Abstract
Background
Tesamorelin, a synthetic analog of human growth hormone-releasing factor, decreases visceral adipose tissue (VAT) in human immunodeficiency virus (HIV)-infected patients with lipodystrophy.
Objectives
1) To evaluate the utility of patient characteristics and validated disease-risk scores, namely indicator variables for the metabolic syndrome defined by the International Diabetes Federation (MetS-IDF) or the National Cholesterol Education Program (MetS-NCEP) and the Framingham Risk Score (FRS), as predictors of VAT reduction during tesamorelin therapy at 3 and 6 months, and 2) To explore the characteristics of patients who reached a threshold of VAT <140 cm2, a level associated with lower risk of adverse health outcomes, after 6 months of treatment with tesamorelin.
Methods
Data were analyzed from two Phase 3 studies in which HIV-infected patients with excess abdominal fat were randomized in a 2:1 ratio to receive tesamorelin 2 mg (n = 543) or placebo (n = 263) subcutaneously daily for 6 months, using ANOVA and ANCOVA models.
Results
Metabolic syndrome (MetS-IDF or MetS-NCEP) and FRS were significantly associated with VAT at baseline. Presence of metabolic syndrome ([MetS-NCEP), triglyceride levels >1.7 mmol/L, and white race had a significant impact on likelihood of response to tesamorelin after 6 months of therapy (interaction p-values 0.054, 0.063, and 0.025, respectively). No predictive factors were identified at 3 months. The odds of a VAT reduction to <140 cm2 for subjects treated with tesamorelin was 3.9 times greater than that of subjects randomized to placebo after controlling for study, gender, baseline body mass index (BMI) and baseline VAT (95% confidence interval [CI] 2.03; 7.44).
Conclusions
Individuals with baseline MetS-NCEP, elevated triglyceride levels, or white race were most likely to experience reductions in VAT after 6 months of tesamorelin treatment. The odds of response of VAT <140 cm2 was 3.9 times greater for tesamorelin-treated patients than that of patients receiving placebo.
Introduction
The long-term use of antiretroviral therapy (ART) in patients with human immunodeficiency virus (HIV) is associated with the variable development of increased visceral adiposity (lipohypertrophy), loss of subcutaneous fat (lipoatrophy), dyslipidemia, and insulin resistance. These changes in body shape and metabolism are sometimes termed ‘HIV-associated lipodystrophy syndrome’ [1–4]. These changes in body shape may negatively impact adherence to ART, as well as patients’ perspective of their body image and their quality of life [5–10]. Of potentially greater concern is an increased risk of type 2 diabetes (T2DM) and cardiovascular diseases (CVD) in patients with this syndrome [11–15]. Increased visceral adipose tissue (VAT) is commonly seen in patients with increased waist circumference (WC). Both of these factors are associated with metabolic abnormalities and are independent predictors of T2DM, CVD and mortality in HIV-negative patients [16–23]. In HIV-infected patients, increased VAT is associated with an adverse lipid profile, elevated Framingham Risk Score (FRS), and increased mortality [24–27]. Furthermore, VAT is positively correlated with greater baseline and greater rate of progression of coronary artery calcium, a surrogate marker of atherosclerosis [28–29].
Tesamorelin (Theratechnologies, Inc., Montreal, Quebec, Canada) is a synthetic analog of human growth hormone-releasing factor (also known as growth hormone-releasing hormone, GHRH), which is indicated for the treatment of excess abdominal fat in HIV-infected patients with lipodystrophy. The basis of the current report was a pooled analysis of the two pivotal, randomized Phase 3 trials in 806 ART-treated patients with HIV and excess abdominal fat, who were randomized 2:1 to receive tesamorelin 2 mg (n = 543) or placebo (n = 263) subcutaneously (sc) daily. Efficacy and safety outcomes of these studies have been described previously [30–32].
