Abstract
Research findings are particularly important for medication choice for first-episode patients as individual prior medication response to guide treatment decisions is unavailable. We describe the first large-scale double-masked randomized comparison with first-episode patients of aripiprazole and risperidone, 2 commonly used first-episode treatment agents. One hundred ninety-eight participants aged 15–40 years with schizophrenia, schizophreniform disorder, schizoaffective disorder or psychotic disorder Not Otherwise Specified, and who had been treated in their lifetime with antipsychotics for 2 weeks or less were randomly assigned to double-masked aripiprazole (5–30mg/d) or risperidone (1–6mg/d) and followed for 12 weeks. Positive symptom response rates did not differ (62.8% vs 56.8%) nor did time to response. Aripiprazole-treated participants had better negative symptom outcomes but experienced more akathisia. Body mass index change did not differ between treatments but advantages were found for aripiprazole treatment for total and low-density lipoprotein cholesterol, fasting glucose, and prolactin levels. Post hoc analyses suggested advantages for aripiprazole on depressed mood. Overall, if the potential for akathisia is a concern, low-dose risperidone as used in this trial maybe a preferred choice over aripiprazole. Otherwise, aripiprazole would be the preferred choice over risperidone in most situations based upon metabolic outcome advantages and some symptom advantages within the context of similar positive symptom response between medications.
Key words: clinical trial, treatment response, negative symptoms, akathisia, metabolic side effects
Introduction
Optimized treatment of the first episode of schizophrenia spectrum disorders offers the potential for better long-term outcomes. Medication choice is usually guided by past response to treatment, the evidence base for treatment options and patient preferences. First-episode patients do not have prior antipsychotic response patterns to guide medication choice; thus, the evidence base becomes especially important for medication decisions. The evidence base ideally should come from first-episode studies as response and side effect patterns differ between first-episode and multiepisode patients.1
Which antipsychotic to try first? Risperidone is the most widely used antipsychotic for first-episode treatment at US community facilities followed in frequency by olanzapine, aripiprazole, paliperidone, and quetiapine.2 Prior randomized first-episode studies comparing olanzapine or quetiapine with risperidone have demonstrated similar efficacy but less weight gain with risperidone3,4 and less dyslipidemia.4 However, even with risperidone, weight gain with first-episode patients is substantial (eg, 11.6% weight increase after 3 months of treatment4). The importance of metabolic side effects in first-episode treatment choice was recently emphasized by the finding from the national RAISE-ETP study that after an average of only 47 days of antipsychotic treatment, approximately half of first-episode patients had dyslipidemia and half were already overweight or obese.5 We addressed the important question is whether antipsychotics such as aripiprazole that produce less metabolic side effects with chronic patients6 could produce equivalent symptom response for first-episode patients as risperidone but with less adverse metabolic effects.
Methods
Settings
The study was conducted at 8 New York City area facilities and one facility each in San Antonio, TX and Calgary, Alberta, Canada. All sites were not-for-profit institutions (either academic centers, community facilities, or public hospitals), were located in urban or suburban areas, and served diverse communities in terms of economic status and racial/ethnic composition. Data were collected from December 2005 until April 2013.
Participants
Inclusion Criteria were: (1) current DSM-IV-defined diagnosis of schizophrenia, schizophreniform, schizoaffective disorder, or psychotic disorder Not Otherwise Specified (NOS); (2) age 15–40; (3) lifetime antipsychotic medication treatment (at any dose) of 2 weeks or less; (4) current positive symptoms rated ≥4 (moderate) on one or more of the Brief Psychiatric Rating Scale-Anchored version (BPRS-A)7 items: conceptual disorganization, grandiosity, hallucinatory behavior, or unusual thought content; (5) for women, a negative pregnancy test and agreement to use a medically accepted birth control method; and (6) competent and willing to provide informed consent or assent for participants under age 18. Exclusion criteria were: (1) meeting DSM-IV criteria for current substance-induced psychotic disorder, psychotic disorder due to a general medical condition, delusional disorder, brief psychotic disorder, shared psychotic disorder, or mood disorder (major depression or bipolar) with psychotic features; (2) serious neurological or endocrine disorder or medical condition/treatment known to affect the brain; (3) medical conditions requiring treatment with a medication with psychotropic effects; (4) medical contraindications to risperidone or aripiprazole treatment; (5) significant risk of suicidal or homicidal behavior; (6) any factor (eg, language limitations) that would preclude participants providing informed consent or participating in study procedures; (7) diagnosis of diabetes (defined as fasting plasma glucose >126mg/dl) or the metabolic syndrome (defined as 3 or more of the following: high blood pressure (≥130/85mm Hg), truncal obesity (waist circumference >40 inches for men and >35 for women), elevated fasting glucose (>110mg/dl), low, high-density lipoprotein (HDL) cholesterol (<40mg/dl for men and <50mg/dl for women), and elevated triglycerides (≥150mg/dl)8; and (8) requiring antidepressant or mood stabilizer treatment.
Consent Procedures
After complete study description, written informed consent was obtained from adult participants and legal guardians of participants under 18 years old, who provided written assent. The study was conducted under the auspices of the Feinstein Institute for Medical Research Institutional Review Board (IRB) as the coordinating center and the IRBs of the clinical sites.
