Abstract
First-episode schizophrenia (FES) spectrum disorders are associated with pronounced cognitive dysfunction across all domains. However, less is known about the course of cognitive functioning, following the first presentation of psychosis, and the relationship of cognition to clinical course during initial treatment. The present longitudinal study examined the magnitude of neurocognitive impairment, using the MATRICS Consensus Cognitive Battery, in patients experiencing their first episode of psychosis at baseline and after 12 weeks of randomized antipsychotic treatment with either aripiprazole or risperidone. At baseline, FES patients evidenced marked impairments in cognitive functioning. Notably, performance on the mazes task of planning and reasoning significantly predicted the likelihood of meeting stringent criteria for positive symptom remission during the first 12 weeks of the trial. Performance on indices of general cognitive function, working memory, and verbal learning improved over time, but these improvements were mediated by improvements in both positive and negative symptoms. We did not detect any differential effects of antipsychotic medication assignment (aripiprazole vs risperidone) on cognitive functioning. Our results suggest that a brief paper-and-pencil measure reflecting planning/reasoning abilities may index responsivity to antipsychotic medication. However, improvements in cognitive functioning over time were related to clinical symptom improvement, reflecting “pseudospecificity.”
Key words: cognition, general cognitive function, psychosis, planning, aripiprazole, risperidone
Introduction
In recent years, cognitive dysfunction has increasingly been considered a core feature of schizophrenia and related psychotic disorders.1–3 Moderate deficits are observed years before the onset of full psychotic symptoms, escalating in severity during adolescence.4,5 By the onset of psychosis, deficits are nearly as severe as those observed in patients with chronic illness; studies of patients in the first episode of illness consistently demonstrate a generalized deficit of 1 SD or more below the mean performance of healthy individuals.6–8 While the presence of severe cognitive deficits in first-episode schizophrenia (FES) spectrum disorders is well established, the relationship between these deficits and early treatment course remains unclear. Most of the studies reviewed in recent meta-analyses of FES patients utilized a cross-sectional design.7,8 Moreover, many of these studies examined patients after clinical stabilization, several months after initiation of antipsychotic treatment.
Due to the relative difficulty in conducting large-scale, controlled treatment trials, only a few prospective studies have examined the course of cognitive deficits in FES patients undergoing initial treatment with antipsychotic medications. Consequently, several critical questions remain unresolved. First, it is unclear to what extent cognitive performance at baseline can predict clinical response to antipsychotic medication. Approximately 40% of patients will fail to respond to the initial antipsychotic trial9 (see also Robinson et al,10 this issue), and prognostic biomarkers are lacking. In treatment of chronic schizophrenia, the risk of nonresponse can often be detected within the first 2 weeks of treatment,11 but early nonresponse is not prognostic for FES patients.12 Baseline predictive indicators can help explicate the neurobiology of antipsychotic response and, ideally, could guide deployment of clinical resources, given that clinical nonresponse accounts for the large majority of total health resource utilization associated with psychosis.13 The Center for Intervention Development and Advanced Research (CIDAR) at the Zucker Hillside Hospital (ZHH; Malhotra,14 this issue) was designed with the primary aim of identifying biomarkers that can predict and explain the heterogeneity of response to antipsychotic treatment in FES.
A second unresolved question is the degree to which antipsychotic medication can ameliorate cognitive deficits in FES. The first prospective studies examining cognitive performance before and after 12 weeks of exposure to initial treatment demonstrated modest improvements (effect sizes between 0.2 and 0.4 SDs) across most cognitive domains.15–17 However, a comparison with healthy controls assessed across the same two time-points indicated that practice effects likely accounted for nearly all of the gains.18 The MATRICS Consensus Cognitive Battery (MCCB) was specifically designed with alternate forms to minimize practice effects for use in controlled treatment trials,19 but to date it has never been examined in an acute trial of FES patients; the present study is the first to do so.
Moreover, the extent to which cognitive improvement after treatment may be secondary to clinical changes (reduction of positive and/or negative symptoms) remains an open question. While studies in chronic patients typically demonstrate modest but significant correlations between cognition and clinical symptomatology (both cross-sectionally and over time20), prior studies in FES patients have yielded mixed results. Some studies have suggested that correlations between cognitive improvement and symptom reduction were specific to first-generation antipsychotics only,16,17 while others have demonstrated broad correlations between cognitive and clinical change.21 Moreover, cross-sectional and longitudinal studies in FES patients have consistently demonstrated correlations of cognitive deficits with negative symptoms.6,22–24
Finally, while studies of cognitive effects have typically demonstrated broad similarities across various second-generation antipsychotic agents,15,21 aripiprazole has not yet been well studied. Only one comparative study has been conducted in a FES cohort; in an unblinded 6-month trial conducted in China, aripiprazole demonstrated broadly similar effects to risperidone, except for a failure to enhance processing speed.25 By contrast, in a comparison of 4 second-generation antipsychotics in a (mostly) chronic cohort, Riedel et al26 reported that aripiprazole demonstrated the greatest ability to improve reaction time and attention. Two other studies in chronic patients27,28 have suggested that aripiprazole may have distinctive cognitive properties (both positive and negative) as a result of its unique mechanism of action (partial agonism rather than pure antagonism at the dopamine D2 receptor). Moreover, aripiprazole (but not risperidone) has demonstrated modestly beneficial effects on negative symptoms (Robinson et al,10 this issue), which may be critical to cognitive change.29 Thus, it is plausible that differential cognitive effects of aripiprazole vs risperidone may also be observed.
The present study was, therefore, designed to prospectively test several unanswered questions in the treatment of FES: (1) Can baseline cognitive measures predict clinical response to antipsychotics?; (2) Can antipsychotic medications ameliorate cognitive deficits in first-episode patients, independent of practice effects and clinical changes?; and (3) Are there differences in the cognitive effects of aripiprazole vs risperidone? As noted above, the present study utilized the MCCB, which is designed to reliably and efficiently assess each of 7 dissociable cognitive factors that have been replicably identified in the schizophrenia literature.30 These questions were addressed in the context of a 12-week, blinded controlled study of aripiprazole vs risperidone, as described in greater detail elsewhere in this volume.10
Methods
Full methodological details regarding the 12-week randomized clinical trial can be found in the accompanying manuscript by Ref: 10. Details of the ZHH CIDAR initiative that are principally relevant to the current study are described below.