In the current analysis, we investigated tools to identify patients who are likely to respond to therapy with tesamorelin, using disease-risk scores previously applied to HIV-infected cohorts, and characterized patients who reached a threshold of VAT <140 cm2 after 6 months of treatment.
Methods
Study Design
These exploratory analyses aimed to construct a statistical model to identify patients likely to respond to tesamorelin after 3 or 6 months of therapy in the clinical setting. The model used easily measurable characteristics and standard disease-risk scores previously applied to HIV-infected cohorts, including indicator variables for metabolic syndrome (MetS), defined by the International Diabetes Federation (MetS-IDF) and defined by the National Cholesterol Education Program (MetS-NCEP), as well as the Framingham Risk Score (FRS) [33–35]. The primary analysis assessed the association between absolute change in VAT from baseline to 3 months and from baseline to 6 months, and baseline covariates. Selected baseline covariates for the analysis were: FRS (continuous and dichotomous: FRS ≥10% vs. FRS <10%), MetS-NCEP (present vs. absent), MetS-IDF (present vs. absent), age, gender, race, body mass index (BMI), WC, waist-to-hip ratio, weight, blood pressure (BP), cholesterol, triglycerides, duration of HIV infection, duration of ART, protease inhibitor (PI)-based highly active antiretroviral therapy (HAART), non-nucleoside reverse transcriptase inhibitor-based HAART, and other medication use. The secondary analysis explored the characteristics of subjects who reached a threshold of VAT <140 cm2 after treatment for 6 months, as this level has been associated with lower risk for adverse outcomes [23, 36, 37].
Subjects
The patient population for both studies pooled for these analyses comprised HIV-infected individuals receiving stable ART aged 18–65 years who had abdominal fat accumulation (defined as patients with a WC of ≥ 95 cm for men and ≥ 94 cm for women plus an elevated waist-to-hip ratio of ≥ 0.94 for men and ≥ 0.88 for women) [38]. Women with a normal mammogram within 6 months of study and not pregnant were included. Subjects were excluded with (1) BMI < 20 kg/m2; (2) HIV-related disease/ infection within 3 months of study; (3) history of malignancy or active neoplasm; (4) prostate specific antigen (PSA) > 5 μg/L; (5) history of pituitary tumor/- surgery or head irradiation; (6) untreated hypothyroidism; (7) prior use of insulin, oral hypoglycemic, or insulin sensitizing agent witin 6 months of study; (8) alanine aminotransferase or aspartate aminotransferase > 3 x normal; (9) creatinine > 133 μmol/L; (10) hemoglobin > 20 g/L below normal; (11) fasting blood glucose ≥ 8.33 mmol/L, known history of Type I diabetes mellitus or Type II diabetes mellitus requiring medication; (12) fasting triglycerides > 11.3 mmol/L or change in lipid-lowering regimen within 3 months before study; (13) untreated hypertension; (14) change in testosterone regimen and/or supraphysiological dose of testosterone; (15) estrogen therapy; (16) anoretics/anorexigenics or anti-obesity agents within 3 months of study; growth hormone (GH), GH secretagogues, GHRH products, insulin-like growth factor-1 (IGF-1), or insulin-like growth factor-binding protein-3 (IGFBP-3) within 6 months of study; (18) drug or alcohol dependence or use of methadone within 6 months of study entry; and (19) participation in a clinical trial with any investigational drug/device within 30 days of screening.
Assessment of Visceral Adipose Tissue
Visceral adipose tissue (VAT) was assessed by computed tomography (CT) scan from a single 5-mm slice obtained at the level of L4-L5 intervertebral disc. Images were analyzed in a blinded fashion at a Central Imaging Reading Centre (Perceptive Informatics, Waltham, MA, USA).
Statistical Analysis
Exploratory analyses were conducted in the intent-to-treat (ITT) populations of two previously reported Phase 3 studies (NCT00123253 and NCT00435136) [39–40]. Both of the Phase 3 studies included a randomized placebo-controlled 6-month primary phase followed by a safety extension. The ITT analysis set was defined as all randomized subjects who received at least one dose of study treatment. The analyses reported here were performed for the integrated ITT populations of both trials. All models included a factor for study to adjust for any differences between trials.