Treatment
Treatment lasted 12 weeks. Participants were stratified by site, previous antipsychotic exposure (none vs any), and diagnosis (psychotic disorder NOS vs other eligible diagnoses) and were randomly assigned on a 1:1 basis to double-masked treatment with either aripiprazole (5–30mg/d) or risperidone (1–6mg/d). Study medication was packaged in identically appearing capsules at 3 different dosing levels (level 1: containing 5mg of aripiprazole or 1mg of risperidone; level 2: 10mg of aripiprazole or 2mg of risperidone; and level 3: 15mg of aripiprazole or 3mg of risperidone). The study allowed for prescription of 1–2 study capsules per day providing a total of 6 possible levels of milligrams of daily study medication (eg, two level 3 capsules provided either 30mg of aripiprazole or 6mg of risperidone daily). Study medication was given at evening but could be moved to other times as needed. Inclusion criteria required all participants to have very limited prior antipsychotic exposure; any antipsychotics being taken at study entry were discontinued. The initial daily dose was 1 study capsule (ie, 5mg of aripiprazole or 1mg of risperidone). Medication doses were advanced according to a titration schedule (level 2 at day 4, level 3 at week 1, two level 2 capsules at week 4, a level 2 and level 3 capsule at week 6, and two level 3 capsules at week 8) until response criteria were achieved or dose-limiting side effects occurred. Study psychiatrists could advance or slow the titration schedule for clinical needs. The initial approach for side effect management was medication dose reduction. Allowed concomitant psychiatric medications were: benztropine for extrapyramidal symptoms (EPS); lorazepam or propranolol for akathisia; lorazepam (followed by sodium amytal or chloral hydrate as alternatives) for agitation or anxiety; lorazepam, zolpidem, or ramelteon for insomnia. Given the metabolic effects of antipsychotics, participants also received a Healthy Lifestyles psycho-educational package based upon materials developed by the National Institute of Diabetes and Digestive and Kidney Diseases’ Weight Control Information Network. Topics included: What is a healthy diet, Tips for healthy eating, What is a healthy weight, Health risks of being overweight, What makes people overweight (with information on potential weight gain with antipsychotic treatment), and Getting active. For participant safety, any participants who fulfilled metabolic syndrome criteria or developed new onset diabetes during the trial were removed from controlled treatment.
Assessments
Initial diagnostic eligibility was established with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID).9 These data were later reviewed in a consensus conference (see Funke et al10) for final diagnostic assignment. Assessments done at baseline, weekly for 4 weeks, and then every 2 weeks were: BPRS-A,7 Hillside clinical trials version of the Scale for the Assessment of Negative Symptoms (SANS),11 Clinical Global Impressions Scale (CGI),12 modified Systematic Assessment for Treatment Emergent Events (Specific Inquiry) (SAFTEE-SI),13 vital signs, Simpson-Angus Scale for EPS,14 and the Barnes Akathisia Scale (BAS).15 At baseline and every 4 weeks, fasting samples for glucose, lipids, insulin, and prolactin were obtained. These metabolic measures were supplemented with 2-hour oral glucose tolerance tests (OGTT) at baseline and end of study. After an overnight fast, subjects were given 100g of glucose. Glucose and insulin levels were obtained at baseline and at 30, 60, 90, and 120 minutes.
To increase assessment uniformity, a central rater team performed the diagnostic and psychopathology assessments, traveling to New York area sites in person or using secure teleconferencing elsewhere. The same rater performed assessments with each participant throughout that individual’s study participation. Intraclass correlation coefficients for the BPRS items comprising the response criteria were for conceptual disorganization 0.94 (95% CI: 0.76, 0.99); grandiosity 0.92 (95% CI: 0.80, 0.98); hallucinatory behavior 0.93 (0.76, 0.99); and unusual thought content 0.92 (95% CI: 0.82, 0.98) and for the SANS global items affective flattening 0.75 (95% CI: 0.43, 0.93); alogia 0.66 (95% CI: 0.36, 0.89); avolition-apathy 0.69 (95% CI: 0.38, 0.91); and asociality-anhedonia 0.52 (95% CI: 0.17, 0.85).
Statistical Analysis
Histograms, q-q plots, and the Shapiro-Wilk test were used to assess distribution of continuous variables. Based on the distribution, an independent-samples t test or Wilcoxon rank-sum test was used to compare the baseline continuous variables between treatment groups. Chi-square test was used for categorical variables. Proportions between the 2 treatment groups were compared using the binomial proportions test. Interrater reliability between the raters was assessed using intraclass coefficients.
Since missing values may be dependent on the observed outcomes, we assumed the missing data to be missing at random, and hence analysis of the longitudinal data was conducted utilizing a mixed-models approach. A random intercept in the mixed-models was used to account for correlation of measurements over time among the participants; the correlational type was assumed to be unstructured. The difference in slopes of the outcomes between the 2 treatment groups was assessed using the group-by-time interaction term of the mixed models. The P values and F-statistics for the main effects of treatment, time, and for the treatment-by-time interaction were based on the type 3 tests of fixed effects. The denominator degrees of freedom for F-statistic in the type 3 tests for fixed effects were computed according to Satterthwaite’s formula, which takes into consideration the variance within the group along with the sample size and is robust against variance heterogeneity.
Symptom Analyses.
