In these samples, dysfunctions of several brain systems were found to be comparable to those in patients with schizophrenia, which is consistent with the continuum hypothesis. However, there are no studies investigating brain mechanisms across different levels of symptom expression in both nonclinical participants and patients with schizophrenia, which would be important to address the continuum vs category debate.
In this context, a major focus of interest lies on the networks underlying reward processing, especially dopaminergic projections with target regions in the ventral striatum (VS) and the orbitofrontal cortex (OFC).11,12 In unmedicated patients with schizophrenia, ventral striatal activation in response to rewarding stimuli is reduced in comparison to healthy participants.13,14 Treatment with atypical antipsychotics seems to attenuate differences between patients and controls.15 Importantly, reduced striatal activation has been associated with negative and positive symptoms in patients with schizophrenia.14,16–18 Apathy seems to be the negative symptom most closely related to blunted reward processing.17,18 More recently, dysfunctional connectivity within the reward system has been reported in patients with schizophrenia.14,19
The aim of the present study was to investigate whether dysfunctional neural reward processing could be a substrate of different levels of psychosis-like symptom (PLS) expression across the general population and clinical symptoms of schizophrenia. For this purpose we measured neural activity during reward anticipation with a modified Cued Reinforcement Reaction Time (CRRT20)-task. Importantly, we recruited both patients with schizophrenia and healthy participants with varying degrees of PLS. The latter group was recruited with a stratified sampling procedure to ensure that all levels of PLS were adequately represented. Our final sample of participants was therefore characterized by a broad distribution of both clinical symptoms and subclinical PLS (supplementary figure S1).
We expected a linear relation between neural reward processing and general symptom expression. A higher symptom load should lead to lower activity in the VS during the anticipation of rewards. Additionally, we expected that apathy would be associated with a reduced activation of the VS during anticipation of a reward. Finally, we investigated connectivity between the VS and other regions within the reward system across healthy participants and patients with schizophrenia.
Methods
Participants
We recruited healthy participants using an internet-based questionnaire assessing subclinical experiences of PLS (Community Assessment of Psychic Experiences, [CAPE21]). The CAPE has been developed to measure positive (20 items), negative (14 items), and depressive symptoms (8 items) in the general population and has been found to possess good validity and reliability.22 The level of symptom expression in participants was determined by combining the frequency score of negative, positive, and depressive symptoms. Out of the 350 participants who filled out the questionnaire, 12 healthy participants with a low PLS level (at least 1 SD below the mean), 14 healthy participants with an average PLS level (deviation of less than 1 SD from the mean), and11 healthy participants with a high PLS level (at least 1 SD above the mean) were invited to participate in an functional magnetic resonance imaging (fMRI) study (supplementary figure S1). Healthy participants had to be right-handed and without any current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) psychiatric disorder and history of psychotic disorder, psychopharmacological treatment, drug abuse, or neurological disorder. Lifetime depressive episodes were reported by 2 subjects in the medium expression group and 4 subjects in the high expression group. Additionally, 23 patients with schizophrenia or schizoaffective disorder were recruited. Patients had to fulfill clinical DSM-IV criteria and the diagnosis was confirmed by M.I.N.I.23 Thus, patients were recruited based on diagnosis and not on CAPE scores. Patients were excluded if another axis-I disorder was present. All patients were medicated with atypical antipsychotics, 5 patients were additionally treated with mood stabilizers, and 7 patients were additionally treated with antidepressants. Chlorpromazine equivalents were calculated following the guidelines provided by Andreasen and colleagues24 as well as Gardner and colleagues.25 The present study complies with the declaration of Helsinki, version 2008, and was approved by the Ethics Committee of the Medical School of the University of Heidelberg. All participants provided written informed consent.