The following methodologies for statistical modeling were pre-specified in a post-hoc statistical analysis plan. ANOVA and ANCOVA models were built for baseline VAT with effects for the dichotomized baseline FRS (<10% vs. ≥10%), study, baseline WC, and the FRS-by-WC interaction effect and their interaction. To test the homogeneity of results between the two trials, the interaction effects were tested at α = 0.1 and omitted from the model if not significant. The ANOVA and ANCOVA modeling were repeated using the composite-risk variables MetS-NCEP and MetS-IDF, in place of FRS, and with BMI replacing WC.
A second ANOVA model was built for absolute change in VAT from baseline to 3 months and 6 months with effects for study, treatment, baseline FRS (<10% vs. ≥10%), and the interaction of baseline FRS and treatment. If the interaction was significant at α = 0.1, subset analyses were conducted by FRS <10% and FRS ≥10%. The ANOVA analyses for absolute change in VAT from baseline to 3 months and 6 months were subsequently repeated, using the composite-risk variables MetS-IDF and MetS-NCEP and other key covariates, instead of FRS.
Ethics Statement
The protocols of the two Phase 3 studies were approved by the institutional review board (IRB) used by the participating sites. These IRBs included: Biomedical Research Ethics Board Montreal General Hospital, Partners Human Research Committee IRB, IRB Services, St. Luke’s-Rooselvet Institute for Health, IRB AIDS Research Consortium of Atlanta, Western IRB, Human Subjects Protection Committee Medical IRB, IRB Rush University Medical Center, AIDS Research Alliance IRB, Kaiser Permanente Northern California IRB, Integrated Scientific and Ethical Review Board, Copernicus Group IRB, UBC Providence Health Care Research Ethics Board, IUPUI and Clarian IRB, New England IRB, Human Subjects Research Committee, Tufts New England Medical Center IRB, Committee for the Protection of Human Subjects, Human Research Protection Program University of California, Dallas VA Medical Center Human Studies Subcommittee, IRB University of Maryland, New York University School of Medicine Institutional Board of Research Associates, Conjoint Health Research Ethics Board University of Calgary Health Region, University of Cincinnati IRB, Research Ethics Board Sunnybrook Health Sciences Centre, Research Ethics Board, Fountain Valley Regional Hospital & Medical Center, Human Research Ethics Committee University of Sherbrooke, Hamilton Health Sciences, Clinical Research Ethics Committee CHUL, University Health Network Research Ethics Board, Florida Department of Health IRB, Office for the Protection of Human Subjects Medical IRB, Office of Human Research Ethics UNC Biomedical IRB, Committee on Human Research, The University of Texas Southwestern Medical Center IRB, IRB University Hospital Case Medical Center, UCSF Committee on Human Research, Tufts medical Center IRB, Ethics Committee CHU Sart-Tilman, CPP Ouest, Ethic Committee CEIC, and London MREC. All participants provided institutionally approved, written informed consent.
Results
Subject Baseline Characteristics
In total, 806 ART-treated subjects (ITT population) with HIV and anthropometric parameters consistent with excess intra-abdominal fat from two studies were included in the exploratory analyses to identify predictors of VAT response after 3 or 6 months of treatment with tesamorelin. Baseline characteristics were comparable for subjects who received tesamorelin and placebo in the two Phase 3 studies [39, 41]. Key baseline characteristics for the pooled analysis population are shown stratified by study in Table 1. Few statistical differences were seen between studies, confirming the similarity of design and allowing for pooling of individual data. No baseline differences between subjects receiving tesamorelin and placebo were revealed in the pooled analysis population after combining the studies. Baseline VAT was available for 802 individuals (n = 540 for tesamorelin and n = 262 for placebo). Subjects had a mean (standard deviation [SD]) VAT of 182.4 cm2 (83.5), consistent with markedly increased VAT. The mean (SD) FRS was 6.5% (4.3), suggestive of a low 1-year risk of CVD. Most subjects were male (85%) and Caucasian (76%). The prevalence of MetS-IDF was 73.4% and 62.8% had mean triglycerides >1.7 mmol/L. MetS-NCEP was present in 42.9% of individuals. Differences in the criteria for MetS-NCEP and MetS-IDF, including WC and number of other predictive components present, may account for the different frequencies of MetS estimated using these criteria [33, 34]. FRS and MetS (MetS-NCEP or MetS-IDF) were significantly associated with baseline VAT, even after controlling for WC (p<0.001 for all).