The primary analysis compared the cumulative 12-week response rates between treatments using standard survival analysis methods, ie, Kaplan-Meier product-limit method and the log-rank test. Response criteria required (1) a rating of 3 (“mild”) or less on all of the following items of the BPRS-A: conceptual disorganization, grandiosity, hallucinatory behavior, and unusual thought content and (2) a CGI Improvement rating of much or very much improved on 2 consecutive rating assessments (time of response was the date of the first of the 2 ratings). Confirmatory positive symptom analyses examined the longitudinal patterns of the sum of the ratings for the BPRS-A items used in the response criterion. A mixed-models approach was used for these analyses and analyses of the longitudinal patterns of negative symptoms that examined the SANS global measures affective flattening, alogia, avolition-apathy, and asociality-anhedonia.
Metabolic Effects.
A mixed-models approach was used to compare across treatments metabolic effects (body mass index [BMI], levels of lipids, glucose, insulin and prolactin, and OGTT results).
Motor Effects.
Parkinsonism was defined as being present if 2 or more of the Simpson-Angus EPS Scale items gait, rigidity of major joints, tremor, akinesia, and akathisia were rated 2 or 1 item was rated 3 or higher. Parkinsonism rates were compared with standard survival analysis techniques. An overall EPS severity score was calculated as the sum of the Simpson-Angus EPS Scale items just listed. Longitudinal patterns of EPS severity and of akathisia as measured by the global item of the BAS were compared using mixed models.
General adverse events from the SAFTEE-SI were characterized by descriptive statistics.
Results
Participants
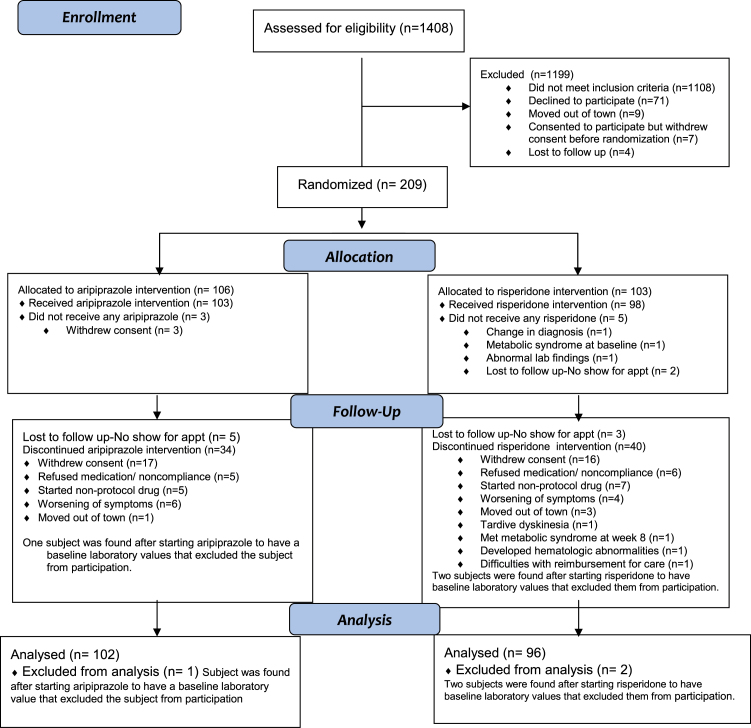
The analysis sample included 198 participants (figure 1). As shown in table 1, participants were young, mostly male (71%), of diverse ethnic backgrounds, and usually from low to lower middle class socioeconomic backgrounds. Participants had psychotic symptoms for a mean of 125.5 weeks before starting antipsychotics, reflecting this two-thirds already met diagnostic criteria for schizophrenia despite having no or minimal treatment. At entry, 27.4% of participants assigned to aripiprazole and 22.4% assigned to risperidone (P = .52) were antipsychotic medication naive. Participants had substantial positive symptoms at study entry (as required by study design). Negative symptoms were less pronounced; the mean ratings on the SANS global items were approximately 2 (mild). Participants assigned to aripiprazole and risperidone did not differ on any baseline characteristics.
Fig. 1.
Study progression.
Table 1.