Participants
Table 1 provides demographic information about the sample. The current study included 175 participants (73% male; mean age of 22.55±5.67 y) at study entry with a schizophrenia spectrum disorder (schizophrenia, n = 123 [67%]; schizophreniform disorder, n = 35 [23%]; schizoaffective disorder, n = 5 [3%]; or psychotic disorder not otherwise specified, n = 12 [7%]). Participants were recruited from a consortium of 10 collaborative medical centers including 8 in the greater New York City area, as well as San Antonio, Texas and Calgary, Alberta, Canada. The sample was racially/ethnically and socioeconomically diverse, as study sites were located in urban or suburban areas and served diverse communities. Study data were collected from December of 2005 through April of 2013. Study inclusion and exclusion criteria are summarized in supplementary table 1. Inclusion criteria required all participants to have less than 2 weeks of prior antipsychotic exposure and 28% of our sample (n = 49) was completely antipsychotic medication naive. Any antipsychotics being taken at the time of study entry were discontinued and participants started randomized medication without a withdrawal period. After complete description of the study, written informed consent was obtained from all adult participants. For participants younger than 18 years, written parental consent and written participant assent were obtained. The study was conducted under the auspices of the Feinstein Institute for Medical Research Institutional Review Board (IRB) as the coordinating center and the IRBs of the clinical sites.
Table 1.
Demographic and Clinical Characteristics of Full Sample (N = 175) at Baseline, and Those Who Did (N = 109) and Did Not (N = 66) Complete the NP Evaluation at Both Time Points
| Full Sample | Completed Full NP | Did Not Complete NP | |||||
|---|---|---|---|---|---|---|---|
| N = 175 | N = 109 | N = 66 | |||||
| N | % | N | % | N | % | P | |
| Male | 128 | 73.14 | 82 | 0.75 | 46 | 0.70 | .424 |
| Racial/ethnic minority | 135 | 77.14 | 84 | 0.77 | 51 | 0.77 | .975 |
| Randomized to aripiprazole | 93 | 53.14 | 60 | 0.55 | 33 | 0.50 | .517 |
| Randomized to risperidone | 82 | 46.86 | 49 | 0.45 | 33 | 0.50 | |
| Mean | SD | Mean | SD | Mean | SD | ||
|---|---|---|---|---|---|---|---|
| Age (y) | 22.55 | 5.67 | 22.10 | 5.40 | 23.29 | 6.07 | .179 |
| Paternal education | 13.18 | 3.34 | 12.69 | 3.44 | 14.31 | 2.81 | .014 |
| Paternal Hollingshead social class | 3.92 | 1.71 | 3.99 | 1.63 | 3.76 | 1.90 | .506 |
| Maternal education | 12.95 | 2.97 | 12.72 | 3.08 | 13.46 | 2.69 | .186 |
| Maternal Hollingshead social class | 3.78 | 2.02 | 3.90 | 2.08 | 3.54 | 1.89 | .363 |
| WRAT-3 estimated premorbid IQ | 97.07 | 12.41 | 95.79 | 12.99 | 99.13 | 11.21 | .127 |
| MCCB general composite, baseline | 33.52 | 9.36 | 33.70 | 9.62 | 33.22 | 9.01 | .749 |
| Handedness (percent right-handed) | 0.89 | 0.32 | 0.85 | 0.36 | 0.95 | 0.21 | .093 |
| BPRS Positive symptoms | 14.55 | 3.59 | 14.48 | 3.35 | 14.67 | 3.97 | .742 |
| SANS Affective flattening | 1.80 | 0.97 | 1.92 | 1.02 | 1.62 | 0.84 | .050 |
| SANS Alogia | 2.03 | 1.06 | 2.08 | 1.08 | 1.94 | 1.04 | .387 |
| SANS Avolition | 2.08 | 1.03 | 2.21 | 1.07 | 1.88 | 0.92 | .041 |
| SANS Anhedonia | 2.13 | 0.97 | 2.15 | 0.98 | 2.11 | 0.95 | .782 |
Note: BPRS, Brief Psychiatric Rating Scale; MCCB, MATRICS Consensus Cognitive Battery; NP, neuropsychological; SANS, Scale for the Assessment of Negative Symptoms; WRAT-3, Wide-Range Achievement Test, 3rd edition.
Clinical Assessment Measures
Initial diagnosis for eligibility was established with the Structured Clinical Interview of Axis I DSM-IV Disorders (SCID).31 These data were later reviewed in a consensus conference for final diagnostic assignment.32 Assessments done at baseline, weekly for 4 weeks, and then every 2 weeks included: Brief Psychiatric Rating Scale-Anchored version (BPRS-A)33; Hillside clinical trials version of the Schedule for Assessment of Negative Symptoms (SANS)34; and the Clinical Global Impression Scale (CGI).35
Treatment Protocol
The acute treatment study phase lasted 12 weeks (85.2±5.7 d elapsed from baseline to follow-up on average). Participants were stratified by site, previous antipsychotic exposure (none vs any), and diagnosis (psychotic disorder Not Otherwise Specified vs other eligible diagnoses) and were randomly assigned on a 1:1 basis to double-masked treatment with either aripiprazole (5–30mg/d) or risperidone (1–6mg/d).
Antipsychotic Treatment Response Criteria
Response to antipsychotic treatment was based on a priori criteria that was defined as follows: (1) a rating of 3 (“mild”) or less on all of the following items of the BPRS-A: conceptual disorganization, grandiosity, hallucinatory behavior, unusual thought content and (2) a CGI Improvement rating of “much improved” or “very much improved.” These criteria were required to be sustained on 2 consecutive rating assessments for a patient to be considered as a responder. Time of response was the date of the first of the 2 consecutive ratings meeting these criteria.
