Abstract
Schizophrenia is a disabling clinical syndrome found across the world. While the incidence and clinical expression of this illness are strongly influenced by ethnic factors, it is unclear whether patients from different ethnicities show distinct brain deficits. In this multicentre study, we used structural Magnetic Resonance Imaging to investigate neuroanatomy in 126 patients with first episode schizophrenia who came from 4 ethnically distinct cohorts (White Caucasians, African-Caribbeans, Japanese, and Chinese). Each patient was individually matched with a healthy control of the same ethnicity, gender, and age (±1 year). We report a reduction in the gray matter volume of the right anterior insula in patients relative to controls (P < .05 corrected); this reduction was detected in all 4 ethnic groups despite differences in psychopathology, exposure to antipsychotic medication and image acquisition sequence. This finding provides evidence for a neuroanatomical signature of schizophrenia expressed above and beyond ethnic variations in incidence and clinical expression. In light of the existing literature, implicating the right anterior insula in bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety, we speculate that the neuroanatomical deficit reported here may represent a transdiagnostic feature of Axis I disorders.
Key words: schizophrenia, neuroanatomy, ethnicity, magnetic resonance imaging, voxel-based morphometry
Schizophrenia is common, severely disabling, and has major socioeconomic impact. Consistent with the current understanding of illness as a collection of heterogeneous clinical syndromes,1,2 epidemiological studies have found that the incidence and clinical expression of schizophrenia vary according to a number of sociodemographic factors including, amongst others, the ethnic origin of the patients under investigation.3–7 For example, patients from Asian ethnicities are more likely to experience visual hallucinations, whereas patients from western cultures and Caucasian ethnicities are more likely to suffer from auditory hallucinations.5 In addition, the content of hallucination and delusions is also strongly influenced by the patient’s ethnic milieus.5 Even within the same geographical area, ethnic origin has been found to strongly influence the incidence and manifestation of the disease. For example, African-Caribbeans living in London are more likely to develop schizophrenia6 and suffer from affective symptoms7 than White Caucasians from the same neighbourhood. Likewise, Mâori people in New Zealand are more likely to present with hallucinations and aggression and less likely to present with depression and self-harm than non-Mâori people from the same area.8
Over the past 2 decades, neuroimaging techniques have enabled greater understanding of the neuroanatomical basis of this illness.9–14 So far, however, the impact of ethnic characteristics on the neuroanatomical basis of schizophrenia has received minimal attention in the existing literature.15 Some neuroimaging studies have recruited ethnically uniform cohorts in order to minimize individual variation within each experimental group16,17; whereas other studies have included participants from a range of ethnic backgrounds based on the assumption that this would not have a significant impact on the results.10,18 Thus, at present, we know very little about whether patients from different ethnicities are characterised by similar or different neuroanatomical deficits. Here, we addressed this question by investigating 4 ethnically distinct samples comprising of (1) White Caucasian, (2) African-Caribbean, (3) Japanese, and (4) Chinese participants. The 4 groups were uniform with respect to the stage of the illness, as all patients had experienced a first episode of schizophrenia within the previous 24 months. In addition, in all 4 groups, each patient was individually matched with a healthy control of the same ethnicity, gender, and age (±1 year) in order to minimize the potential impact of these demographic variables. As the 4 groups were scanned at different sites using different scanners and acquisition sequences (see supplementary table S1 for detail), any discrepancy between datasets could be due to methodological differences. For the purpose of the present investigation, therefore, we focussed on differences between patients and controls that were expressed consistently across the 4 groups. As we preprocessed and analysed each dataset independently, using ethnic-specific templates, our investigation can be thought of as a series of replication studies across 4 independent datasets. We hypothesised that the 4 groups would show consistent neuroanatomical reductions in specific prefrontal, parietal, and limbic regions that are implicated in existing animal19 and human11,20–24 models of schizophrenia. This would provide evidence for a neuroanatomical signature of schizophrenia expressed above and beyond ethnic differences in incidence and clinical expression.