Demographic and Clinical Characteristics of Participantsa
| Characteristic | All Participants | Aripiprazole-Treated Participants | Risperidone-Treated Participants | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Men | 140 | 71 | 72 | 71 | 68 | 71 |
| Women | 58 | 29 | 30 | 29 | 28 | 29 |
| Ethnic background | ||||||
| African-American | 73 | 37 | 38 | 38 | 35 | 37 |
| Asian | 39 | 20 | 22 | 22 | 17 | 18 |
| Caucasian | 48 | 24 | 21 | 21 | 27 | 28 |
| Hispanic | 20 | 10 | 11 | 11 | 9 | 9 |
| Other/mixed | 16 | 9 | 9 | 9 | 7 | 7 |
| Marital status | ||||||
| Never married | 182 | 92 | 92 | 92 | 90 | 94 |
| Married | 4 | 2 | 1 | 1 | 3 | 3 |
| Remarried | 1 | 0.5 | 1 | 1 | 0 | 0 |
| Divorced | 9 | 4.5 | 6 | 6 | 3 | 3 |
| Unknown | 2 | 1 | 2 | 2 | - | - |
| Diagnosis | ||||||
| Schizophrenia | 131 | 66 | 72 | 71 | 59 | 62 |
| Schizophreniform disorder | 45 | 23 | 23 | 22 | 22 | 23 |
| Schizoaffective disorder | 6 | 3 | 2 | 2 | 4 | 4 |
| Psychotic disorder NOS | 16 | 8 | 5 | 5 | 11 | 11 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (y) | 22.1 | 5.6 | 22.4 | 5.8 | 21.8 | 5.4 |
| Highest educational level (1 = postgraduate; 7 = grade school) | 3.9 | 1.2 | 4.0 | 1.4 | 3.8 | 1.1 |
| Hollingshead social class | ||||||
| Participant | 4.4 | 2.5 | 4.2 | 0.8 | 4.6 | 3.4 |
| Parent | 3.4 | 2.7 | 3.3 | 1.3 | 3.5 | 3.6 |
| Age at first psychiatric symptoms (y) | 18.1 | 6.0 | 18.2 | 5.9 | 18.1 | 6.0 |
| Age at first psychotic symptoms (y) | 19.7 | 5.3 | 19.8 | 5.0 | 19.7 | 5.6 |
| Duration of psychiatric symptoms before study entry (wk) | 203.9 | 282.1 | 218.0 | 317.6 | 188.8 | 238.9 |
| Duration of psychotic symptoms before study entry (wk) | 125.5 | 208.8 | 138.2 | 243.3 | 112.0 | 164.4 |
| Least square means estimates of symptom severity | ||||||
| BPRS-A total score at study entry | 45.89 | 0.86 | 44.38 | 0.92 | ||
| Total of BPRS-A items in response criterion at study entry | 14.66 | 0.38 | 14.42 | 0.40 | ||
| CGI severity score at study entry | 4.95 | 0.11 | 4.94 | 0.12 | ||
| SANS global scores at study entry | ||||||
| Affective flattening | 1.85 | 0.09 | 1.74 | 0.09 | ||
| Alogia | 2.08 | 0.08 | 1.96 | 0.09 | ||
| Avolition-apathy | 2.16 | 0.10 | 2.02 | 0.10 | ||
| Asociality-anhedonia | 2.19 | 0.08 | 2.07 | 0.09 | ||
Note: BPRS-A, Brief Psychiatric Rating Scale-Anchored version; CGI, Clinical Global Impressions Scale; NOS, Not Otherwise Specified; SANS, Scale for the Assessment of Negative Symptoms.
aParticipants assigned to aripiprazole and risperidone did not differ on any characteristic.
Duration of Treatment and Dose
Aripiprazole and risperidone participants had similar lengths of time on controlled treatment (mean length was 8.3 [SD = 4.9] wk with aripiprazole and 8.2 [SD = 4.9] wk with risperidone [t = 0.15, P = .88]). Three subjects (all on risperidone) were removed from controlled treatment before 12 weeks for safety concerns: one developed metabolic syndrome, one fulfilled Research Diagnosis of Tardive Dyskinesia16 criteria, and one had hematologic abnormalities. The prescribed capsule dosing level did not differ between medications. The mean modal level was 2.96 (SD = 1.2) for aripiprazole and 3.16 (SD = 1.49) for risperidone (P = .20) corresponding to a daily milligram dose of 14.8 (SD = 6.0) for aripiprazole and 3.2 (SD = 1.5) for risperidone. Concomitant medication use rates did not differ across conditions. The number of aripiprazole- vs risperidone-treated participants receiving a concomitant medication were: benzodiazepines 66 (66.7%) vs 71 (74.0%) (P = .34); anticholinergic medications 30 (29.4%) vs 35 (36.5%) (P = .35); beta blockers 15 (14.7%) vs 9 (9.4%) (P = .36); and medications specifically for sleep 2 (2.0%) vs 2 (2.1%) (P = .99).
Symptom Response
Response Rates.
Cumulative response rates did not differ between aripiprazole (62.8%; 95% CI: 50.8%, 74.8%) and risperidone (56.8%; 95% CI: 43.9%, 69.9%) treatment (log-rank test, χ2 = 0.26, P = .61). Mean time to response with aripiprazole was 8.0 (95% CI: 7.9, 8.1) weeks and 8.2 (95% CI: 7.3, 9.2) weeks with risperidone.
Symptom Levels.
Positive Symptoms There were no significant differences between medications and no medication-by-time interactions in longitudinal analyses of the positive symptom BPRS score (F = 0.34, df = 8,1134, P = .96). Scores improved markedly over time (F = 116.65, df = 8,1134, P < .0001).