Psychopathological Assessment
In both groups, negative symptoms were measured using the Scale for the Assessment of Negative Symptoms (SANS).26 We calculated the SANS total score by summing all global rating items excluding the attention subscale. For the SANS apathy score we summed the global rating items from the apathy and anhedonia subscale, and for the SANS diminished expression the global rating items from the alogia and affective flattening subscales.27 Apathy was assessed using the clinician version of the Apathy Evaluation Scale (AES28). Positive symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS29). For the assessment of delusions in patients, we used the Psychotic Symptom Rating Scales,30 while in healthy participants we used the Magical Ideation Scale.31 Depression was assessed using the Calgary depression scale in patients with schizophrenia32 and the Beck Depression Inventory (BDI) in healthy participants.33 Anhedonia was assessed using the Chapman scales for physical and social anhedonia34 as well as the temporal experience of pleasure scale.35 Premorbid intelligence was assessed using a vocabulary-based test.36
fMRI Task
We employed a modified version of the CRRT.20 The CRRT-task allows an examination of brain activation during the anticipation of monetary gains and losses, which are dependent on performance in a simple discrimination task (figure 1). We adopted a 2:1 ratio between amounts won and lost, because the individual value function for losses is generally steeper than for gains, ie, smaller potential losses acquire the same behavioral relevance as higher potential gains.37 The amount of money won or lost during each trial was calculated using an algorithm incorporating the participant’s previous reaction times (see supplementary figure S2). Error trials were defined as wrong reaction or omission of reaction and were not included in the subsequent analysis. Participants engaged in two 12-min sessions, each consisting of 80 trials. The maximum amount to be won was €36. To increase statistical efficiency, trials were separated by jittered intertrial intervals which varied between 1 and 9 s, with a mean duration of 3.5 s. Each participant completed 25 practice trials before the task. The main behavioral outcome measure of the task was reward-related speeding, which was calculated by subtracting the reaction time during the neutral condition from the reaction time during experimental conditions. Subjects were paid out the amount of money won during the task, and no additional financial compensation was offered.
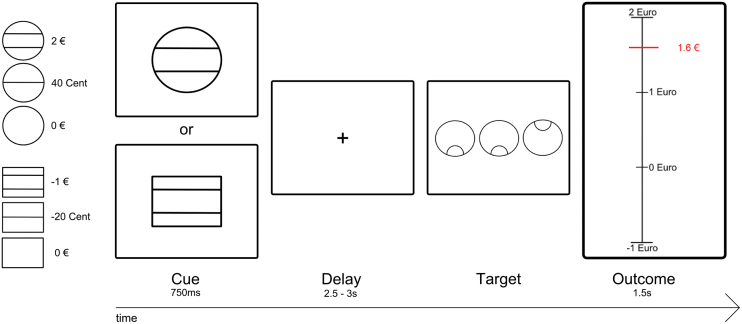
Fig. 1.
Cued reinforcement reaction time task (CRRT). In each trial, participants saw 1 of 6 geometric figures (“cue”; duration of .750 s), which indicated maximum possible monetary amount to be won (2 euro, 40 cent, 0 euro, depicted using circles) or lost (−1 euro, −20 cent, 0 cent, depicting using squares). After a delay period (varying between 2.5 and 3 s), participants had to identify an outlier out of an array of 3 circles via button press (either left or right button). Immediately after target presentation, participants received feedback about the amount of money they had won/lost during the respective trial (feedback, 1.5 s). A red horizontal line indicated the amount of money won during the respective trial. The precise amount was presented adjacent to the red line.
fMRI Acquisition
Images were collected using a 1.5-Tesla Siemens Magnetom AvantoMRI system (Siemens Healthcare, Erlangen, Germany). 365 T2*-weighted, echo-planar images of the entire brain were acquired in each of the 2 runs. In order to minimize susceptibility artifacts in the orbitofrontal cortex, 27 oblique slices of 4mm thickness with a 30° angle relative to the anterior–posterior-commissure axis were acquired with the following parameters: TR 2280ms, TE 40ms, flip angle 90°, field of view 192×192mm, matrix 64×64, and voxel size 3×3 × 4mm3. Participants viewed visual stimuli on a projection screen via a mirror fixed to the head coil and responded with the right hand using a button box. Following the functional scans, high resolution T1 weighted images were acquired using the three-dimensional MPRAGE sequence with isotropic voxel size (160 slices, voxel size 1×1 × 1mm, TR 1810ms, TE 3.07ms, 15° flip angle, and field of view 256×256mm) for anatomical reference.
fMRI Preprocessing
fMRI data were analyzed using SPM5 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology). Functional images were checked manually for artifacts and corrected for differences in slice acquisition timing. Furthermore, all images were realigned with the allowed motion limited to ±4mm translation and to ±3° rotation over the whole experiment, and unwarped to correct for artifacts due to susceptibility-by-movement interaction. Both functional and structural images were normalized to the Montreal Neurological Institute (MNI) T1 template. Functional images were spatially smoothed with a kernel of 8mm full-width at half maximum. Low-frequency signal drifts were removed using a 128-s high-pass filter.