Cognitive Evaluation With the MCCB
The MCCB is a battery of neuropsychological tests that were rigorously identified for inclusion as a standardized approach to the serial assessment of key cognitive deficits in medication trials for patients with schizophrenia and related disorders.36 The MCCB includes 7 cognitive domains: Speed of Processing (semantic fluency [animal naming], Brief Assessment of Cognition in Schizophrenia [BACS] symbol coding, and part A of the Trail Making test); Working Memory (letter-number span and spatial span); Reasoning and Problem Solving (Neuropsychological Assessment Battery [NAB] Mazes); Verbal Learning (Hopkins Verbal Learning Test-Revised [HVLT-R]); Visual Learning (Brief Visuospatial Memory Test-Revised [BVMT-R]); Attention/Vigilance (Continuous Performance Test, Identical Pairs [CPT-IP]); and Social Cognition (Mayer-Salovey-Caruso Emotional Intelligence Test [MSCEIT], Managing Emotions). In addition to the 7 domain scores, the MCCB provides an overall composite score that indexes general cognitive performance across domains. Trained psychometricians administered the battery, and administration of all MCCB tests adhered to procedures described in the test manual.36
Statistical Procedures
Quality Control.
Raw data for continuous variables were plotted using standard procedures (eg, histograms and Q-Q plots) and examined for normality. Extreme outlier data points (defined as a value whose distance from the nearest quartile was greater than 1.5 times the interquartile range in either direction) were removed.37 Cognitive performance data were adjusted for age, sex, site (using 2 site indicator variables; one representing the largest site [ZHH] as the reference group and one representing the Calgary site, which had significantly higher cognitive scores compared to all other sites, as the reference group), and racial/ethnic minority status.
Baseline Cognitive Predictors of Response to Treatment.
Initial analyses compared the rates of antipsychotic treatment response (based on criteria defined above) over the 12-week trial as a function of cognitive performance at study entry using the MCCB. These analyses used Cox regression procedures, under a proportional hazards model, to assess clinical response status and time as a function of baseline cognitive status and relevant covariates.
Change in Cognitive Performance in Relation to Antipsychotic Medication and Psychosis Symptomatology.
Next, we examined whether cognitive performance in FES is: (1) stable, declines, or improves after 3 months time; (2) dissimilar in trajectory depending on whether patients were randomized to aripiprazole (a partial dopamine agonist) vs risperidone (a dopamine antagonist); and (3) mediated by improvements in positive and negative symptoms (ie, if changes could be due to “pseudospecificity,” simply reflecting antipsychotic effects on symptoms). Analyses of longitudinal patterns of change in cognitive performance as a function of antipsychotic drug and psychosis symptomatology were carried out using a series of linear mixed-model approaches in SAS.38 Factors in the models were time, medication type, time × medication type interaction, the sum of BPRS remission items, and the 4 global SANS scores. A random intercept in the mixed models was used to account for correlation of measurements over time among the participants; the correlational type was assumed to be unstructured. The difference in slopes of the outcomes between the 2 treatment groups was assessed using a group-by-time interaction term in the mixed models.
Results
Premorbid IQ estimated from the word reading subtest of the Wide-Range Achievement Test, 3rd edition (WRAT-3) was in the average range (mean = 97.07±12.41), and participants were predominantly right-handed (89% ± 32%). As shown as part of table 1, 109 of 175 participants completed cognitive evaluations at baseline and follow-up. Completers and noncompleters were not significantly different in key clinical variables including age, race/ethnicity, antipsychotic type, IQ, and most symptom measures, except for affective flatting and avolition, which were slightly higher in completers. As shown in table 2, our FES cohort demonstrated marked impairments in cognitive functioning across domains on the MCCB at baseline, with an average T score of 33.8±11.8, more than 1.5 SDs below the general population mean. On average, scores improved slightly from baseline to 3-month follow-up across domains, although performance was still markedly impaired with an average T score of 35.5±11.1. Deficits in processing speed were the most pronounced of all domains, and social cognition was least impaired relative to the other domains. Table 2 also provides summaries of the raw score data from each individual test across time.
Table 2.
MCCB Standardized Domain Scores and Raw Subtest Scores at Baseline
| Baseline | Week 12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min. | Max. | N | Mean | SD | Min. | Max. | |
| MCCB domain (T scores) | ||||||||||
| General cognitive function | 172 | 33.39 | 9.39 | 8.20 | 57.00 | 109 | 35.49 | 8.61 | 10.50 | 58.29 |
| Speed of processing | 170 | 30.18 | 15.05 | −3.00 | 71.00 | 108 | 30.96 | 12.25 | −5.00 | 64.00 |
| Working memory | 165 | 32.45 | 14.38 | −10.00 | 63.00 | 106 | 37.92 | 12.38 | 6.00 | 76.00 |
| Reasoning/problem solving | 164 | 36.85 | 10.85 | 15.00 | 62.00 | 105 | 38.75 | 10.83 | 16.00 | 63.00 |
| Verbal learning | 172 | 35.39 | 8.79 | 17.00 | 72.00 | 111 | 37.56 | 8.94 | 19.00 | 67.00 |
| Visual learning | 168 | 32.51 | 12.47 | −3.00 | 61.00 | 109 | 33.98 | 12.51 | −1.00 | 59.00 |
| Attention/vigilance | 125 | 32.12 | 10.51 | 6.00 | 57.00 | 88 | 31.85 | 11.64 | 6.00 | 62.00 |
| Social cognition | 152 | 37.21 | 13.07 | 4.00 | 67.00 | 101 | 37.43 | 11.99 | 14.00 | 63.00 |
| MCCB subtest (raw scores) | ||||||||||
| BACS symbol coding | 172 | 44.93 | 13.71 | 11.00 | 80.00 | 111 | 45.86 | 11.66 | 9.00 | 80.00 |
| Semantic fluency (animals) | 172 | 19.09 | 7.20 | 3.00 | 38.00 | 109 | 18.91 | 5.76 | 8.00 | 41.00 |
| Trails A | 170 | 40.13 | 19.57 | 16.72 | 109.12 | 111 | 37.01 | 14.91 | 15.56 | 83.65 |
| Letter-number span | 166 | 11.96 | 4.38 | 1.00 | 22.00 | 107 | 13.07 | 3.62 | 4.00 | 21.00 |
| Spatial span | 170 | 13.68 | 4.22 | 3.00 | 25.00 | 111 | 15.41 | 4.01 | 7.00 | 28.00 |
| NAB mazes | 164 | 16.29 | 6.84 | 0.00 | 26.00 | 105 | 17.69 | 6.28 | 0.00 | 26.00 |
| HVLT-R total score, trials 1–3 | 172 | 20.33 | 5.74 | 3.00 | 35.00 | 111 | 21.79 | 5.55 | 5.00 | 34.00 |
| BVMT-R total score, trials 1–3 | 168 | 19.80 | 7.02 | 0.00 | 35.00 | 109 | 20.70 | 7.07 | 1.00 | 35.00 |
| CPT-IP dʹ | 125 | 1.95 | 0.72 | −0.07 | 3.64 | 88 | 1.95 | 0.76 | 0.27 | 3.94 |
| MSCEIT managing emotions | 152 | 84.44 | 11.47 | 54.54 | 109.15 | 101 | 83.97 | 10.84 | 54.58 | 107.96 |
Note: Domain scores are presented as T scores (M = 50, SD = 10) and domain subtest scores are presented as raw data scores (eg, number of seconds to complete Trails A). BACS, Brief Assessment of Cognition in Schizophrenia; BVMT-R, Brief Visuospatial Memory Test - Revised; CPT-IP, Continuous Performance Test - Identical Pairs; HVLT-R, Hopkins Verbal Learning Test - Revised; MCCB, MATRICS Consensus Cognitive Battery; NAB, Neuropsychological Assessment Battery; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test.