Negative Symptoms
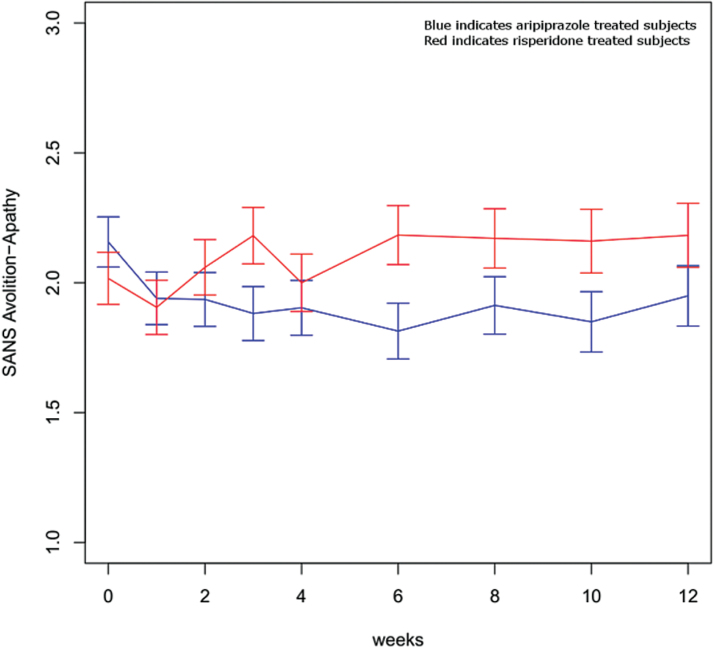
There were no significant differences between medications and no medication-by-time interactions in longitudinal analyses of the SANS global items affective flattening and alogia. Alogia scores improved over time (F = 25.42, df = 8,1156, P < .0001) but affective flattening did not. In contrast, analyses revealed a significant medication-by-time interaction (F = 2.18, df = 8,1162, P = .03) with avolition-apathy and a nonsignificant but trend-level interaction (F = 1.76, df = 9,1149, P = .08) for asociality-anhedonia. Asociality-anhedonia significantly improved over time (F = 3.29, df = 8,1149, P = .001). Avolition-apathy improved with aripiprazole and worsened with risperidone treatment but the differences were modest (a similar pattern was seen with asociality-anhedonia) (figure 2).
Fig. 2.
SANS Avolition-Apathy Global Score. Medication-by-time interaction, F = 2.18, df = 8,1162, P = .03. SANS, Scale for the Assessment of Negative Symptoms.
Global Symptom Measures
There were no significant differences between medications and no medication-by-time interactions in longitudinal analyses of the total BPRS (F = 1.17, df = 8,1095, P = .32) and also of CGI Severity (F = 0.84, df = 8,1137, P = .57). Scores improved markedly over time for both the total BPRS (F = 115.31, df = 8,1095, P < .0001) and CGI (F = 105.13, df = 8,1137, P < .0001) measures.
Motor Side Effects
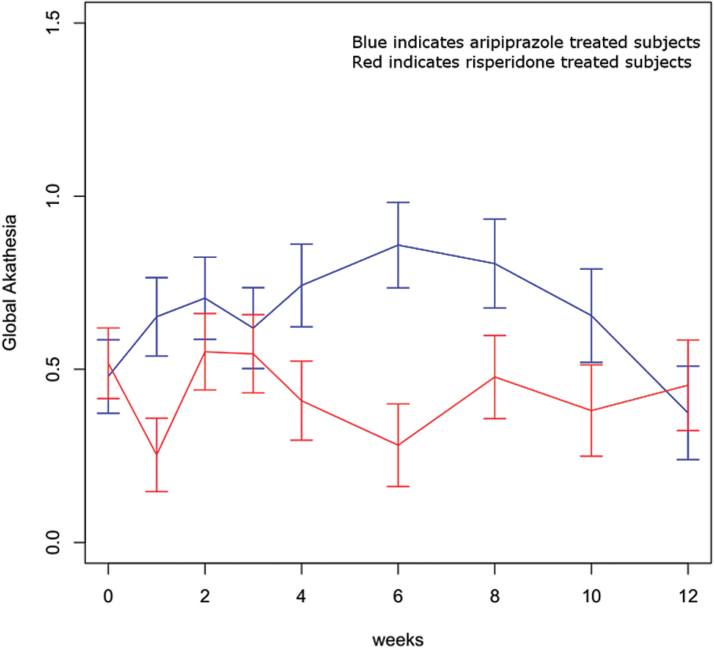
Analysis of the BAS global item revealed a significant medication-by-time interaction (F = 2.21, df = 8,1127, P = .03) (figure 3). Akathisia scores were significantly more severe with aripiprazole compared with risperidone at weeks 1, 4, and 6. Longitudinal analysis of the presence/absence of Parkinsonism revealed no significant medication, time, or treatment-by-time effects. Based upon a survival analysis, the cumulative rates of Parkinsonism were 14.8% (95% CI: 7.4%, 22.1%) with aripiprazole and 15.5% (95% CI: 7.1%, 24.0%) with risperidone (log-rank test, χ2 = 0.11, P = .75). Analyses of the extrapyramidal symptom severity score found no time or treatment-by-time effects but a significant medication effect (F = 5.61, df = 1,197, P = .02); scores were significantly worse with aripiprazole compared with risperidone at weeks 1, 2, and 4 (supplementary figure 1). Our a priori definition of extrapyramidal side effects included akathisia. Post hoc, we recalculated an extrapyramidal severity score excluding akathisia. Analyses of this severity score without akathisia again revealed no treatment-by-time or time effects while the medication effect became only trend level (F = 3.13, df = 1,201, P = .08).
Fig. 3.
Global Barnes Akathisia Scores. Medication-by-time interaction, F = 2.21, df = 8,1127, P = .03.
Relationship Between Levels of Extrapyramidal Symptoms and Avolition-Apathy.
Because extrapyramidal symptoms can be a cause of secondary negative symptoms, post hoc we examined the correlation at each assessment point between severity of avolition-apathy and the extrapyramidal severity score. Correlations except at week 3 were low (≤0.18) and not significant (P > .05). The week 3 correlation was significant (P < .001) but still low (0.36).