Table 1. Baseline Characteristics of each Study and the Pooled Analysis Population.
| Study 1 (N = 410) | Study 2 (N = 396) | Pooled Analysis Population (N = 806) | |
|---|---|---|---|
| Demographics | |||
| Age, years, mean (SD) | 47.6 (7.4) | 47.6 (7.6) | 47.6 (7.5) |
| Males, N (%) | 352 (85.9) | 333 (84.1) | 685 (85.0) |
| Race, N (%) | |||
| Caucasian | 308 (75.1) | 305 (77.0) | 613 (76.0) |
| Asian | 2 (0.5) | 3 (0.8) | 5 (0.6) |
| African American | 59 (14.4) | 46 (11.6) | 105 (13.0) |
| Hispanic | 34 (8.3) | 35 (8.8) | 69 (8.6) |
| Other | 7 (1.7) | 7 (1.8) | 14 (1.7) |
| Disease and treatment history | |||
| Duration of HIV, years, mean (SD) a | 13.3 (5.3) | 14.0 (5.6) | 13.6 (5.4) |
| Duration of ART, months, mean (SD) | 53.7 (35.5) | 52.8 (36.4) | 53.3 (35.9) |
| PI-HAART, N (%) | 229 (55.9) | 216 (54.6) | 445 (55.2) |
| Body metrics | |||
| Weight, kg, mean (SD) | 89.8 (13.9) | 88.4 (14.3) | 89.1 (14.1) |
| BMI, kg/m2, mean (SD) | 29.2 (4.2) | 28.8 (4.2) | 29.0 (4.2) |
| BMI categories, kg/m2, N (%) | |||
| Normal (18.5 to <25) | 53 (12.9) | 70 (17.7) | 123 (15.3) |
| Overweight (25 to <30) | 214 (52.2) | 189 (47.7) | 403 (50.0) |
| Obese (≥30) | 143 (34.9) | 137 (34.6) | 280 (34.7) |
| WC, cm, mean (SD) | 104.4 (9.5) | 104.8 (9.0) | 104.6 (9.3) |
| WC categories, N (%) | |||
| High (>102 cm men, >88 cm women) | 217 (52.9) | 231 (58.3) | 448 (55.6) |
| Low/moderate (≤102 cm men, ≤88 cm women) | 193 (47.1) | 165 (41.7) | 358 (44.4) |
| Waist-to-hip ratio, mean (SD) | 1.05 (0.06) | 1.05 (0.07) | 1.05 (0.07) |
| VAT, cm2, mean (SD) a | 175.9 (76.9) | 189.2 (89.5) | 182.4 (83.5) |
| Disease risk scores | |||
| FRS, mean (SD) a | 6.1% (4.1%) | 6.9% (4.4%) | 6.5% (4.3%) |
| FRS categories, N (%) a | |||
| FRS <10% | 307 (74.9) | 310 (78.3) | 617 (76.6) |
| FRS ≥10% | 43 (10.5) | 78 (19.7) | 121 (15.0) |
| Missing a | 60 (14.6) | 8 (2.0) | 68 (8.4) |
| MetS-NCEP, N (%) | 176 (42.9) | 170 (42.9) | 346 (42.9) |
| MetS-IDF, N (%) | 296 (72.2) | 296 (74.8) | 592 (73.4) |
| Laboratory assessments | |||
| Triglycerides, mg/dL, mean (SD) | 236.5 (153.9) | 233.4 (230.5) | 234.9 (195.5) |
| Triglycerides >1.7 mmol/L, N (%) | 265 (64.6) | 241 (60.9) | 506 (62.8) |
| Vital signs | |||
| Systolic BP, mmHg, mean (SD) a | 124.6 (14.3) | 122.2 (12.1) | 123.4 (13.3) |
| Diastolic BP, mmHg, mean (SD) | 79.0 (8.9) | 78.3 (7.8) | 78.7 (8.4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; BP, blood pressure; FRS, Framingham Risk Score; HIV, human immunodeficiency virus; MetS-IDF, International Diabetes Foundation definition of metabolic syndrome; MetS-NCEP, National Cholesterol Education Program defined metabolic syndrome; PI–HAART, protease inhibitor-based highly active antiretroviral therapy; SD, standard deviation; VAT; visceral adipose tissue; WC, waist circumference.