Baseline Predictors of Response
In Cox regression analyses, after controlling for potential demographic confounds, as well as the sum of BPRS remission items and SANS negative symptoms at study entry, general cognitive performance at baseline significantly predicted 12-week response rates to antipsychotic treatment (Wald statistic = 4.62, P = .032). Further, adding this term significantly improved model fit (χ2 change: 4.73, P = .030). To determine if a specific process drove the general cognitive effect, subsequent analyses examined the 7 MCCB domains individually for association to treatment response. After strict Bonferroni correction for multiple comparisons, baseline performance in the MCCB reasoning domain (as measured by the NAB mazes subtest) was a significant and robust predictor of response to antipsychotic treatment (Wald statistic = 10.02, P = .002) when entered into the model (χ2 change: 9.94, P = .002). As shown in table 3, in both instances, individuals who evidenced better cognitive functioning in general and specifically in the domain of reasoning at baseline responded faster to subsequent antipsychotic treatment as compared to patients who were relatively more impaired.
Table 3.
Baseline Cognitive Predictors of Treatment Response
| Variable | B | SE | Wald | P value | OR | 95% CI lower | 95% CI upper | |
|---|---|---|---|---|---|---|---|---|
| Block 1 | Sex | −0.050 | 0.023 | 4.807 | .028 | 0.951 | 0.909 | 0.995 |
| Age | 0.311 | 0.269 | 1.338 | .247 | 1.365 | 0.806 | 2.311 | |
| Minority status | 0.179 | 0.294 | 0.372 | .542 | 1.196 | 0.673 | 2.127 | |
| Site indicator 1 (ZHH) | −0.028 | 0.242 | 0.013 | .909 | 0.973 | 0.605 | 1.563 | |
| Site indicator 2 (Calgary) | 1.347 | 0.653 | 4.251 | .039 | 3.844 | 1.069 | 13.825 | |
| Block 2 | BPRS Positive symptoms | −0.048 | 0.036 | 1.826 | .177 | 0.953 | 0.889 | 1.022 |
| SANS Affective flattening | 0.029 | 0.126 | 0.054 | .816 | 1.030 | 0.804 | 1.318 | |
| SANS Alogia | 0.037 | 0.127 | 0.086 | .770 | 1.038 | 0.809 | 1.332 | |
| SANS Avolition | 0.058 | 0.147 | 0.158 | .691 | 1.060 | 0.795 | 1.414 | |
| SANS Anhedonia | −0.215 | 0.167 | 1.66 | .198 | 0.807 | 0.582 | 1.119 | |
| Block 3 | General cognitive function | 0.03 | 0.014 | 4.622 | .032 | 1.03 | 1.003 | 1.059 |
| Block 1 | Age | −0.064 | 0.033 | 3.723 | .054 | 0.938 | 0.879 | 1.001 |
| Sex | 0.206 | 0.370 | 0.309 | .578 | 1.229 | 0.595 | 2.539 | |
| Minority status | 0.818 | 0.401 | 4.166 | .041 | 2.265 | 1.033 | 4.968 | |
| Site indicator 1 (ZHH) | −0.059 | 0.305 | 0.037 | .847 | 0.943 | 0.519 | 1.714 | |
| Site indicator 2 (Calgary) | 1.535 | 0.706 | 4.725 | .030 | 4.642 | 1.163 | 18.533 | |
| Block 2 | BPRS Positive symptoms | −0.101 | 0.048 | 4.477 | .034 | 0.904 | 0.823 | 0.993 |
| SANS Affective flattening | −0.037 | 0.151 | 0.060 | .806 | 0.964 | 0.717 | 1.295 | |
| SANS Alogia | 0.034 | 0.172 | 0.039 | .843 | 1.035 | 0.739 | 1.448 | |
| SANS Avolition | 0.138 | 0.184 | 0.562 | .453 | 1.148 | 0.801 | 1.645 | |
| SANS Anhedonia | −0.334 | 0.204 | 2.686 | .101 | 0.716 | 0.481 | 1.068 | |
| Block 3 | Speed of processing | 0.002 | 0.016 | 0.009 | .926 | 1.002 | 0.970 | 1.034 |
| Working memory | 0.018 | 0.017 | 1.038 | .308 | 1.018 | 0.984 | 1.053 | |
| Verbal memory | 0.000 | 0.021 | 0.000 | .987 | 1.000 | 0.960 | 1.041 | |
| Visual memory | 0.007 | 0.020 | 0.113 | .737 | 1.007 | 0.968 | 1.047 | |
| Attention/vigilance | −0.019 | 0.018 | 1.079 | .299 | 0.981 | 0.947 | 1.017 | |
| Social cognition | −0.013 | 0.013 | 0.895 | .344 | 0.987 | 0.962 | 1.014 | |
| Block 4 | Reasoning/problem solving | 0.051 | 0.018 | 8.081 | .004 | 1.053 | 1.016 | 1.091 |
Note: The upper half of the table displays the results of general cognitive function, and the lower half of the table displays the results of reasoning after controlling for the other 6 cognitive domains. B, regression coefficient. Bold values indicate significant P values for Table 3; BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; SE, standard error of B; ZHH, Zucker Hillside Hospital.