Metabolic Outcomes
BMI increased over time (F = 124.09, df = 8,1102, P < .0001) but there were no treatment-by-time or treatment effects. Estimated mean baseline vs 12-week BMI was 23.02 (95% CI: 22.22, 23.82) vs 24.81 (95% CI: 24.01, 25.62) for aripiprazole-treated participants and 23.22 (95% CI: 22.42, 24.02) vs 24.98 (95% CI: 24.17, 25.80) for risperidone-treated participants. Analyses of weight revealed the same pattern. Estimated kilogram increase in weight from baseline to 12 weeks with aripiprazole was 5.04 (95% CI: 4.16, 5.93) and 6.12 (95% CI: 4.11, 8.14) with risperidone.
Significant treatment-by-time interactions favoring aripiprazole were revealed in analyses of total cholesterol and low-density lipoprotein (LDL) cholesterol but not in analyses of HDL cholesterol or triglycerides (table 2). Analysis of fasting glucose but not fasting insulin levels also revealed a significant treatment-by-time interaction, again favoring aripiprazole treatment. Analysis of the OGTT area under the curve for glucose and for insulin revealed no treatment, time, or treatment-by-time effects. Analyses of prolactin levels separately by sex revealed significant treatment-by-time interactions with both women and men. Prolactin levels were significantly less at all time points after baseline for aripiprazole compared with risperidone.
Table 2.
Metabolic Outcomes: Estimated Least Squares Means and SE
| Measure | Condition | Baseline | SE | Week 4 | SE | Week 8 | SE | Week 12 | SE | Treatment- by-Time Interaction |
|---|---|---|---|---|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | F = 4.73, df = 3,376, P = .003 | |||||||||
| Aripiprazole | 158.25 | 3.15 | 157.81* | 3.41 | 162.98* | 3.74 | 163.02** | 3.74 | ||
| Risperidone | 157.01 | 3.30 | 168.62* | 3.62 | 175.92* | 3.77 | 178.36** | 4.10 | ||
| LDL cholesterol (mg/dl) | F = 4.36, df = 3,369, P < .01 | |||||||||
| Aripiprazole | 86.37 | 2.60 | 84.97* | 2.78 | 90.31** | 3.02 | 91.89** | 3.02 | ||
| Risperidone | 88.25 | 2.77 | 95.49* | 3.02 | 104.10** | 3.14 | 105.62** | 3.41 | ||
| HDL cholesterol (mg/dl) | F = 0.83, df = 3,372, P = .48 | |||||||||
| Aripiprazole | 55.99 | 1.37 | 56.59 | 1.47 | 56.25 | 1.60 | 55.14 | 1.60 | ||
| Risperidone | 53.23 | 1.40 | 56.60 | 1.52 | 55.05 | 1.57 | 54.21 | 1.69 | ||
| Triglycerides (mg/dl) | F = 0.66, df = 3,352, P = .59a | |||||||||
| Aripiprazole | 80.00 | 4.48 | 80.78 | 4.90 | 87.52 | 5.44 | 87.35 | 5.44 | ||
| Risperidone | 77.41 | 4.50 | 86.12 | 4.96 | 88.24 | 5.17 | 93.17 | 5.64 | ||
| Fasting glucose (mg/dl) | F = 3.18, df = 3,367, P = .03 | |||||||||
| Aripiprazole | 85.06 | 0.69 | 86.26 | 0.76 | 87.18 | 0.87 | 84.22** | 0.89 | ||
| Risperidone | 84.60 | 0.89 | 85.81 | 1.03 | 86.61 | 1.09 | 88.24** | 1.22 | ||
| Fasting insulin (uU/ml) | F = 0.59, df = 3,372, P = .63 | |||||||||
| Aripiprazole | 12.39 | 1.10 | 13.06 | 1.23 | 13.79 | 1.40 | 14.65 | 1.39 | ||
| Risperidone | 13.10 | 1.38 | 12.60 | 1.54 | 14.14 | 1.67 | 12.18 | 1.85 | ||
| Prolactin (ng/ml) | ||||||||||
| Female participants | Aripiprazole | 61.84 | 7.61 | 11.58*** | 8.41 | 12.11*** | 8.97 | 9.72** | 11.60 | F = 11.62, df = 3,52.8, P < .0001 |
| Risperidone | 65.52 | 12.88 | 129.49*** | 14.45 | 103.50*** | 15.25 | 101.18** | 20.81 | ||
| Male participants | Aripiprazole | 28.72 | 3.05 | 9.05*** | 3.21 | 10.92*** | 3.67 | 6.59*** | 4.56 | F = 34.13, df = 3,205, P < .0001 |
| Risperidone | 31.50 | 3.24 | 54.52*** | 3.70 | 63.06*** | 4.00 | 54.23*** | 4.60 |
Note: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
aMain effect of time F = 3.84, df = 3,352, P = .01.
*Difference between treatments, P < .05, **P < .01, ***P < .0001.
Other Side Effects
The longitudinal SAFTEE-SI assessment results (supplementary table 1) showed a high rate of side effects with both agents for sleep disturbances and sedation, appetite and weight changes, and motor side effects. The SAFTEE-SI event with the greatest difference between treatments was depression. Post hoc, we examined longitudinal change in the BPRS item depressive mood. This analysis showed a medication-by-treatment interaction (F = 2.43, df = 8,1088, P = .02), as with the SAFTEE-SI data, favoring aripiprazole (supplementary figure 2).