Statistical Analysis
On the first level of analysis, fMRI data were analyzed with a general linear model approach. Separate regressors were included for the 5 different anticipation phases (anticipation of €2, anticipation of 40 cents, anticipation of minus €1, anticipation of minus 20 cent, and anticipation of €0) and the 5 corresponding outcome phases, as explanatory variables convolved with the gamma-variate function described as implemented in SPM. Targets and error trials were included as additional regressors of no interest. For the analysis of reward anticipation, individual contrast images corresponding to the effects of interest were subsequently constructed. This involved contrasting the anticipation of a high reward (€2) with the anticipation of €0 and the anticipation of a high loss (minus €1) with the anticipation of €0. Group comparisons were performed with a region of interest approach focusing on the VS. In addition, we performed a whole-brain analysis to characterize reward related activity in the whole sample (patients and healthy participants, N = 60) using a one sample t test. Results of the whole brain analysis are reported at a family-wise error corrected threshold of P < .05.
Region of Interest Analysis
In order to assess brain activation in the VS—our a priori region of interest—we used anatomical voxel-masks taken from a publication-based probabilistic MNI atlas as in previous studies.18,38 Mean percent signal change was extracted for the VS ROI using MarsBaR.39 In order to assess differences in reward related activity between all 4 groups, we performed ANOVA analyses with these activation estimates as dependent variables. Least Significant Difference post hoc tests were performed to assess differences in activation between groups. Furthermore, we employed Pearson correlations to investigate the associations between VS signal change and clinical variables.
Connectivity Analysis
We employed a psychophysiological interaction analysis (PPI) to assess functional coupling of the VS with each voxel in the whole brain during the anticipation of a high reward (€2) vs €0, corresponding to changes in experimental conditions.40 The interaction term was defined by using the contrast “anticipation of a high reward (€2) vs €0.” We used an anatomical voxel mask of the VS (see ROI-analysis) as a seed mask at the individual level. To reduce effects induced by task-related variance or correlations with the seed-ROI regardless of task, we included both the task regressor and the VS time course as regressors of no interest. The resulting statistical maps were then used in a random effects group analysis. Voxels showing a significant coupling with the VS were reported (thresholded at P < .05 cluster level corrected, cluster defining threshold P < .001 uncorrected). To further specify the relation between PLS and connectivity with the VS, we performed a covariate analysis by including the group-level (ie, coding of each participant according to his or her group affiliation) as covariate-of-interest in our statistical design. We extracted parameter estimates from these regions in order to compute an ANOVA to assess group differences.
Results
Demographic and Clinical Data
Demographic, clinical, and behavioral data are summarized in table 1. There were no significant differences between groups with respect to age, education, reaction times, or premorbid intelligence level. Apathy, total negative symptoms, and positive symptoms differed between groups with the low PLS group showing the lowest level of symptoms and the schizophrenia group the highest score.
Table 1.