We then examined the specificity of reasoning relative to the other cognitive domains. After controlling for age, sex, minority status, and site on step 1, baseline SANS negative symptoms on step 2, and the 6 other MCCB cognitive domains on step 3, adding reasoning on step 4 significantly improved the model fit (χ2 change = 8.03, P = .005), and reasoning continued to evidence a significant association to treatment response (B = 0.051, SE = 0.018, Wald = 8.08, P = .004). Thus, reasoning/problem solving at baseline specifically predicted treatment response, as no other cognitive domains were significant (P > .29; table 3). Further, the association of general cognitive function to treatment response was primarily driven by reasoning/planning performance on the mazes task.
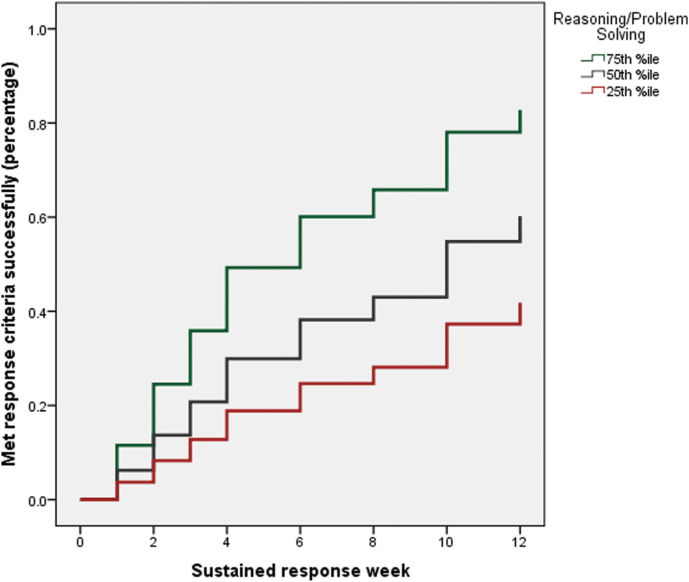
To better understand the association between MCCB reasoning and treatment response, we examined the trajectory of sustained response rates across the 12-week treatment phase. In order to perform a graphical examination, we classified individuals as high performers on NAB mazes (defined as those scoring at or above the 75th percentile within the sample), average performers (those who scored below the 75th percentile and above the 25th percentile), and low performers (those who performed at or below the 25th percentile). As shown in figure 1, only 20% of patients in the low performing group responded favorably to antipsychotic treatment by the midpoint of the trial (week 6) compared to 60% of the high performing group. By week 12, 40% of the low group responded to treatment compared to 80% of patients in the high group. Response rates for the average/middle group fell in-between the high and low groups.
Fig. 1.
Result of Cox regression analysis of MATRICS Consensus Cognitive Battery reasoning/problem solving performance at baseline in relation to 12-week treatment response rates. As can be seen, ~50% of the patients who performed relatively well on the task at baseline (defined as those scoring at or above the >75th percentile within the sample) successfully responded to antipsychotic treatment after 6 weeks, whereas only ~20% of poor performers (ie, those who performed at or below the 25th percentile) met criteria after the same period of time.
Changes in Cognitive Performance
During the 3 months of treatment, general cognitive function improved by approximately 2 points, working memory improved by 5 points, and verbal learning improved by 2 points. No other MCCB domain evidenced significant change from baseline to follow-up (supplementary table 2). In the sections below, we report the results of examining how these changes in cognition were influenced by clinical variables, specifically medication type and symptom change.
Comparison of Aripiprazole and Risperidone in Cognitive Performance Change
No differential effects of aripiprazole vs risperidone on cognitive performance were observed. Specifically, there were no statistically significant antipsychotic medication type (aripiprazole vs risperidone) × time interactions for any cognitive variables (all P > .35).
Changes in Cognitive Performance in Relation to Symptom Improvement
As described above and in supplementary table 2, general cognitive function, working memory, and verbal learning improved from baseline to 12-week follow-up. To test whether these improvements were dependent upon reductions in clinical symptomatology, we conducted a series of mediation analyses. For these analyses, we employed the causal-steps approach,39,40 which states that 4 steps in the causal process must be true for full mediation to be present: (1) The total effect of the predictor on the outcome must be significant; (2) The effect of the predictor on the mediator must be significant; (3) The effect of the mediator on the outcome controlling for the predictor must be significant; and (4) The direct effect of the predictor on the outcome adjusting for the mediator must be nonsignificant.39,40 We used the BPRS remission items and SANS global measures of affective flattening, alogia, avolition-apathy, and asociality-anhedonia as mediating variables in these models, adding them after we examined the main, unconditional effect of antipsychotic treatment.