a Statistically significant difference between Study 1 and Study 2 (p<0.05 from Student’s t-test for continuous or Fisher’s exact test for categorical data). No differences between the treatment and placebo groups were seen within the pooled analysis population after combining the studies.
Absolute Change in VAT
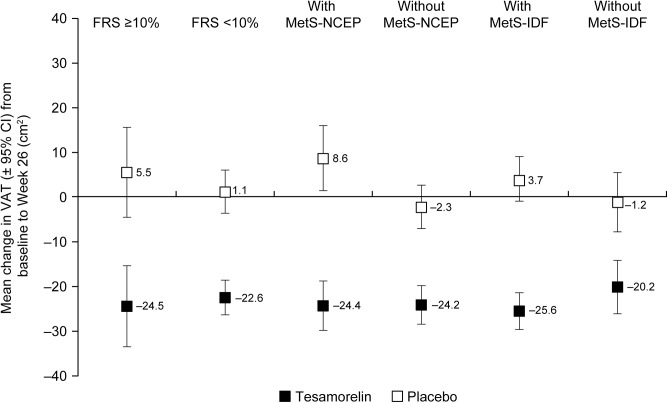
Mean changes in VAT from baseline to 6 months by treatment and risk category of FRS, MetS-NCEP and MetS-IDF are shown in Fig 1. The test of interaction showed that the treatment effect of tesamorelin versus placebo with respect to mean absolute change in VAT from baseline to 6 months was significantly greater (p = 0.054; α = 0.1) in patients with MetS-NCEP (–24.4 vs. 8.6 cm2) compared to those without MetS-NCEP (–24.2 vs. –2.3 cm2). For MetS-IDF, the interaction test did not reach statistical significance (p = 0.124; α = 0.1). However, the treatment effect of tesamorelin versus placebo was numerically greater for patients with MetS-IDF (–25.6 vs. 3.7 cm2) compared to those without MetS-IDF (–20.2 vs. –1.2 cm2). The treatment effect at 6 months was not statistically different for patients with baseline FRS ≥10% and those with baseline FRS <10% (p = 0.435; α = 0.1).
Fig 1. Mean change from baseline to 6 months in VAT by treatment and baseline disease-risk category.
Baseline risk score (by FRS, MetS-NCEP or MetS-IDF) and the interaction between baseline risk score and treatment showed that the treatment effect of tesamorelin versus placebo was greater in patients with MetS-NCEP compared with those without MetS-NCEP (interaction p = 0.054). Abbreviation: FRS, Framingham Risk Score; MetS-IDF, International Diabetes Foundation definition of metabolic syndrome; MetS-NCEP, National Cholesterol Education Program-defined metabolic syndrome; VAT: visceral adipose tissue.