Demographical Data, Psychopathology Ratings, and Task Performance
| Healthy Controls | |||||
|---|---|---|---|---|---|
| Low Symptom Expression | Average Symptom Expression | High Symptom Expression | Patients with Schizophrenia | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Difference Between Groups | |
| Age (years) | 25.5 (8.5) | 26.4 (5.3) | 28 (9) | 30.3 (7.3) | F = 1.4 |
| N, Gender | N = 12. 8 females | N = 14. 8 females | N = 11. 8 females | N = 23. 8 females | X2 = 5.8 |
| MWT-B | 30 (4) | 28 (3) | 30 (3) | 28 (5) | F = .9 |
| Education (years) | 14.9 (2) | 16.3 (1.8) | 15.8 (3.9) | 15.4 (4.2) | F = .4 |
| Number of smokers | 1 | 1 | 1 | 11 | F = 4.9 |
| Chlorpromazine equivalent | 585 (402) | ||||
| PAS | 7 (6) | 10 (6) | 10 (7) | 11 (7) | F = .8 |
| SAS | 6 (5) | 9 (5) | 13 (7) | 11 (6) | F = 3.4* |
| TEPS general | 84 (7) | 80 (10) | 78 (10) | 76 (13) | F = 1.6 |
| TEPS anticipation | 49 (4) | 42 (5) | 43 (6) | 42 (7) | F = 3.8* |
| TEPS consummation | 39 (6) | 38 (6) | 38 (6) | 36 (7) | F = .4 |
| CAPE frequency | 1.2 (0.1) | 1.7 (0.2) | 2.2 (0.2) | 1.9 (0.3) | F = 31.6*** |
| CAPE severity | 1.1 (0.1) | 1.4 (0.2) | 1.9 (0.3) | 1.6 (0.3) | F = 11.1*** |
| AES | 7.7 (4) | 11.6 (5.5) | 14.1 (6.5) | 17.7 (7.2) | F = 7.5*** |
| PANSS positive | 8.3 (1.1) | 8.9 (1.9) | 9.2 (2.2) | 10.5 (3.2) | F = 3.3* |
| PANSS negative | 7.6 (1.2) | 9.2 (2.5) | 9.9 (3) | 14.8 (8.5) | F = 6.9*** |
| PANSS general | 16.4 (0.8) | 18.2 (2) | 20.6 (5.4) | 24.3 (6.2) | F = 11.8*** |
| SANS total | 0.7 (1.1) | 1.4 (1.9) | 2.6 (3.7) | 5.2 (4.1) | F = 10.4*** |
| SANS apathy | 0.3 (0.6) | 0.6 (1.6) | 1.2 (2) | 2.3 (2) | F = 5** |
| SANS dimexp | 0.4 (1) | 0.7 (1.1) | 1.5 (2.4) | 2.7 (2.5) | F = 5.3** |
| BDI | 1.6 (1.4) | 4.6 (3.7) | 10.8 (9.6) | ||
| Calgary | 4.2 (4) | ||||
| MIS | 2.1 (2.9) | 2.5 (1.8) | 5.5 (2.5) | ||
| PSYRATS | 5 (5.5) | ||||
| RRS win | −0.035 (0.045) | −0.05 (0.055) | −0.029 (0.026) | −0.042 (0.032) | F = .9 |
| RRS lose | −0.029 (0.043) | −0.041 (0.052) | −0.027 (0.024) | −0.027 (0.027) | F = .5 |
| Total win (euros) | 29 (6) | 29 (6) | 29 (4) | 28 (7) | F = .1 |
Note: Values given as mean ± SD. MWT-B, vocabulary-based test for the assessment of premorbid intelligence; RRS, reward related speeding; PAS/SAS, Physical and social anhedonia scales; TEPS, Temporal experience of pleasure scale; CAPE, Community assessment of psychotic experiences; AES, Apathy evaluation scale; PANSS, Positive and negative symptom scale; SANS, Scale for the assessment of negative symptoms; BDI, Beck depression inventory; MIS, Magic ideation scale; PSYRATS, Psychotic symptom rating scales.
***P < .001, **P < .01, *P < .05.
Behavioral Data
There were no significant differences between groups with respect to the total amount of money earned. Participants in all groups showed reward-related speeding in the win and loss conditions (supplementary figure S4). No difference in reward-related speeding between groups was observed (Fs < 1.5, P > .1).
fMRI—Local Activation in the VS
The focus of this analysis was regional activation in the VS ROI. The results of the whole-brain analysis are given in figure 2A and supplementary table S1 and reflect expected regions of the reward system.
Fig. 2.
(A) Activation map of the contrast high reward (€2) anticipation vs no reward anticipation for all pooled participants. The threshold was set at P < .05 FWE corrected. The T-Map was overlaid on a normalized structural image averaged across all participants. The VS ROI is outlined on the activation map. (B) Group differences in VS signal change for high reward vs no reward anticipation, error bars depict SD.
We observed significant group differences in VS activity during the anticipation of €2 compared with €0 (F = 2.97, P = .04, figure 2B), suggesting lower VS activity in groups with higher PLS. Post hoc tests suggest a significant difference between the low PLS group and the high PLS group (95% CI: 0.05, 0.37), as well as between the low PLS group and patients with schizophrenia (95% CI: 0.03, 0.33). We did not observe a significant difference between the high PLS group and patients with schizophrenia (95% CI: −.017, 0.11).