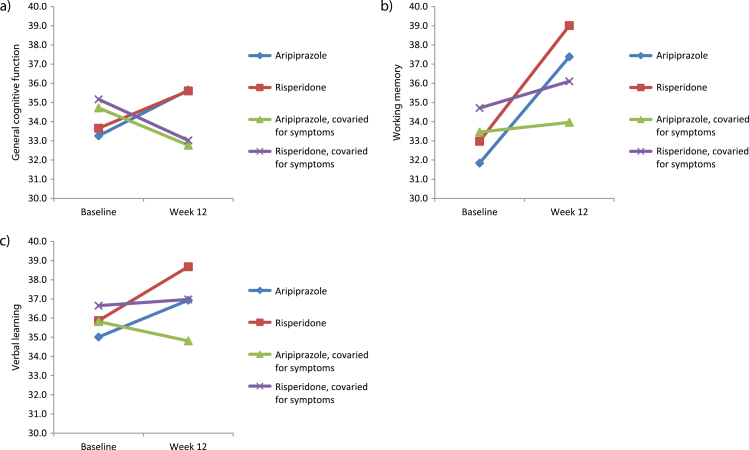
Details of the mediation analysis are provided in figure 1 and supplementary table 3. In all cases, changes in cognitive performance were either partially or fully mediated by changes in BPRS remission scores and/or global alogia as rated on the SANS. Specifically, improvements in general cognitive functioning were partially mediated by changes in positive symptoms and fully mediated by improvements in alogia. Improvements in working memory were fully mediated by reductions in BPRS remission scores and partially mediated by reductions in alogia. Finally, the mediating effect of BPRS remission scores on improvements in verbal learning was inconclusive because the direct effect of BPRS on verbal learning controlled for time was not significant; however, improvements in verbal learning were fully mediated by reductions in alogia. These results are displayed pictorially in figure 2, which show (1) that antipsychotic medication type had no differential affect on cognition and (2) that the simple slopes of the change from baseline to follow-up flatten out over time after covarying for clinical improvement. Additional results of this analysis are presented in supplementary table 4 for the other cognitive domains. Notably, after controlling for symptoms, social cognition declined significantly over time (P = .032).
Fig. 2.
Results of mediation analysis of clinical symptoms on cognitive change. Improvements in clinical symptoms partially and/or fully mediated improvements in (a) general cognition, (b) working memory, and (c) verbal learning.
Discussion
In this study, we examined baseline cognitive predictors of antipsychotic treatment response in a large sample of medication-naive or minimally treated FES patients. We found that better general cognitive functioning at baseline, which was driven by performance on a test of planning and reasoning, was associated with a faster rate of positive symptom response. We also compared the effectiveness of aripiprazole and risperidone in improving cognitive function, and our results indicate that differential medication effects were not detectable. Lastly, small but significant improvements in general cognitive function, working memory, and verbal learning were seen over time, but these findings were mediated by improvements in clinical symptomatology.
The most striking result of our study was the ability of baseline cognitive performance, specifically the MCCB reasoning subtest, to predict 12-week clinical response to risperidone or aripiprazole. Our clinical response measure was strictly defined as an absence of psychotic-level positive symptoms observed over 2 consecutive assessments (see Ref: 10, this issue). As demonstrated in figure 1, patients in the top quartile of scores on MCCB reasoning were twice as likely to respond as those in the bottom quartile. Notably, our results were highly specific to the reasoning domain score: (1) Similar results were not observed for other cognitive domains and (2) Multiple regression demonstrated no attenuation of results for the reasoning test when all other domain scores were entered into the model first. This specificity is striking, given that the large majority of the cognitive variance in schizophrenia is accounted for by generalized, as opposed to specific, deficits.41,42
While pretreatment cognitive performance has not been widely studied as a clinically relevant prognostic biomarker, a few studies have suggested that this may be a promising avenue for future research. For example, in an early study of FES patients treated with fluphenazine, we demonstrated that an “attention” domain score (which included working memory measures as well) significantly predicted positive symptom response within the first year.43 More recently, a small (n = 28) naturalistic study indicated that first-episode patients with higher baseline scores on MCCB attention and verbal memory scales (but not reasoning) were more likely to meet remission criteria after 6 months of treatment.44 Another small study (n = 55, divided into 3 medication groups) also found that verbal memory, as well as a novel measure of planning, predicted acute (8wk) positive symptom response.45 Differing sample characteristics and test batteries may account for the failure to converge on a single prognostic measure, but collectively these studies strongly suggest that brief paper-and-pencil measures of neurocognitive functioning may provide significant prognostic information.
Given the relative paucity of functional neuroimaging studies examining maze performance, which forms the basis of the MCCB reasoning scale, a neurobiological interpretation of this result is not straightforward. To our knowledge, only 1 neuroimaging study has been conducted using this paradigm; as expected, results demonstrated a robust activation of a dorsal frontoparietal attention network.46 Notably, the study also found activations of the basal ganglia and cortical motor areas, despite using a nonmotoric (imaginary performance only) version of the task. Relatedly, specific deficits in maze performance were reported in a study of neurological patients with basal ganglia infarcts.47 Thus, it is possible that MCCB reasoning performance may serve to index dopamine-sensitive frontostriatal circuitry relevant to the mechanism of action of antipsychotic medications. Such a conclusion is consistent with our recent observation that frontostriatal connectivity, measured with resting-state functional magnetic resonance imaging, is a powerful (sensitivity = 80% and specificity = 75%) and replicable prognostic biomarker of antipsychotic response in both first-episode and multiepisode patients.48
As noted, we found changes in general cognition, working memory, and verbal learning that were highly significant for a time effect when no symptom covariates were included, but these associations became nonsignificant when time-varying symptom dimensions were included. In all cases, BPRS remission score and SANS global alogia were consistently significant in the models, implicating a mediation effect of these symptoms on cognition.39 However, it should be noted that such “pseudospecificity” (ie, cognitive improvement that could result from reductions in other aspects of the illness) cannot be fully explicated with post hoc statistical approaches.49
Although the introduction of second-generation antipsychotics held the promise of nootropic effects, our longitudinal cognitive data are consistent with a growing body of evidence that second-generation antipsychotics provide no specific cognitive benefit.50 While several cognitive scores improved modestly over the course of our 12-week trial, gains were entirely eliminated when controlling for changes in positive and negative symptoms. These results are consistent with other recent studies reporting a significant correlation between cognitive change and symptom remission in FES patients.21,44,51 Indeed, 1 study concluded that verbal memory was a state marker of psychosis remission52; our use of a mediation model provides formal statistical support for this assertion.