Methods
Participants
We recruited a total of 126 patients (age range: 18–44; all right-handed) who had experienced a first episode of schizophrenia within the previous 24 months and 126 healthy controls (age range: 18–44; all right-handed). The patient group comprised 4 distinct samples differing in terms of self-ascribed ethnicity; these included 29 White Caucasians, 19 African-Caribbean, 28 Japanese, and 50 Chinese. The White Caucasian and African-Caribbean samples were recruited from the South London and Maudsley Foundation Trust and scanned at the Institute of Psychiatry (United Kingdom) between 2008 and 2012. The Japanese sample was recruited from and scanned at University of Tokyo Hospital (Japan) between 2010 and 2013; a subset of this sample was included in a previous publication.25 The Chinese sample was recruited from the West China Hospital of Sichuan University and scanned at the Huaxi MR Research Center in Chengdu (China) between 2009 and 2012. Diagnosis of schizophrenia was formulated by an experienced psychiatrist using the ICD-10 criteria (for the White Caucasians, African-Caribbean, and Chinese samples) or the DSM-V criteria (for the Japanese sample). In addition, psychotic symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS)26 on the day of scanning (for the White Caucasians, African-Caribbean, and Chinese samples) or within 7 days (for the Japanese sample). All patients in the Chinese sample were medication naïve at the time of scanning; in contrast, patients in the White Caucasian, African-Caribbean and Japanese samples were taking first- or second-generation antipsychotic medication, or a combination of the two (see table 1 for chlorpromazine-equivalent dose). Each patient group was recruited as part of a larger single-center project, and comprised a mixture of in- and outpatients. The healthy controls were recruited through local advertisement from the same geographical areas as patients, and were individually matched to patients on age (±1 year), gender, and ethnicity. A screening tool (the Psychosis Screening Questionnaire27 for the White Caucasians, African-Caribbean, and Chinese samples; the Mini-International Neuropsychiatric Interview28 for the Japanese sample) was used to exclude the presence of psychotic symptomatology or a history of psychotic illness. Additional exclusion criteria for all participants included learning disabilities (based as an IQ < 70), current or past neurological illness, brain injury with loss of consciousness for more than 1 hour and suspected or confirmed pregnancy. Ethical approval was granted from the relevant Ethics Committees, and written informed consent was obtained from all participants.
Table 1.
Sociodemographic, Clinical, and Treatment Characteristics of Participants
| Japanese (n = 28 Pairs) | African-Caribbean (n = 18 Pairs) | White Caucasian (n = 29 Pairs) | Chinese (n = 50 Pairs) | |||||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | |
| Age | 25 (0.71) | 25.1 (1.07) | 26.6 (1.62) | 26.3 (1.45) | 26.2 (0.97) | 26.5 (0.96) | 24.3 (0.87) | 24.3 (0.87) |
| Gender (male) | 19 (67.8%) | 19 (67.8%) | 5 (26%) | 5 (26%) | 15 (51%) | 15 (51%) | 25 (50%) | 25 (50%) |
| Education | 16 (0.4) | 13.5 (0.5) | 14.6 (0.52) | 14 (0.66) | 15.4 (1.29) | 13.8 (0.85) | 13.4 (0.54) | 12.1 (0.49) |
| PANSS tot | NA | NA | NA | 63.6 (3.49)a | NA | 61.9 (2.68)b | NA | 97.5 (2.33) |
| PANSS Pos | NA | 15.32 (0.95) | NA | 14.6 (1.4)a | NA | 15.5 (0.99)b | NA | 25.3 (0.74) |
| PANSS Neg | NA | 18.9 (0.98) | NA | 18.3 (1.41)a | NA | 16 (1.17)b | NA | 18.8 (1.18) |
| PANSS GP | NA | 34.1 (1.51) | NA | 30.7 (1.61)a | NA | 30.4 (1.55)b | NA | 47.4 (1.14) |
| Duration of illness (mo) | 0 | 7.33 (1.66) | 0 | 1.70 (0.22) | 0 | 2.03 (0.25) | 0 | 8.26 (1.22) |
| Chlorpromazine-equivalent dose (mg/d) | 0 | 595 (489) | 0 | 202.5 (157.2) | 0 | 140.9 (149.2) | 0 | 0 |
| Duration of antipsychotic exposure (mo) | 0 | 1.55 (9.22) | 0 | 1.51 (0.31) | 0 | 2.42 (0.96) | 0 | 0 |
Note: PANSS, positive and negative syndrome scale; Pos, positive symptoms; Neg, negative symptoms; GP, general psychopathology; NA, not assessed. Values denote mean (with SE in brackets).
aData available for 16 out of 18 patients.
bData available for 22 out of 29 patients.
Image Acquisition
At all sites, volumetric MR images were acquired using a T1- weighted protocol. All sites used General Electric scanners with a field strength of 3T. The details of the image acquisition sequence varied between scanners, as reported in supplementary table S1.
Statistical Analysis of Sociodemographic and Clinical Parameters
Differences in demographics and clinical profile between groups were examined using 1-way ANOVA for parametric data and a chi-square test for nonparametric data, as implemented in the Statistical Package for the Social Sciences 17.0 (SPSS 17.0 for Windows).