There were no significant effects of gender, smoking, education years, or premorbid intelligence as covariates. Moreover, there was no significant correlation between chlorpromazine equivalents and VS activity during the reward anticipation in the patient group (r = −.14, P = .521). Additionally, we performed an explorative ANOVA to compare whole brain activity during the anticipation of €2 compared with €0 between groups. We did not observe any additional group differences.
We observed a similar pattern during the anticipation of a possible loss of €1 compared with €0, which however only reached trend-level significance (F = 2.2, P = .1).
Correlation Between Ventral Striatal Activation and Apathy
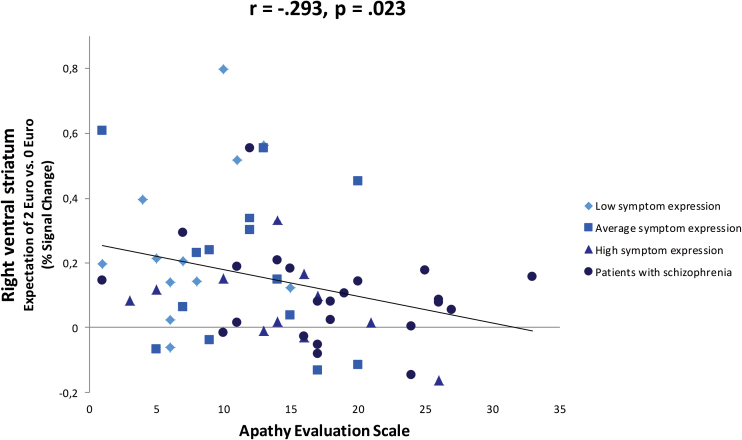
We observed a negative correlation between percent signal change in the right VS ROI during the anticipation of €2 vs no reward, and the level of apathy (AES: r = −.293, P = .023, SANS apathy subscale: r = −.336, P = .009) (figure 3). There were no significant correlations between PANSS positive symptoms and VS activation. In addition, there were no significant correlations between VS activation during loss anticipation and any psychopathological variable. VS activity was also negatively correlated with CAPE scores (see supplementary results S1). Furthermore, we observed a negative correlation between BDI scores and VS activity in healthy participants (see supplementary results S1).
Fig. 3.
Correlation between percent signal change in the right VS ROI during the anticipation of €2 vs no reward and apathy scores
Ventral Striatal Connectivity During the Anticipation of a Reward
Connectivity analysis in the whole sample showed a significant functional coupling of the right VS with the bilateral thalamus (left thalamus: −12, −18, 8, t = 5.31, right thalamus: 15, −15, 4, t = 4.3), and anterior cingulate cortex (−9, 15, 32, t = 4.25). Restricting the connectivity analysis to the group of healthy participants (N = 37) revealed additional areas in the medial OFC (mOFC) (−12, 63, 0, t = 4.81), dorsal striatum (right DS, 15, 9, 12, t = 5.08), bilateral insula (left insula: −45, 12, 4, t = 5.41, right insula: 39, 21, 4, t = 4.51), and dorsal lateral prefrontal cortex (left, −39, 48, 24, t = 5.49).
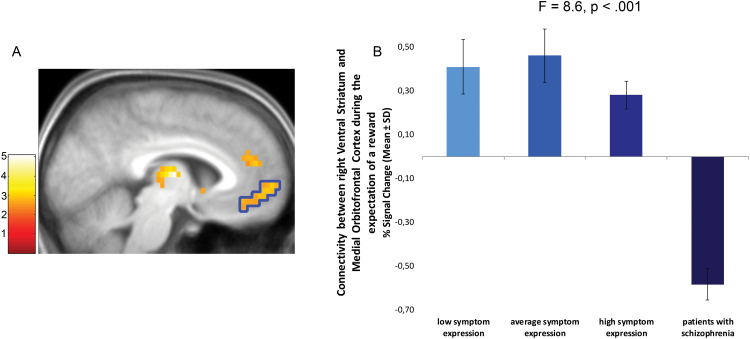
Furthermore, our regression analysis revealed that connectivity with the mOFC (−12, 63, 0, t = 4.2), left thalamus (−6, −12, 8, t = 5.04), and right DS (18, 9, 12, t = 5.09) was modulated by the group-regressor; lower PLS was related to stronger connectivity with these regions (figure 4A). An ANOVA revealed significant group differences in the mOFC using extracted parameter estimates (F = 8.6, P < .001, figure 4B). Post hoc tests revealed that the significant effect in the mOFC was mainly driven by pronounced differences in connectivity between patients and the different healthy control groups (Ps < 0.002), the three healthy groups did not differ (Ps > 0.05). The group effect remained significant after controlling for the effect of smoking (F = 3.6, P = .019) and differences in variability of local VS activation (F = 6.4, P = .003).