Our results do not support a special role for aripiprazole, as compared to other second-generation antipsychotic agents, in the treatment of cognitive deficits. Despite a putative difference in mechanism of action, there is no difference in the effects of aripiprazole and risperidone on cognitive performance, just as there was no difference in positive symptom response (Ref: 10, this issue). Differences in negative symptom change and motoric side effects reported elsewhere in this issue were not reflected in cognitive scores in any domain.
Several limitations of the present study should be noted. First, although comparisons between completers and noncompleters suggested that the 2 groups did not differ on most clinical and demographic variables, paternal education was higher in noncompleters, and SANS global affect and avolition were lower in noncompleters. However, it is unlikely that these results influenced our findings. Second, we did not include a control group to examine practice effects. However, the MCCB was designed to ameliorate this potential confound, and the fact that no cognitive domain change scores were significant after controlling for symptom change suggests that practice effects were limited. Nonetheless, it is plausible that the extent of overall change in the MCCB reflected learning/practice effects, and that reduction of psychosis may permit patients to successfully learn from practice. In the schizophrenia literature, psychotic symptomatology is not strongly correlated with concurrent measures of cognitive performance,53 but it may more strongly interfere with learning ability over time, with significant implications for cognitive remediation.54 An alternative interpretation is that the ability to solve problems (as measured by the mazes subtest of the MCCB) predicts the ability to learn with practice, which may or may not be mediated by clinical response. Finally, our mediation models were not designed to explain the direction of the cognitive-symptom change interactions, although it is intuitive that symptom reduction causally preceded cognitive improvement.
Nevertheless, the present results suggest that planning and reasoning skills in FES might hold prognostic value in helping to determine which patients are likely to rapidly benefit from second-generation antipsychotic treatment. Moreover, severe cognitive deficits remain a substantial problem in the treatment of FES, and novel pharmacologic and/or behavioral strategies are needed to ameliorate them.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported in part by the National Institutes of Health (R01MH060004 to D.G.R., K23MH100264 to J.A.G., P30MH090590 to J.M.K., and P50MH080173 to A.K.M.) and by a NARSAD Young Investigator Grant to J.A.G. from the Brain & Behavior Research Foundation. Medication supplies were donated by Bristol-Myers Squibb and by Janssen Pharmaceuticals. Dr. Lencz has received grant support from the National Institute of Mental Health, the US-Israel Binational Science Fund, and the Brain & Behavioral Research Foundation. Dr. DeRosse has received grant support from the National Institute of Mental Health. Dr. Gallego has received grant support from National Institute of Mental Health and Brain & Behavioral Research Foundation. Dr. Petrides has received research support from St. Jude Medical, Astra Zeneca, Proteus, Ai-Cure, Corcept Therapeutics and Amgen. Dr. Hassoun has been a consultant and/or advisor to or has received honoraria from Otsuka, Lundbeck, Bristol-Myers Squibb and Sunovion. Dr. Zhang has received grant support from the National Institute of Mental Health, Brain & Behavioral Research Foundation, and Genomind, Inc. Dr. Kellner receives royalties from Cambridge University Press, and honoraria from UpToDate, Psychiatric Times, and North Shore-LIJ Health System. Dr. Tohen was a full time employee at Lilly (1997 to 2008), and has received honoraria from, or consulted for, Abbott, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Sunovion, Forest, Geodon Richter Plc, Roche, Elan, Alkermes, Lundbeck, Teva, Pamlab, Wyeth and Wiley Publishing; his spouse was a full-time employee at Lilly (1998-2013). Dr. Burdick has served as an advisory board member for Dainippon Sumitomo Pharmaceutical and for Takeda Lundbeck. Dr. Goldberg has consulted for Neurocog Trials, and receives royalties for use of a cognitive test battery in clinical trials, the BACS. Dr. Kane has been a consultant for Alkermes, Amgen, Bristol-Myers Squibb, Eli Lilly, EnVivo Pharmaceuticals (Forum), Forest, Genentech, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, Merck, Novartis, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion and Teva. Dr. Kane has received honoraria for lectures from Bristol-Myers Squibb, Janssen, Genentech, Lundbeck and Otsuka. Dr. Kane is a Shareholder in MedAvante, Inc. and the Vanguard Research Group. Dr. Robinson has been a consultant to Asubio, Shire and Otsuka, and he has received grants from Bristol Meyers Squibb, Janssen, and Otsuka. Dr. Malhotra has received grant support from the National Institute of Mental Health and the Brain & Behavioral Research Foundation. Dr. Malhotra is a consultant to Genomind, Inc and FORUM Pharmaceuticals.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Gold JM, Dickinson D. “Generalized cognitive deficit” in schizophrenia: overused or underappreciated? Schizophr Bull. 2013;39:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keefe RSE, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;213:11–37. [DOI] [PubMed] [Google Scholar]
- 3. Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. [DOI] [PubMed] [Google Scholar]
- 4. Bilder RM, Reiter G, Bates J, et al. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28:270–282. [DOI] [PubMed] [Google Scholar]
- 5. MacCabe JH, Wicks S, Löfving S, et al. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70:261–270. [DOI] [PubMed] [Google Scholar]
- 6. Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. [DOI] [PubMed] [Google Scholar]
- 7. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 8. Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158:156–162. [DOI] [PubMed] [Google Scholar]
- 9. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163:2096–2102. [DOI] [PubMed] [Google Scholar]
- 10. Robinson DG Gallego JA John M et al . A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophr Bull. 2015:sbv125. doi:10.1093/schbul/sbv125. [DOI] [PMC free article] [PubMed]
- 11. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–163. [DOI] [PubMed] [Google Scholar]
- 12. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72:1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76. [DOI] [PubMed] [Google Scholar]