Statistical Analysis of MRI Data
To minimize the impact of ethnicity and acquisition sequence on the results, we preprocessed and analysed each dataset independently; thus, our investigation can be thought of as a series of replication studies carried out on 4 independent datasets. Our aim was to identify differences in gray matter volume (GMV) between patients and controls that would be expressed consistently across all 4 datasets; in contrast, we did not test for differences across datasets as these would not be easily interpretable due to inconsistencies in sample size and acquisition sequence. The T1-weighted volumetric images were preprocessed using voxel-based morphometry as implemented in SPM8 software (http://www.fil.ion.ucl.ac.uk/spm) running under Matlab 7.1 (Math Works). In particular we used the Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) SPM8 toolbox29; this involves the creation of a dataset-specific template and the segmentation of each individual image using such template, with the aim of maximizing accuracy and sensitivity.30 The following steps were followed: (1) checking for scanner artifacts and gross anatomical abnormalities for each subject; (2) setting the image origin to the anterior commissure; (3) using the DARTEL toolbox to produce a high-dimensional normalization protocol; (4) checking for homogeneity across the sample; and (5) using standard smoothing (ie, 8mm). A “modulation step” was also included in the normalization in order to preserve the information about the absolute gray matter values.31 After the preprocessing, we obtained smoothed, modulated, normalized images for each of our 4 datasets. These were used to compare patients and controls using a 2-sample t test, with age, gender and total GMV modeled as covariates of no interest, for each dataset independently.
This analysis focused on 22 regions of interest that are implicated in contemporary animal19 and human11,20–24 models of schizophrenia; see table 2 for complete list. The identification of these regions was carried out using the Automated Anatomical Labeling atlas as implemented in PickAtlas software (http://fmri.wfubmc.edu/software/PickAtlas). Statistical inferences were made at P < .05 with Bonferroni correction for multiple comparisons resulting in a corrected P value < .0022. For each region and each dataset, we also report Cohen’s d to provide an indication of the effect size.32
Table 2.
Regions of Interest and Corresponding Coordinates, P Values and Cohen’s d for the Comparison “Controls > Patients” in Each Dataset
| Japanese | African-Caribbean | White Caucasian | Chinese | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | P a | d b | Coordinates | P a | d b | Coordinates | P a | d b | Coordinate | P a | d b | ||
| Anterior cingulate cortex | R | NS | NS | NS | 9 35 −6 | .0006 | 0.67 | ||||||
| L | NS | NS | NS | NS | |||||||||
| Inferior frontal gyrus | R | 27 38 −11 | .0001 | 1.06 | 41 20 −15 | .0011 | 1.08 | NS | 29 35 −19 | .0003 | 0.64 | ||
| L | −48 29 12 | .0007 | 0.90 | −51 15 0 | <.0001 | 1.75 | NS | −48 24 8 | .001 | 0.71 | |||
| Anterior insula | R | 35 18 −14 | .0001 | 1.05 | 41 18 −15 | .0014 | 1.15 | 45 6 6 | .0004 | 0.93 | 32 24 −20 | .0021 | 0.57 |
| L | −31 12 −9 | <.0001 | 1.28 | −45 16 −4 | .0007 | 1.05 | NS | NS | |||||
| Anterior hippocampus | R | 26 −4 29 | <.0001 | 1.18 | NS | NS | NS | ||||||
| L | NS | NS | NS | NS | |||||||||
| Posterior hippocampus | R | 29 −30 −11 | .0001 | 0.82 | NS | NS | NS | ||||||
| L | NS | NS | NS | NS | |||||||||
| Parahippocampus | R | 26 −4 −33 | .0006 | 1.44 | 20 −9 −27 | .0016 | 1.04 | NS | NS | ||||
| L | −28 0 −35 | .0006 | 0.92 | NS | −24 3 −32 | <.0001 | 1.15 | NS | |||||
| Middle temporal gyrus | R | 62 −48 6 | <.0001 | 1.43 | NS | 54 −54 10 | .001 | 0.84 | NS | ||||
| L | −63 −45 5 | <.0001 | 1.37 | NS | NS | NS | |||||||
| Superior temporal gyrus | R | 60 −48 17 | <.0001 | 1.37 | NS | 51 −30 17 | <.0001 | 2.42 | NS | ||||
| L | −64 −28 21 | <.0001 | 1.23 | NS | −45 −22 0 | <.0001 | 2.86 | NS | |||||
| Supramarginal gyrus | R | 66 −18 18 | .0001 | 1.03 | 49 −43 31 | <.0001 | 1.38 | 64 −46 30 | .0001 | 1 | NS | ||
| L | −66 −24 23 | <.0001 | 1.36 | NS | NS | NS | |||||||
| Thalamus | R | NS | NS | NS | NS | ||||||||
| L | NS | NS | NS | NS | |||||||||
| Caudatus | R | NS | NS | NS | NS | ||||||||
| L | −15 17 −8 | .0004 | 0.95 | NS | NS | NS | |||||||
Note: R, right; L, left; NS, not significant.
aStatistically significant after correction for multiple comparisons.
bCohen’s effect size.