Discussion
To our knowledge, our data are the first presented from a large double-masked randomized trial comparing aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related conditions.
What do the results suggest about choosing between aripiprazole and risperidone as the initial antipsychotic? Regarding symptom improvement, we found mostly equivalent efficacy with a potential advantage for aripiprazole. Response rates and levels of positive symptom improvement were equivalent. Avolition-apathy improved with aripiprazole and worsened with risperidone but the differences were modest. Extrapyramidal symptoms can be a cause of secondary negative symptoms but the lack of correlation in our post hoc analyses between levels of avolition-apathy and extrapyramidal symptoms suggests that the avolition-apathy findings were not driven by this mechanism. Our SAFTEE-SI data and post hoc analyses raise the possibility of an advantage for aripiprazole for depression. The medications did differ on side effects. Akathisia was more severe with aripiprazole compared with risperidone. BMI increase did not differ but aripiprazole was associated with better outcomes than risperidone on total and LDL cholesterol and prolactin. Aripiprazole was also associated with better outcomes for fasting glucose; this advantage was not seen with fasting insulin or OGTT results.
Comparable first-episode data are very limited. Zhang and colleagues17 reported on a randomized, but open-label, trial comparing aripiprazole, paliperidone, and ziprasidone for first-episode schizophrenia. Paliperidone treatment was associated with more improvement in Positive and Negative Syndrome Scale (PANSS)18 scores at 13 weeks of treatment than aripiprazole or ziprasidone. Aripiprazole was associated with more weight gain than either paliperidone or ziprasidone. An unusual feature of this trial was the very low dosing of aripiprazole (5mg) and ziprasidone (20mg) in comparison with paliperidone (6mg). How this may have affected the results is unknown. Correll and colleagues19 followed for 12 weeks 505 youth first starting an antipsychotic; antipsychotic choice was by clinician decision. The sample included 168 patients taking risperidone and 47 aripiprazole. The analyses compared baseline values and 12-week values for each agent studied (no direct medication comparisons). 95% CIs of change differed between aripiprazole and risperidone for triglycerides but not for BMI, glucose, insulin, or cholesterol.
How do our results compare with data from trials with multiepisode patients? Contrary to our symptom findings, a recent multiple-treatments meta-analysis6 of acute trials found better efficacy for improvement in total PANSS or BPRS scores with risperidone compared with aripiprazole. However, in a meta-analysis20 of acute trials focused solely on direct comparisons of the agents this advantage was not present. Consistent with our findings, this meta-analysis found an advantage for end point negative symptoms with aripiprazole. Our findings differ more with the multiepisode studies on side effects, possibly related to an increased vulnerability with young patients first starting treatment. We did not find differences in BMI change across agents in contrast with consistent findings with multiepisode patients.6,20 Multiepisode studies have found more extrapyramidal symptoms and akathisia with risperidone.20 Stroup and colleagues21 reported outcomes for 72 patients taking risperidone who had preexisting metabolic risk factors and who were randomly assigned to remain on risperidone or switch to aripiprazole. Participants switched to aripiprazole at 24 weeks of follow-up had better triglyceride and fasting insulin values but not for total cholesterol or LDL cholesterol. Our prolactin findings favoring aripiprazole over risperidone are consistent with those from multiepisode comparison studies6,20 and from studies of prolactin levels following switching from risperidone to aripiprazole.22
Our study had limitations. Our trial lasted 12 weeks which has been a widely used duration in first-episode acute trials (eg, Lieberman et al23) but a longer trial may have resulted in higher response rates.24 Our Healthy Lifestyles education program was designed to model services that are available in routine clinical settings. It is possible that our metabolic findings may be less severe than would be encountered in clinics treating patients without any program. Further, our metabolic data capture only the initial effects and not long-term effects of the antipsychotics studied. Our participants were required to have moderate or more severe positive symptoms at entry. Our negative symptom analyses therefore only address negative symptoms in the initial context of positive symptoms and may not necessarily generalize to negative symptoms in the absence of positive symptoms.
Our data have several clinical implications. One is the need to follow guideline recommendations (eg, Marder et al25) for laboratory testing for patients treated with antipsychotics as our data show that metabolic differences between antipsychotics can occur in the absence of BMI change differences. As in other first-episode studies (eg, McEvoy et al4), we found high rates of adverse events suggesting the need for close side effect monitoring with first-episode patients. Regarding which antipsychotic to choose, we found no difference in positive symptom response between agents. Aripiprazole had an advantage for negative symptoms but a disadvantage for akathisia, possibly reflecting an overall activation effect. Even though we did not find a difference in all metabolic outcomes, the differences found all favored aripiprazole. Overall, if the potential for akathisia is a concern, low-dose risperidone as used in this trial maybe a preferred choice over aripiprazole. Otherwise, aripiprazole would be the preferred choice over risperidone in most situations based upon metabolic outcome advantages and some symptom advantages within the context of similar results between medications on positive symptom response. We did not anticipate a difference between agents in depressive symptoms. Our SAFTEE-SI and post hoc BPRS analyses may be useful for hypothesis generation for future depressive symptom focused schizophrenia studies.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the National Institutes of Health (R01 MH060004 to D.G.R., K23MH100264 to J.A.G., P30MH090590 to J.M.K. and P50MH080173 to A.K.M.) and by a NARSAD Young Investigator Grant to J.A.G. from the Brain & Behavior Research Foundation. Medication supplies were donated by Bristol-Myers Squibb and by Janssen Pharmaceuticals. D.G.R. has received grants from Bristol-Meyers Squibb, Janssen, and Otsuka. G.P. has received research support from St. Jude Medical, Astra Zeneca, Proteus, Ai-Cure, Corcept Therapeutics, and Amgen. J.-P.Z. has received grant support from Genomind, Inc. L.L. has received grant funding from Janssen. C.U.C. has received grant support from Bristol-Myers Squibb, Janssen/J&J, Novo Nordisk A/S, Otsuka, and Takeda.