- 14. Malhotra AK. Dissecting the heterogeneity of treatment response in first-episode schizophrenia. Schizophr Bull. 2015:sbv117. doi:10.1093/schbul/sbv117. [DOI] [PMC free article] [PubMed]
- 15. Keefe RS, Sweeney JA, Gu H, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. [DOI] [PubMed] [Google Scholar]
- 16. Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry. 2005;162:1888–1895. [DOI] [PubMed] [Google Scholar]
- 17. Keefe RS, Seidman LJ, Christensen BK, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. [DOI] [PubMed] [Google Scholar]
- 18. Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. [DOI] [PubMed] [Google Scholar]
- 19. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 20. Keefe RS, Bilder RM, Davis SM, et al. ; CATIE Investigators; Neurocognitive Working Group. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. [DOI] [PubMed] [Google Scholar]
- 21. Davidson M, Galderisi S, Weiser M, et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST). Am J Psychiatry. 2009;166:675–682. [DOI] [PubMed] [Google Scholar]
- 22. Rund BR, Melle I, Friis S, et al. Neurocognitive dysfunction in first-episode psychosis: correlates with symptoms, premorbid adjustment, and duration of untreated psychosis. Am J Psychiatry. 2004;161:466–472. [DOI] [PubMed] [Google Scholar]
- 23. Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG. Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res. 2004;68:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Chang WC, Hui CL, Chan SK, Lee EH, Wong GH, Chen EY. Relationship between diminished expression and cognitive impairment in first-episode schizophrenia: a prospective three-year follow-up study. Schizophr Res. 2014;152:146–151. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Hu M, Guo X, Wu R, Li L, Zhao J. Cognitive effects of atypical antipsychotic drugs in first-episode drug-naïve schizophrenic patients. Neural Regen Res. 2013;8;277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riedel M, Schennach-Wolff R, Musil R, et al. Neurocognition and its influencing factors in the treatment of schizophrenia - Effects of aripiprazole, olanzapine, quetiapine and risperidone. Hum Psychopharmacol. 2010;25:116–125. [DOI] [PubMed] [Google Scholar]
- 27. Hori H, Yoshimura R, Katsuki A, et al. The cognitive profile of aripiprazole differs from that of other atypical antipsychotics in schizophrenia patients. J Psychiatr Res. 2012;46:757–761. [DOI] [PubMed] [Google Scholar]
- 28. Yasui-Furukori N, Kaneda A, Sugawara N, Tomita T, Kaneko S. Effect of adjunctive treatment with aripiprazole to atypical antipsychotics on cognitive function in schizophrenia patients. J Psychopharmacol (Oxford). 2012;26:806–812. [DOI] [PubMed] [Google Scholar]
- 29. Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. [DOI] [PubMed] [Google Scholar]
- 31. First MB, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis 1 DSM-IV Disorders Patient Edition. New York, NY: New York Biometrics Research Department, New York State Psychiatric Institute: 1998. [Google Scholar]
- 32. Funke B, Finn CT, Plocik AM, et al. Association of the DTNBP1 locus with schizophrenia in a U.S. population. Am J Hum Genet. 2004;75:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 1988;24:112–117. [PubMed] [Google Scholar]
- 34. Robinson D, Woerner M, Schooler N. Intervention research in psychosis: issues related to clinical assessment. Schizophr Bull. 2000;26:551–556. [DOI] [PubMed] [Google Scholar]
- 35. Guy W. The clinical global impression scale. In: ECDEU Assessment Manual for Psychopharmacology, revised. Rockville, MD: US Dept. of Health, Education and Welfare, ADAMHA, NIMH Psychopharmacology Research Branch: 1976;218–222. [Google Scholar]
- 36. Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery. Los Angeles, CA: MATRICS Assessment, Inc.; 2006. [Google Scholar]
- 37. Trampush JW, Lencz T, Knowles E, et al. Independent evidence for an association between general cognitive ability and a genetic locus for educational attainment. Am J Med Genet Part B Neuropsychiatr Genet. 2015;168:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. SAS/STAT User’s Guide, Version 9.1. Cary, NC: SAS Institute Inc. Vol. 3 1999. [Google Scholar]
- 39. Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 41. Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reilly JL, Sweeney JA. Generalized and specific neurocognitive deficits in psychotic disorders: utility for evaluating pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophr Bull. 2014;40:516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156:544–549. [DOI] [PubMed] [Google Scholar]
- 44. Torgalsbøen AK, Mohn C, Rishovd Rund B. Neurocognitive predictors of remission of symptoms and social and role functioning in the early course of first-episode schizophrenia. Psychiatry Res. 2014;216:1–5. [DOI] [PubMed] [Google Scholar]
- 45. Zhou FC, Xiang YT, Wang CY, et al. Predictive value of prospective memory for remission in first-episode schizophrenia. Perspect Psychiatr Care. 2013;50:102–110. [DOI] [PubMed] [Google Scholar]
- 46. Kirsch P, Lis S, Esslinger C, et al. Brain activation during mental maze solving. Neuropsychobiology. 2006;54:51–58. [DOI] [PubMed] [Google Scholar]
- 47. Vakil E, Blachstein H, Soroker N. Differential effect of right and left basal ganglionic infarctions on procedural learning. Cogn Behav Neurol. 2004;17:62–73. [DOI] [PubMed] [Google Scholar]
- 48. Sarpal DK, Argyelan M, Robinson DG, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. [DOI] [PubMed] [Google Scholar]
- 50. Keefe RSE. The longitudinal course of cognitive impairment in schizophrenia: An examination of data from premorbid through posttreatment phases of illness. J Clin Psychiatry. 2014;75(suppl 2):8–13. doi:10.4088/JCP.13065su1.02. [DOI] [PubMed] [Google Scholar]
- 51. Olivier MR, Killian S, Chiliza B, et al. Cognitive performance during the first year of treatment in first-episode schizophrenia: a case-control study. Psychol Med. 2015;22:1–11. [DOI] [PubMed] [Google Scholar]
- 52. Benoit A, Bodnar M, Malla AK, Joober R, Bherer L, Lepage M. Changes in memory performance over a 12-month period in relation to achieving symptomatic remission after a first-episode psychosis. Schizophr Res. 2014;153:103–108. [DOI] [PubMed] [Google Scholar]
- 53. Ventura J, Thames AD, Wood RC, Guzik LH, Hellemann GS. Disorganization and reality distortion in schizophrenia: a meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophr Res. 2010;121:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy-what works and could we do better? Schizophr Bull. 2011;37(suppl 2):S80–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.