Abstract
People with schizophrenia typically experience auditory hallucinations or delusions during acute episodes. Although effective drug treatments are available, many have intractable symptoms that do not recover between acute episodes. One proposed alternative to drug treatments is transcranial magnetic stimulation (TMS). To date, many research trials to assess effectiveness of TMS for people with symptoms of schizophrenia have been conducted worldwide. However, there is a lack of consensus on whether TMS should be recommended to be adopted in routine clinical practice. We conducted a systematic review of the literature for all relevant randomized controlled trials (RCTs) comparing TMS with sham or standard treatment. Forty-one trials (1473 participants) survived eligibility criteria and had extractable data. We found significant differences in favor of temporoparietal TMS compared with sham TMS for global state (7 RCTs, n = 224, MD: -0.5, 95% CI: -0.76 to -0.23) and for positive symptoms measured on the Positive and Negative Syndrome Scale (5 RCTs, n = 127, MD: -6.09, 95% CI: -10.95 to -1.22). However, we also found that the quality of trial reporting was frequently suboptimal and the risks of bias were strong or unascertainable for many trial aspects; this led to many results being graded as very low-quality evidence. On that basis, we were unable to definitively support or refute the routine use of TMS in clinical practice. Future definitive trials of TMS with rigorous processes and high-quality reporting are needed.
Key words: schizophrenia, transcranial magnetic stimulation, auditory hallucinations
Background
People with schizophrenia often experience symptoms which fail to fully respond to antipsychotic medication. Transcranial magnetic stimulation (TMS) has been proposed as a new treatment for people with schizophrenia, especially those who experience persistent auditory hallucinations.
Objectives
To estimate the effects of TMS alone, compared with sham TMS or with “standard management” and any other comparison interventions in reducing psychotic symptoms associated with schizophrenia.
Search Methods
We searched the Cochrane Schizophrenia Group Trials Register (June 2006, June 2008, and April 2013).
Selection Criteria
We included all randomized controlled trials (RCTs) recruiting at least 5 participants and comparing TMS with sham TMS or any other treatment for people with schizophrenia.
Data Collection and Analysis
We extracted data independently. For dichotomous data, we calculated relative risks (RRs) and their 95% confidence intervals (CIs). For continuous data, we calculated mean differences (MD) and 95% CI. We used a fixed-effect model. We assessed overall quality of the evidence using the GRADE approach.
Main Results
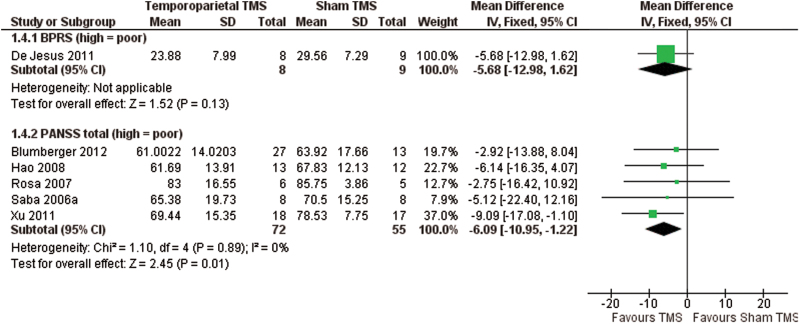
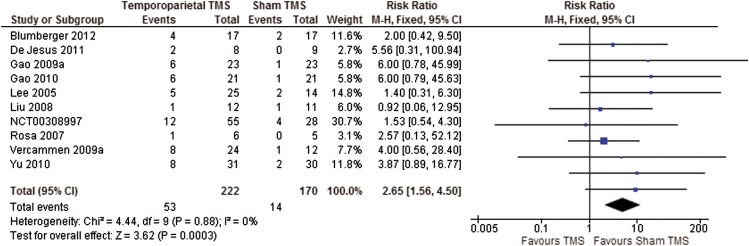
We included 41 studies with 1473 participants in the review. We found significant differences in favor of temporoparietal TMS compared with sham TMS for global state measured on the Clinical Global Impression Scale (7 RCTs, n = 224, MD: −0.5, 95% CI: −0.76 to −0.23, very low-quality evidence) and positive symptoms measured on the Positive and Negative Syndrome Scale (PANSS; 5 RCTs, n = 127, MD: −6.09, 95% CI: −10.95 to −1.22, very low-quality evidence, figure 1). Participants experienced significantly more headaches in the temporoparietal TMS group (10 RCTs, n = 392, RR: 2.65, 95% CI: 1.56 to 4.50, very low-quality evidence, figure 2). However, no more participants left the study early from the TMS group than from the sham group (very low-quality evidence). Cognitive state was assessed using 39 different measures, and all were equivocal (very low-quality evidence).