Fig. 4.
(A) Group differences in functional coupling of the VS with the mOFC calculated with a covariate analysis (inclusion of the group-level as covariate of interest in the statistical design, P < .05 cluster level corrected, cluster defining threshold P < .001 uncorrected). The T-Map was overlaid on a normalized structural image averaged across all participants. (B) Strength of connectivity for each group, error bars depict SD. The ROI used for the extraction of parameter estimates is outlined on the activation map.
Discussion
To our knowledge, this is the first neuroimaging study to include healthy participants with different levels of PLS and patients with manifest schizophrenia. We show that higher PLS were associated with lower activation in the VS during the anticipation of rewards, irrespective of diagnostic status. Across groups, apathy—a core negative symptom—was the symptom most strongly associated with ventral striatal activation. However, in contrast to local activation of the VS, its connectivity with the medial orbitofrontal cortex was specifically altered in patients with schizophrenia.
In our healthy participants, ventral striatal activation became smaller with increasing PLS as assessed by the CAPE. Thus, the continuous expression of PLS across the different groups was paralleled by decreased ventral striatal activation. Importantly, healthy participants with high PLS seem to differ from those with low PLS, but not from patients with schizophrenia. This suggests that with respect to local brain response to reward anticipation in the VS, patients with schizophrenia do not show a qualitative difference to participants without a psychotic disorder, but rather lie at one end of the general population distribution.
The finding that patients with schizophrenia did not show lower ventral striatal activation than healthy participants with high PLS was somewhat unexpected. However, this lack of difference may relate to treatment effects. First, as a consequence of treatment, patients with schizophrenia did show higher symptom levels only in some scales. Second, treatment with atypical antipsychotics has been shown to normalize responses of the reward system.15
Furthermore, we found that across groups, lower activity in the VS during the anticipation of a reward is related to apathy. This observation extends previous findings in patients with schizophrenia.17,18 In functional terms, the VS has mainly been considered to code the incentive salience or “wanting” of a reward, while it might also have a role in coding the “liking” of a reward.41 A dysfunctional coding of incentive salience has been discussed as a possible underlying factor for reduced goal-directed behavior in patients with schizophrenia.17 In contrast to previous studies, we assessed negative symptoms and apathy not only in patients, but also in healthy participants with different levels of PLS. While negative symptoms have traditionally been thought to be specific to schizophrenia, it has more recently been suggested that negative symptoms can be observed outside the schizophrenia spectrum.5 Our data show not only a considerable overlap in negative symptom expression across groups, but also a continuous association between apathy and ventral striatal activation.
Group differences in ventral striatal activation and the correlation with apathy were more pronounced during win than during loss anticipation. This suggests that overall the effects were mainly driven by the association of ventral striatal response with reward and negative symptoms, specifically apathy.16,18 One possible explanation for the stronger relationship of apathy with rewards relates to the focus on reward seeking and approach motivation in most apathy scales. It is an open question whether impaired motivation to avoid punishment should also be considered as a part of apathy, which would be expected to be more closely related to ventral striatal responses to loss anticipation.42
In addition, there was no association of positive symptoms with striatal response. Thus, these findings do not directly support an explanation in terms of aberrant salience, which would associate positive symptoms with both wins and losses.43 However, the low variance in positive symptom expression might account for these negative findings. In addition, it is possible that the 2:1 ratio between amounts won and lost adopted in our study failed to induce sufficient behavioral relevance or salience of potential losses.