Following the identification of regional differences between patients and controls across all 4 datasets, we performed a series of follow-up analyses to explore the relationship between GMV in these regions and 8 variables of interest including age, gender, years of education, PANSS score total, PANSS score positive, PANSS score negative, PANSS score general, and exposure to antipsychotic medication (ie, chlorpromazine-equivalent dose). For each dataset, this involved extracting the GMV from the peak voxels in each ethnic sample and performing a total of 8 correlation analyses with age, gender and total GMV as covariates of no interest, using SPSS (Statistical Package for Social Sciences – version 23). In these exploratory analyses, statistical inferences were made at P < .05 (uncorrected).
Results
Sociodemographic and Clinical Parameters
The sociodemographic and clinical characteristics of our samples are reported in Table 1.
With respect to sociodemographic parameters, within each group patients and controls were individually matched for ethnicity, gender and age (±1 year); hence these variables did not differ between patients and controls in any of the 4 groups (P > .05). Years of education differed between patients and controls in the Japanese group (t = 4.02, P < .001) but not in the African-Caribbean (t = 0.63, P = .53), White Caucasian (t = 1.03, P = .331), and Chinese (t = 1.8, P = .08) groups. Between groups, there were no significant differences in age (F[3,122] = 1.58, P = .19). However, there was a difference in education (F[3,122] = 6.39, P < .001) and gender (Pearson χ2 = 15.6, df = 3, P = .001). Newman-Keuls post hoc tests on education revealed that the Chinese group had fewer years of education than the Japanese (P = .019), African-Caribbean (P = .034), and White Caucasian (P = .057) groups. Chi-square tests on pairwise comparisons on gender indicated differences between the Japanese and African-Caribbean groups (P < .0001), between the African-Caribbean and Chinese groups (P = .007), and between the African-Caribben and White Caucasian (P = .01) groups. In addition, there were differences at trend level between the Japanese and Chinese groups (P = .054) and between the Japanese and White Caucasian groups (P = .079). In contrast, there were no differences between the Chinese and White Caucasian groups (P = .97)
With respect to clinical parameters, there was a difference in PANSS total (F[3,122] = 4165, P < .001), PANSS positive (F[3,122] = 35.08, P < .001), and PANSS general (F[3,112] = 36.82, P < .001) scores across groups (table 1). Post hoc t tests indicated that these differences were driven by the Chinese group having higher scores in PANSS total, PANSS positive, and PANSS general than the Japanese, African-Caribbean, and White Caucasian groups (P < .001); in contrast, the 4 groups did not differ in terms of PANSS negative scores (F[3,112] = 1, P = .39). In addition, there was a difference in duration of illness (F[3,122] = 6.76, P = .0003) across groups (table 1). Post hoc t tests indicated that these differences were driven by the Japanese group having longer duration of illness than the African-Caribbean (P = .01) and the White Caucasian (P = .007) groups, and by the Chinese group having longer duration of illness than the African-Caribbean (P = .005) and the White Caucasian (P = .004) groups. In contrast, there were no statistically significant differences in duration of illness between the Japanese and Chinese groups (P = .63) and between the African-Caribbean and White Caucasian groups (P = .83).
The 4 groups differed with respect to daily exposure to Chlorpromazine-equivalent dose of antipsychotic medication (F[3,122] = 34.7, P < .001; table 1). Newman-Keuls post hoc test revealed that the Chinese patients, who were all medication-naïve, differed from the other groups who were receiving medication (for all comparisons, P < .001). In addition, the Japanese group was receiving a higher dose than the African-Caribbean (P < .01) and the White Caucasian (P < .01) group; in contrast, the African-Caribbean and the White Caucasian groups did not differ between them (P = .36). In addition, the 4 groups differed with respect to duration of antipsychotic exposure (F[3,122] = 6.74, P = .0003). Newman-Keuls post hoc test revealed that the Chinese patients, who were all medication-naïve, differed from the other groups who had received medication; in contrast, there were no statistically significant differences in duration of antipsychotic exposure between the Japanese and the African-Caribbean (P = .82) groups; between the White Caucasian and the African-Caribbean (P = .11) groups; and between the White Caucasian and the Japanese (P = .1) groups.