Supplementary Material
Acknowledgments
We are indebted to the many staff at the sites and the study participants whose commitment made the study possible. Dr Margaret Woerner provided expert input about study design and initiation. Dr Robinson has been a consultant to Asubio, Shire, and Otsuka. Dr Hassoun has been a consultant and/or advisor to or has received honoraria from Otsuka, Lundbeck, Bristol-Myers Squibb, and Sunovion. Dr Kellner receives royalties from Cambridge University Press and honoraria from UpToDate, Psychiatric Times, and North Shore-LIJ Health System. Dr Tohen was a full time employee at Lilly (1997–2008). He has received honoraria from, or consulted for, Abbott, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Sunovion, Forest, Gedeon Richter Plc., Roche, Elan, Alkermes, Lundbeck, Teva, Pamlab, Wyeth, and Wiley Publishing. His spouse was a full time employee at Lilly (1998–2013). Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Actavis, Alkermes, Bristol-Myers Squibb, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Merck, Otsuka, Pfizer, ProPhase, Reviva, Roche, Sunovion, Supernus, Takeda, and Teva. Dr Kane has been a consultant for Alkermes, Amgen, Bristol-Myers Squibb, Eli Lilly, EnVivo Pharmaceuticals (Forum), Forest, Genentech, H. Lundbeck. Intracellular Therapies, Janssen Pharmaceutica, Johnson and Johnson, Merck, Novartis, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, and Teva; has received honoraria for lectures from Bristol-Myers Squibb, Janssen, Genentech, Lundbeck, and Otsuka; and is a Shareholder in MedAvante, Inc. and the Vanguard Research Group. Dr Malhotra has been a consultant to Genomind, Inc. and Forum Pharmaceuticals. Drs Gallego, John, Braga, Sevy, Addington, and Lencz and Ms Naraine, Bennett, and Greenberg have no conflicts of interest in relation to the subject of this study. Clinical trials registration: NCT00320671.
References
- 1. Robinson DG, Woerner MG, Delman HM, Kane JM. Pharmacological treatments for first-episode schizophrenia. Schizophr Bull. 2005;31:705–722. [DOI] [PubMed] [Google Scholar]
- 2. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163:2096–2102. [DOI] [PubMed] [Google Scholar]
- 4. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1050–1060. [DOI] [PubMed] [Google Scholar]
- 5. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71:1350–1363. [DOI] [PubMed] [Google Scholar]
- 6. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. [DOI] [PubMed] [Google Scholar]
- 7. Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 1988;24:112–117. [PubMed] [Google Scholar]
- 8. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 9. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis 1 DSM-IV Disorders Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 1998. [Google Scholar]
- 10. Funke B, Finn CT, Plocik AM, et al. Association of the DTNBP1 locus with schizophrenia in a U.S. population. Am J Hum Genet. 2004;75:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson D, Woerner M, Schooler N. Intervention research in psychosis: issues related to clinical assessment. Schizophr Bull. 2000;26:551–556. [DOI] [PubMed] [Google Scholar]
- 12. Guy W, Bonato RR. CGI: Clinical Global Impressions. In: ECDEU Assess Manual Psychopharmacol-Revised. Rockville, MD: US Department of Health, Education and Welfare, ADAMHA, MIMH Psychopharmacology Research Branch; 1976:217–222. [Google Scholar]
- 13. Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- 14. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 15. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 16. Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39:486–487. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Dai G. Efficacy and metabolic influence of paliperidone ER, aripiprazole and ziprasidone to patients with first-episode schizophrenia through 52 weeks follow-up in China. Hum Psychopharmacol. 2012;27:605–614. [DOI] [PubMed] [Google Scholar]
- 18. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 19. Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khanna P, Suo T, Komossa K, et al. Aripiprazole versus Other Atypical Antipsychotics for Schizophrenia. Cochrane Database of Systematic Reviews [Internet] John Wiley & Sons, Ltd; 1996. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006569.pub5/abstract Assessed April 6, 2015. [Google Scholar]
- 21. Stroup TS, McEvoy JP, Ring KD, et al. ; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Byerly MJ, Marcus RN, Tran QV, Eudicone JM, Whitehead R, Baker RA. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009;107:218–222. [DOI] [PubMed] [Google Scholar]
- 23. Lieberman JA, Tollefson G, Tohen M, et al. ; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. [DOI] [PubMed] [Google Scholar]
- 24. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72:1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–1349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.