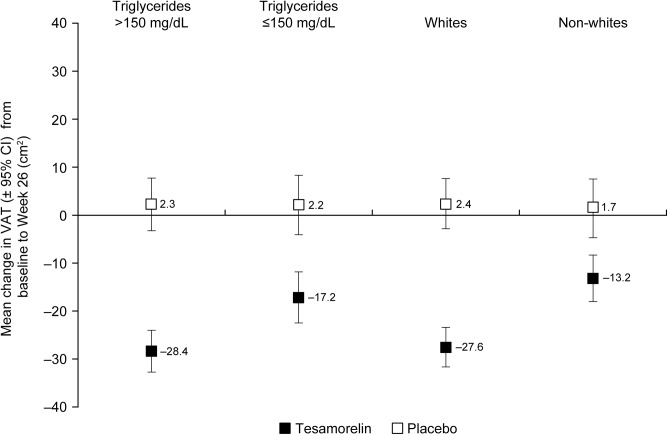
Baseline triglycerides (>1.7 mmol/L vs. ≤1.7 mmol/L) and race (white vs. non-white) were the only covariates that impacted treatment effect (interaction p = 0.063 and p = 0.025, respectively). As shown in Fig 2, the difference in mean absolute change in VAT from baseline to 6 months for tesamorelin versus placebo was larger for patients with triglycerides >1.7 mmol/L (–28.4 vs. 2.3 cm2) than for those with triglycerides ≤1.7 mmol/L (–17.2 vs. 2.2 cm2), and larger for white patients (–27.6 vs. 2.4 cm2) compared with non-white patients (–13.2 vs. 1.7 cm2). At 3 months, no significant interactions were observed between treatment and baseline covariates or disease-risk scores.
Fig 2. Mean change from baseline to 6 months by race and baseline triglyceride levels.
The difference in mean absolute change in VAT from baseline to 6 months for tesamorelin versus placebo was greater for patients with triglycerides > 1.7 mmol/L than for those with triglycerides ≤ 1.7 mmol/L (interaction p = 0.063), and greater for white patients compared with non-white patients (interaction p = 0.025).
Change in VAT to <140 cm2
The proportion of patients reaching a threshold of VAT <140 cm2 after 6 months of treatment is shown in Table 2. Different patterns of change in VAT were observed in the two treatment groups (p<0.0001). Of subjects with VAT ≥140 cm2 at baseline, 23% (n = 80 of 350) of those treated with tesamorelin for 6 months reduced their VAT to <140 cm2 compared with 9% (n = 14 of 163) of those receiving placebo. Furthermore, 44% of those with baseline VAT 140–200 cm2 and 7% of those with baseline VAT >200 cm2 had a follow-up VAT measurement of <140 cm2 following 6 months of treatment with tesamorelin, compared with 17% and 2% of those receiving placebo, respectively. Overall, of the 203 and 94 subjects receiving tesamorelin and placebo, respectively, who had baseline VAT ≥200 cm2, a greater proportion of those receiving tesamorelin achieved a reduction in VAT to <200 cm2 after 6 months (tesamorelin 34% [n = 70 of 203], placebo 16% [n = 15 of 94]). When stratified by BMI categories, 29% of those who were overweight and had a VAT ≥140 cm2 reduced their VAT to <140 cm2 after 6 months of treatment with tesamorelin compared with 24% and 15% of those who were normal weight or obese, respectively. For those receiving placebo, 11% of the overweight subjects who had a VAT ≥140 cm2 achieved a VAT reduction to <140 cm2 compared with 7% of subjects who were obese. The odds of a VAT reduction to <140 cm2 for subjects receiving tesamorelin was 3.9-times greater than that of subjects randomized to placebo after controlling for study, gender, baseline BMI and baseline VAT (95% confidence interval [CI] 2.03; 7.44). The odds of response of VAT <140 cm2 after 6 months in overweight subjects was 2.1 times greater than that of obese subjects after controlling for study, gender, baseline BMI, baseline VAT and treatment (95% CI 1.2; 3.8).