Gray Matter Volume
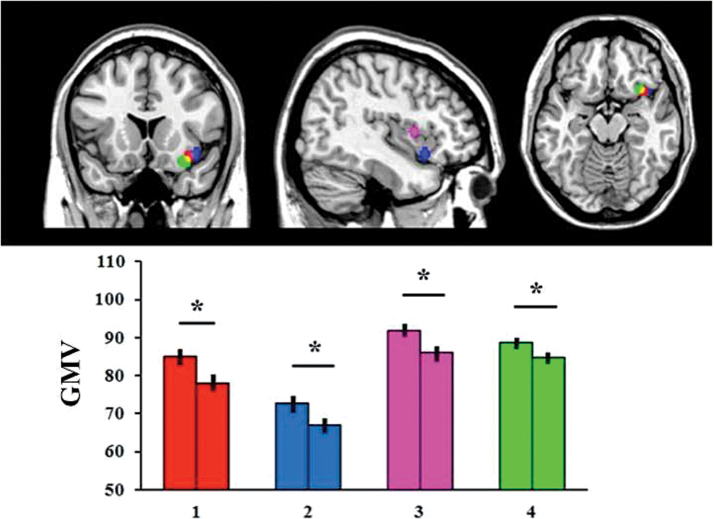
The right anterior insula showed decreased GMV in patients relative to controls (P < .05 corrected) consistently in all 4 groups (figure 1; table 2). Follow-up analyses indicated that gray matter in this region was not associated with age, gender, or years of education in any of the 4 samples (P < .05). Furthermore, gray matter in this region was not associated with any of the PANSS scores or exposure to antipsychotic medication (ie, chlorpromazine-equivalent dose) in any of the patient groups (P > .05).
Fig. 1.
Region of the right anterior insula that showed reduced gray matter volume in patients relative to healthy controls consistently in each of the 4 datasets. The asterisk (*) indicates a statistically significant difference at P < .05 with Bonferroni correction for multiple comparisons. GMV = gray matter volume measured as mm3 of gray matter per voxel. 1 = Japanese; 2 = African-Caribbean; 3 = White Caucasian; 4 = Chinese.
In addition, 3 regions (the left and right inferior frontal gyrus and the right supramerginal gyrus) showed decreased GMV in patients relative to controls (P < .05 corrected) in 3 out of the 4 groups (table 2); 6 regions (the left anterior insula, right middle temporal gyrus, left and right superior temporal gyrus, and left and right parahippocampus) showed decreased GMV in patients relative to controls (P<0.05 corrected) in 2 out of the 4 groups (table 2); and 6 regions (left caudatus, left supramarginal gyrus, left middle temporal gyrus, right anterior hippocampus, right posterior hippocampus, and right anterior cingulate cortex) showed decreased GMV in patients relative to controls (P < .05 corrected) in just 1 out of the 4 groups (table 2). Again, none of these regions showed an association with medication (ie, chlorpromazine-equivalent dose) in any of the 3 groups who had received pharmacological treatment (White Caucasian, African-Caribbean, and Japanese; P > .05).
The P value and effect size for the comparison “controls > patients” in each of our region of interest and each dataset are reported in table 2; in addition, mean and SE of GMV in these regions are reported for patients and controls separately in supplementary table S2.
Discussion
The clinical expression and even the incidence of schizophrenia vary depending on the ethnic origin of the patients under investigation; at present, it is unclear whether schizophrenia is characterized by a neuroanatomical signature that is expressed above and beyond these ethnic differences. Here we tested for consistent neuroanatomical alternations in 4 ethnically diverse groups of patients with first episode schizophrenia; as we preprocessed and analysed each dataset independently, using ethnic-specific templates, our investigation can be thought of as a series of replication studies carried out on 4 independent datasets. A strength of the present investigation is that we individually matched patients and controls for gender and age (±1 year); given the impact of these variables on GMV,33 this is likely to have increased the sensitivity of our statistical analysis. A further strength is that all participants were at the early stage of the illness, in contrast with the majority of previous studies that included patients at different stages of illness; again, this may have increased the sensitivity of our statistical analysis by minimizing the neuroanatomical variability associated with illness progression.34 We found a consistent reduction in the GMV of the right anterior insula; remarkably, this reduction was detected across all 4 ethnic groups despite differences in symptomatology, exposure to antipsychotic medication and image acquisition sequence. Furthermore, gray matter in this region was not associated with chlorpromazine-equivalent dose in any of the 3 groups who had received pharmacological treatment. This is consistent with the notion of a neuroanatomical signature of early schizophrenia above and beyond ethnic differences in the manifestation of the disease.