Fig. 1.
Comparison: temporoparietal transcranial magnetic stimulation (TMS) vs sham TMS. Outcome: mental state: general—average total score (various scales).
Fig. 2.
Comparison: temporoparietal transcranial magnetic stimulation (TMS) vs sham TMS. Outcome: adverse effects: headache.
We included only 2 trials which compared temporoparietal TMS with standard treatment. In both trials, the participants received first- and second-generation antipsychotic medication in both treatment groups; therefore, TMS was used an adjunctive therapy to medication. We found no significant differences in the number of participants that showed clinical improvement in global state (1 RCT, n = 100, RR: 1.19, 95% CI: 0.91 to 1.57) or left the study early (2 RCTs, n = 140, RR: 0.33, 95% CI: 0.08 to 1.46) (both very low-quality evidence). No studies reported on global state score, mental state, cognitive state, and adverse effects.
For prefrontal TMS compared with sham TMS, global state was measured on 3 different scales, all of which presented equivocal results (very low-quality evidence). We could not pool data for mental state on the PANSS due to high heterogeneity. Cognitive state was assessed using 19 different measures, with 15/19 being equivocal (very low-quality evidence). Prefrontal TMS caused more headaches (6 RCTs, n = 164, RR: 2.77, 95% CI: 1.22 to 6.26, very low-quality evidence) but there was no difference in the number of participants leaving the study early (very low-quality evidence). No studies reported data for clinical improvement.
We found a significant difference in favor of prefrontal theta burst stimulation TMS compared with sham TMS for mental state on the PANNS (3 RCTs, n = 108, MD: −5.71, 95% CI: −9.32 to −2.10, very low evidence). We found no difference for clinical improvement, cognitive state, number of headaches, and leaving the study early (very low-quality evidence).
None of the included studies reported satisfaction with care.
Authors’ Conclusions
Based on this review, there is insufficient evidence to support or refute the use of TMS to treat symptoms of schizophrenia. Although some evidence suggests that TMS, and in particular temporoparietal TMS, may improve certain symptoms (such as auditory hallucinations and positive symptoms of schizophrenia) compared with sham TMS, the results were not robust enough to be unequivocal across the assessment measures used. There was insufficient evidence to suggest any added benefit with TMS used as an adjunctive therapy to antipsychotic medication.
The overall quality of evidence was graded as very low due to risk of bias, and this was accompanied by an imprecision in estimates due to the relatively small number of participants in the studies. Thus, consideration is required in improving the quality of trial processes, as well as the quality of reporting of ongoing and future TMS trials, so as to facilitate accurate future judgments in assessing risk of bias. Differences in TMS techniques in relation to stimulation intensity, stimulation length, brain areas stimulated, and variations in the design of sham TMS contributed to the heterogeneity of study findings and limited the interpretation and applicability of the results. In addition, the trials assessed their outcomes with a variety of scales, and usable data were limited. Therefore, to better evaluate the treatment effects of TMS in people with schizophrenia, we favor the use of standardized treatment protocols and outcome measures. Full details are reported in the Cochrane review.1
Funding
Gordon Small Charitable Trust for Research in Old Age Psychiatry, UK (to N.D.).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Reference
- 1. Dougall N, Maayan N, Soares-Weiser K, McDermott LM, McIntosh A. Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database Syst Rev. 2015;(Issue 8):CD006081. doi:10.1002/14651858.CD006081. [DOI] [PMC free article] [PubMed] [Google Scholar]