During anticipation of a reward vs no reward, we found aberrant connectivity between the VS and the mOFC specifically in the patient group. In contrast to local brain activation in the VS, connectivity did not differ significantly between healthy groups with different levels of PLS. Thus, dysfunctional connectivity, which has been put forward as a core neurophysiological mechanism underlying schizophrenia,44,45 shows a pattern consistent with a categorical difference between patients with schizophrenia and persons without a psychotic disorder. Dysfunctional frontostriatal connectivity has been related to maladaptive reinforcement learning,46 which has been consistently observed in patients with schizophrenia.47,48 Abnormal functional integration within the reward system may therefore represent a pathognomonic aspect of schizophrenia.
Our results for local striatal activation during the anticipation of a reward support a dimensional view, which is consistent with a number of studies suggesting a continuous association with dimensions of human behavior and personality.49,50 These findings have mostly been interpreted in terms of variations in dopaminergic neurotransmission. In contrast, the connectivity analysis supports a categorical difference between “healthy” persons and patients with schizophrenia. Structural white-matter changes and N-Methyl-D-aspartate (NMDA)-receptor dysfunction have been proposed as pathomechanisms underlying aberrant functional coupling.45 Whether the latter mechanisms show any specificity for schizophrenia remains to be elucidated.
There are several limitations to our study. First, because we used a web-based questionnaire to identify healthy participants with differing degrees of PLS, we had no guarantee as to whether or not participants were accurately describing their own experiences. Nonetheless, the subsequent psychometric assessment confirmed the validity of our groups. Second, splitting the healthy participants into groups with different PLS led to relatively small cell sizes, which reduced the power to detect differences between groups. Third, because we did not correct post hoc tests and correlational analyses for multiple comparisons, our findings should be considered as exploratory and need to be replicated. Fourth, all patients were medicated with antipsychotics, which might have affected differences between healthy and patient groups, although chlorpromazine equivalent dosage was not related to ventral striatal activation or connectivity. It is nevertheless important to consider the possibility that the influence of medication could be categorical and not linear. Fifth, it has to be noted that although we included both the task regressor and VS time course as regressors of no interest in our PPI analysis, unaccounted task-related variance stemming from the unmodeled outcome phase could have influenced the observed activation in the mOFC.
In conclusion, the present study provides evidence for both continuous and discontinuous neural substrates of symptom expression across patients with schizophrenia and the general population. Dysfunctional local activation of the VS depends less on diagnostic status than on the degree of symptom expression. Lifetime PLS, current negative symptoms as well as symptoms of depression in nonclinical participants were associated with reduced striatal activation. Thus, local activation of the VS shows a pattern consistent with a psychosis continuum. In contrast, aberrant connectivity in the reward system is specific to patients with schizophrenia, thereby supporting a categorical view. The investigation of samples across a broad range of nonclinical and clinical symptom expression is a promising avenue for addressing the neural basis of symptom distributions observed in epidemiological samples.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
University of Heidelberg (to J.J.S.).]
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Rössler W, Riecher-Rössler A, Angst J, et al. Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res. 2007;92:1–14. [DOI] [PubMed] [Google Scholar]
- 2. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 3. Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. 2011;37:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 5. Kaiser S, Heekeren K, Simon JJ. The negative symptoms of schizophrenia: category or continuum? Psychopathology. 2011;44:345–353. [DOI] [PubMed] [Google Scholar]
- 6. Linscott RJ, van Os J. Systematic reviews of categorical versus continuum models in psychosis: evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annu Rev Clin Psychol. 2010;6:391–419. [DOI] [PubMed] [Google Scholar]
- 7. David AS. Why we need more debate on whether psychotic symptoms lie on a continuum with normality. Psychol Med. 2010;40:1935–1942. [DOI] [PubMed] [Google Scholar]
- 8. Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25:295–305. [DOI] [PubMed] [Google Scholar]