The right anterior insula has ipsilateral reciprocal connections with several prefrontal and temporal cortical regions,35 as well as with subcortical structures including the thalamus and the amygdala.36 It is considered part of the limbic system, and as such has been implicated in self-awareness,37 emotional regulation,38 and the processing of salience,39 3 aspects of mental functioning that are known to be affected in schizophrenia.40 There is a wealth of neuroimaging evidence suggesting that the right anterior insula is structurally and functionally impaired both in prodromal and established schizophrenia.23,24,41–48 For example, GMV in the right anterior insula is inversely related to both negative and positive symptomatology in people with an at-risk mental state, and is associated with individual risk of transition to psychosis42; in addition, a reduction of GMV in this region can be observed in patients with first episode and chronic schizophrenia relative to healthy controls,20–24,49 and is associated with severity of positive symptoms.50 The vast majority of above studies, however, were typically carried out in people of White Caucasian origin. The present investigation extends the existing literature by showing that gray matter reduction in the right anterior insula can be detected in the early stages of the illness irrespective of the ethnic characteristics of the patients. It should be noted, however, that the exact location of this reduction varied slightly from one dataset to the other (figure 1 and table 2). This is not surprising given that the 4 datasets were acquired using different scanners and acquisition parameters and were processed separately using ethnic-specific templates.
At present, we have no reliable biomarkers to identify individuals with schizophrenia, and the diagnosis of this disorder is still entirely reliant on clinical presentation and psychometric measures.51 The finding of consistent gray matter reduction in the right anterior insula, evident after a single episode of the illness irrespective of exposure to antipsychotic medication, suggests that this region might provide valuable information that could be used to inform diagnostic evaluations. Future studies could examine the clinical applicability of this finding in terms of accuracy, sensitivity, and specificity, using supervised machine learning techniques that allow inferences at individual rather than group level.52 Ideally, any evaluation of clinical applicability should go beyond the comparison between patients and controls, and consider the usefulness of the right anterior insular deficit reported here for distinguishing between alternative diagnostic options. For example, a recent meta-analytic investigation, including 193 published studies and a total of 15892 participants, found that gray matter loss in the right anterior insula is not specific to schizophrenia but can also be seen in bipolar disorder, depression, addiction, obsessive-compulsive disorder and anxiety.53 In light of the existing literature, therefore, we speculate that the right anterior insular deficit reported here may represent a transdiagnostic feature of most if not all Axis I disorders. Consistent with this interpretation, we note that schizophrenia’s diagnostic boundaries are not clear cut, and that its etiology, psychopathology and treatment tend to overlap with those of other Axis I disorders.54 In particular, right anterior insular alteration could reflect cognitive impairment and/or emotional dysfunction—2 deficits have been associated with anterior insular alteration and that tend to co-occur across developmental and adult disorders53,55,56
The observation of a neuroanatomical signature of schizophrenia in the right anterior insula across different ethnic groups, does not exclude the presence of further neuroanatomical alterations associated with the ethnic origin of the patients. For example, different sets of genes might confer vulnerability to schizophrenia in different ethnic groups, resulting in ethnicity-specific neuroanatomical alterations.57 Furthermore, the different patterns of symptoms expressed in different ethnic groups might be associated with different patters of neuroanatomical alteration. Indeed the observation of several regions showing gray matter differences in some but not all the 4 groups would appear to support this notion. We speculate that at least some of the group-specific effects reported in table 2 reflect real differences across ethnicities rather than the use of different scanners and acquisition sequences. In order to address this issue, one would need to scan all participants at a single centre or use a harmonised acquisition sequence.58 A further limitation of the present study is that there were a number of methodological differences across the 4 datasets; for example, the diagnosis of schizophrenia was made using the ICD-10 criteria in 3 groups and the DSM-V criteria in 1 group (see Material and Methods); exposure to antipsychotic medication differed between some of the groups (table 1); and, although all datasets were acquired using a 3T scanner, the specific acquisition sequence differed between some of the sites (supplementary table S1). Given the focus of the present study on effects that were expressed consistently across datasets, these methodological differences are most unlikely to account for our main finding in the right anterior insula. However, it is possible that these methodological differences prevented us from detecting further regions expressed consistent alterations across ethnicities. A third limitation relates to the definition of ethnicity. While race is defined based on a combination of genetic and cultural factors, ethnicity is considered an entirely social-political construct, referring to the sharing of a common culture, including origin, psychological attitudes, language, religion, and traditions.59 For this reason, we used self-ascribed ethnicity as a valid and reliable indicator of ethnicity, consistent with the existing literature.15,60,61 However, we acknowledge that self-declared ethnicity may not be fully accurate for a multitude of possible reasons including, for example, nonpaternity and failing to recognize or appreciate mixed heritage in previous generations.61 A final limitation is that, although patients and controls were matched for ethnicity, age and gender, other potential confounding variables were not controlled for. For example, parental socioeconomic status contributes to health disparities in most countries and accounts for some of the executive deficits observed in patients with schizophrenia relative to healthy controls62; however, we note that a recent investigation of the impact of this variable on brain structure did not detect a significant effect in the right anterior insula.62
In conclusion, our investigation provides evidence for a right anterior insular deficit in first episode schizophrenia, expressed above and beyond ethnic variations in incidence and clinical expression. In light of the existing literature, implicating this region in bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety, we speculate that this deficit may represent a transdiagnostic feature of Axis I disorders.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
QG was supported by the Chinese National Natural Science Foundation (Grant Nos. 81030027, 81227002 and 81220108013) and a CMB Distinguished Professorship Award by the Institute of International Education, USA (Award No. F510000/ G16916411). PD was supported by a King’s College London Translational Research Grant, NARSAD and the Psychiatry Research Trust. KK was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (23118001, 20118004, CBSN) and the Japan Agency for Medical Research and Development (H25-002, H26-012, Brain/MINDS). PD, CS, TRM, RMM, ASD and AM were supported by the UK National Institute of Health Research Biomedical Research Centre at the South London & Maudsley NHS Foundation Trust and the Institute of Psychiatry, Psychology & Neuroscience, King’s College London. The acquisition of the Japanese dataset was carried out under Grants-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network and Adolescent Mind & Self-Regulation [23118001 & 23118004]) and “Development of biomarker candidates for social behavior” of the Strategic Research Program for Brain Sciences by the MEXT. RMM and ASD are also supported by the UK National Institute of Health Research Biomedical Research Centre at the South London & Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology & Neuroscience, King’s College London.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fischer BA, Carpenter WT., Jr Will the Kraepelinian dichotomy survive DSM-V? Neuropsychopharmacology. 2009;34:2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalra G, Bhugra D, Shah N. Cultural aspects of schizophrenia. Int Rev Psychiatry. 2012;24:441–449. [DOI] [PubMed] [Google Scholar]
- 4. Lim CS, Subramaniam M, Poon LY, Chong SA, Verma S. Cross-ethnic differences in severity of symptomatology of individuals with first-episode schizophrenia spectrum disorder. Early Interv Psychiatry. 2011;5:242–248. [DOI] [PubMed] [Google Scholar]
- 5. Suhail K, Cochrane R. Effect of Culture and Environment on the Phenomenology of Delusions and Hallucinations. Int J Soc Psychiatry. 2002;48:126–138. [DOI] [PubMed] [Google Scholar]
- 6. Fearon P, Kirkbride JB, Morgan C, et al. Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP Study. Psychol Med. 2006;36:1541–1550. [DOI] [PubMed] [Google Scholar]
- 7. Hutchinson G, Takei N, Sham P, Harvey I, Murray RM. Factor analysis of symptoms in schizophrenia: differences between White and Caribbean patients in Camberwell. Psychol Med. 1999;29:607–612. [DOI] [PubMed] [Google Scholar]
- 8. Tapsell R, Mellsop G. The Contributions of Culture and Ethnicity To New Zealand Mental Health Research Findings. Int J Soc Psychiatry. 2007;53:317–324. [DOI] [PubMed] [Google Scholar]
- 9. Mechelli A, Riecher-Rössler A, Meisenzahl EM, et al. Neuroanatomical Abnormalities That Predate the Onset of Psychosis. Arch Gen Psychiatry. 2011;68:489–495. [DOI] [PubMed] [Google Scholar]
- 10. Benetti S, Pettersson-Yeo W, Hutton C, et al. Elucidating neuroanatomical alterations in the at risk mental state and first episode psychosis: a combined voxel-based morphometry and voxel-based cortical thickness study. Schizophr Res. 2013;150:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Smieskova R, Serafini G, Politi P, Borgwardt S. Neuroanatomical markers of genetic liability to psychosis and first episode psychosis: a voxelwise meta-analytical comparison. World J Biol Psychiatry. 2014;15:219–228. [DOI] [PubMed] [Google Scholar]
- 13. Koutsouleris N, Gaser C, Jager M, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39:1600–1612. [DOI] [PubMed] [Google Scholar]
- 14. Nenadic I, Gaser C, Sauer H. Heterogeneity of brain structural variation and the structural imaging endophenotypes in schizophrenia. Neuropsychobiology. 2012;66:44–49. [DOI] [PubMed] [Google Scholar]
- 15. Morgan KD, Dazzan P, Morgan C, et al. Differing patterns of brain structural abnormalities between black and white patients with their first episode of psychosis. Psychol Med. 2010;40:1137–1147. [DOI] [PubMed] [Google Scholar]
- 16. Chen Z, Deng W, Gong Q, et al. Extensive brain structural network abnormality in first-episode treatment-naive patients with schizophrenia: morphometrical and covariation study. Psychol Med. 2014;44:2489–501. [DOI] [PubMed] [Google Scholar]