- 9. Corlett PR, Fletcher PC. The neurobiology of schizotypy: fronto-striatal prediction error signal correlates with delusion-like beliefs in healthy people. Neuropsychologia. 2012;50:3612–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumari V, Antonova E, Geyer MA. Prepulse inhibition and “psychosis-proneness” in healthy individuals: an fMRI study. Eur Psychiatry. 2008;23:274–280. [DOI] [PubMed] [Google Scholar]
- 11. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirvonen J, van Erp TG, Huttunen J, et al. Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry. 2005;62:371–378. [DOI] [PubMed] [Google Scholar]
- 13. Nielsen MØ, Rostrup E, Wulff S, et al. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71:898–905. [DOI] [PubMed] [Google Scholar]
- 14. Schlagenhauf F, Sterzer P, Schmack K, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–1039. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen MO, Rostrup E, Wulff S, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69:1195–1204. [DOI] [PubMed] [Google Scholar]
- 16. Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. [DOI] [PubMed] [Google Scholar]
- 17. Waltz JA, Schweitzer JB, Gold JM, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon JJ, Biller A, Walther S, et al. Neural correlates of reward processing in schizophrenia–relationship to apathy and depression. Schizophr Res. 2010;118:154–161. [DOI] [PubMed] [Google Scholar]
- 19. Gradin VB, Waiter G, O’Connor A, et al. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2013;211:104–111. [DOI] [PubMed] [Google Scholar]
- 20. Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30:1362–1373. [DOI] [PubMed] [Google Scholar]
- 21. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. [DOI] [PubMed] [Google Scholar]
- 22. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006;114:55–61. [DOI] [PubMed] [Google Scholar]
- 23. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33; quiz 34. [PubMed] [Google Scholar]
- 24. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 26. Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: The University of Iowa; 1983. [Google Scholar]
- 27. Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the scale for the assessment of negative symptoms. Psychol. Assess. 1996;8:269–280. [Google Scholar]
- 28. Lueken U, Seidl U, Schwarz M, et al. Psychometric properties of a German version of the apathy evaluation scale. Fortschr Neurol Psychiatr. 2006;74:714–722. [DOI] [PubMed] [Google Scholar]
- 29. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 30. Kronmüller KT, von Bock A, Grupe S, et al. Psychometric evaluation of the Psychotic Symptom Rating Scales. Compr Psychiatry. 2011;52:102–108. [DOI] [PubMed] [Google Scholar]
- 31. Hoppe L. Die “Magical ideation scale”: Itemanalyse und Validierung durch eine Untersuchung der Vigilanzleistung (Doctoral dissertation) 1991. [Google Scholar]
- 32. Müller MJ, Marx-Dannigkeit P, Schlösser R, Wetzel H, Addington D, Benkert O. The Calgary Depression Rating Scale for Schizophrenia: development and interrater reliability of a German version (CDSS-G). J Psychiatr Res. 1999;33:433–443. [DOI] [PubMed] [Google Scholar]
- 33. Hautzinger M, Keller F, Kühner C. BDI-II. Beck-Depressions-Inventar Revision —Manual. Frankfurt: Harcourt Test Services; 2006. [Google Scholar]
- 34. Burgdörfer G, Hautzinger M. [Psychological and social anhedonia. Evaluation of a research instrument for measuring a basic psychopathologic disorder]. Eur Arch Psychiatry Neurol Sci. 1987;236:223–229. [DOI] [PubMed] [Google Scholar]
- 35. Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Personal. 2006;40:1086–1102. [Google Scholar]
- 36. Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91:335–345. [DOI] [PubMed] [Google Scholar]
- 37. Tversky A, Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J. Risk Uncertain. 1992;5:297–323. [Google Scholar]
- 38. Nielsen FA, Hansen LK. Automatic Anatomical Labeling of Talairach Coordinates and Generation of Volumes of Interest via tbe Brainmap Database NeuroImage, vol. 16. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available on CD-Rom. 2002. [Google Scholar]
- 39. Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 2002;16:S497. [Google Scholar]
- 40. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. [DOI] [PubMed] [Google Scholar]
- 41. Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. [DOI] [PubMed] [Google Scholar]
- 42. Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. [DOI] [PubMed] [Google Scholar]
- 43. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 44. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 45. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernacer J, Corlett PR, Ramachandra P, et al. Methamphetamine-induced disruption of frontostriatal reward learning signals: relation to psychotic symptoms. Am J Psychiatry. 2013;170:1326–1334. [DOI] [PubMed] [Google Scholar]
- 47. Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simon JJ, Walther S, Fiebach CJ, et al. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49:1868–1874. [DOI] [PubMed] [Google Scholar]
- 50. Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage. 2014;84:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.