- 17. Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21. [DOI] [PubMed] [Google Scholar]
- 18. Sheffield JM, Williams LE, Woodward ND, Heckers S. Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res. 2013;143:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer F, Louilot A. Consequences at adulthood of transient inactivation of the parahippocampal and prefrontal regions during early development: new insights from a disconnection animal model for schizophrenia. Front Behav Neurosci. 2014;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–2333. [DOI] [PubMed] [Google Scholar]
- 21. Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. [DOI] [PubMed] [Google Scholar]
- 22. Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwashiro N, Suga M, Takano Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res. 2012;137:124–131. [DOI] [PubMed] [Google Scholar]
- 26. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 27. Bebbington P, Nayani T. The Psychosis Screening Questionnaire. Int J Methods Psychiatr Res. 1995;5:11–19. [Google Scholar]
- 28. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 29. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 30. Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–327. [DOI] [PubMed] [Google Scholar]
- 31. Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Curr Med Imaging Rev. 2005;1:105–113. [Google Scholar]
- 32. Cumming G. The new statistics: why and how. Psychol Sci. 2014;25:7–29. [DOI] [PubMed] [Google Scholar]
- 33. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 34. Mane A, Falcon C, Mateos JJ, et al. Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res. 2009;114:136–143. [DOI] [PubMed] [Google Scholar]
- 35. Jakab A, Molnar PP, Bogner P, Beres M, Berenyi EL. Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain Topogr. 2012;25:264–271. [DOI] [PubMed] [Google Scholar]
- 36. Mufson EJ, Mesulam M-M, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. [DOI] [PubMed] [Google Scholar]
- 37. Straube T, Miltner WH. Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage. 2011;54:2534–2538. [DOI] [PubMed] [Google Scholar]
- 38. Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 39. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. The Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hasenkamp W, James GA, Boshoven W, Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res. 2011;125:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smieskova R, Fusar-Poli P, Aston J, et al. Insular volume abnormalities associated with different transition probabilities to psychosis. Psychol Med. 2012;42:1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pu W, Li L, Zhang H, et al. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141:15–21. [DOI] [PubMed] [Google Scholar]
- 45. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi T, Wood SJ, Soulsby B, et al. Diagnostic specificity of the insular cortex abnormalities in first-episode psychotic disorders. Prog Neuropsychopharmacology Biol Psychiatry. 2009;33:651–657. [DOI] [PubMed] [Google Scholar]
- 47. Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. [DOI] [PubMed] [Google Scholar]
- 48. Shapleske J, Rossell SL, Chitnis XA, et al. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex. 2002;12:1331–1341. [DOI] [PubMed] [Google Scholar]
- 49. Takahashi T, Wood SJ, Soulsby B, et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr Res. 2009;108:49–56. [DOI] [PubMed] [Google Scholar]
- 50. Cascella NG, Gerner GJ, Fieldstone SC, Sawa A, Schretlen DJ. The insula-claustrum region and delusions in schizophrenia. Schizophr Res. 2011;133:77–81. [DOI] [PubMed] [Google Scholar]
- 51. Prata D, Mechelli A, Kapur S. Clinically meaningful biomarkers for psychosis: a systematic and quantitative review. Neurosci Biobehav Rev. 2014;45:134–141. [DOI] [PubMed] [Google Scholar]
- 52. Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140–1152. [DOI] [PubMed] [Google Scholar]
- 53. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fusar-Poli P, Carpenter WT, Woods SW, McGlashan TH. Attenuated psychosis syndrome: ready for DSM-5.1? Annu Rev Clin Psychol. 2014;10:155–192. [DOI] [PubMed] [Google Scholar]
- 55. Takeuchi H, Taki Y, Sassa Y, et al. Regional gray matter density associated with emotional intelligence: evidence from voxel-based morphometry. Hum Brain Mapp. 2011;32:1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giuliani NR, Drabant EM, Bhatnagar R, Gross JJ. Emotion regulation and brain plasticity: expressive suppression use predicts anterior insula volume. Neuroimage. 2011;58:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gelernter J, Kranzler HR, Sherva R, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bauer CM, Jara H, Killiany R. Whole brain quantitative T2 MRI across multiple scanners with dual echo FSE: applications to AD, MCI, and normal aging. Neuroimage. 2010;52:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ford ME, Kelly PA. Conceptualizing and categorizing race and ethnicity in health services research. Health Services Res. 2005;40(Pt 2):1658–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amminger GP, Schafer MR, Klier CM, et al. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol Psychiatry. 2012;17:1150–1152. [DOI] [PubMed] [Google Scholar]
- 61. Thomas RH, Drew CJ, Wood SE, Hammond CL, Chung SK, Rees MI. Ethnicity can predict GLRA1 genotypes in hyperekplexia. J Neurol Neurosurg Psychiatry. 2015;86:341–343. [DOI] [PubMed] [Google Scholar]
- 62. Yeo RA, Martinez D, Pommy J, et al. The impact of parent socio-economic status on executive functioning and cortical morphology in individuals with schizophrenia and healthy controls. Psychol Med. 2014;44:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]