Table 2. Proportion of Patients Reaching a Threshold of VAT <140 cm2 by Treatment and BMI Category.
| Baseline VAT (cm 2 ) | |||||||
| n (%) | Placebo (N = 263) | Tesamorelin (N = 543) | |||||
| Week 26 VAT (cm 2 ) a | <140 | ≥140 | <140 | ≥140 | |||
| <140 | 86 (86.0%) | 14 (8.6%) | 185 (95.9%) | 80 (22.9%) | |||
| <140 | 140 to <200 | ≥200 | <140 | 140 to <200 | ≥200 | ||
| <140 | 86 (86.0%) | 12 (17.4%) | 2 (2.1%) | 185 (95.9%) | 65 (44.2%) | 15 (7.4%) | |
| 140 to <200 | 12 (12.0%) | 44 (63.8%) | 13 (13.8%) | 7 (3.6%) | 70 (47.6%) | 55 (27.1%) | |
| ≥200 | 2 (2.0%) | 13 (18.8%) | 79 (84.0%) | 1 (0.5%) | 12 (8.2%) | 133 (65.5%) | |
| Total | 100 | 69 | 94 | 193 | 147 | 203 | |
| Baseline VAT (cm 2 ) | |||||||
| n/N (%) | Placebo (N = 263) | Tesamorelin (N = 543) | |||||
| Week 26 VAT (cm 2 ) | BMI Category (kg/m 2 ) | <140 | ≥140 | <140 | ≥140 | ||
| <140 | Normal (18.5 to <25) | 20/24 (83.3%) | 1/19 (5.3%) | 33/35 (94.3%) | 11/45 (24.4%) | ||
| Overweight (25 to <30) | 40/48 (83.3%) | 9/85 (10.6%) | 98/101 (97.0%) | 49/169 (29.0%) | |||
| Obese (≥30) | 26/28 (92.9%) | 4/59 (6.8%) | 54/57 (94.7%) | 20/136 (14.7%) | |||
Abbreviations: BMI, body mass index; VAT; visceral adipose tissue.
a Statistically significant difference in the pattern of change in VAT for patients on tesamorelin treatment vs. placebo (p<0.0001 from exact likelihood ratio test of association).
Discussion
Tesamorelin has been shown to reduce VAT in HIV-infected patients with lipodystrophy. In this analysis, we identify some predictors of response to tesamorelin. Presence of MetS-NCEP, high triglycerides, and white race were associated with a greater likelihood of responding to 6 months of tesamorelin treatment. Although VAT was significantly correlated with both MetS-NCEP and MetS-IDF at baseline, even after controlling for WC, and both measures showed the same pattern of response to treatment, only MetS-NCEP reached the pre-specified level of significance. It appears that MetS-NCEP was a more sensitive indicator of response to tesamorelin treatment than MetS-IDF in our study population. Central obesity, as defined by WC, is a required component of the IDF definition, whereas WC is only one of five factors that define MetS-NCEP [42]. However, based on the inclusion criteria for these two Phase 3 trials, all participants with MetS-NCEP had high WC. Our data demonstrating that patients with MetS-IDF responded to tesamorelin treatment similarly to patients with MetS-NCEP suggest that presence of MetS-IDF can be used in the clinical setting to identify patients eligible to tesamorelin therapy. We also observed an apparent VAT increase in placebo-treated subjects with MetS or a high FRS over the 6-month period. This observation is indicative of a trend for increasing VAT in the untreated, at risk population. In a summary of eight recent studies in treatment-naïve, HIV-infected patients initiating highly active antiretroviral therapy (HAART), VAT increases in response to first-line treatment were variable, which led the author to conclude that VAT may still increase with current regimens [43]. Our finding demonstrating that presence of high triglyderides was a predictor of response to tesamorelin could be a reflexion of the documentated positive association between increased VAT and high triglycerides and WC in HIV-infected patients [44]. To our knowledge, there are no data in the scientific literature demonstrating genetic variability in the pharmacodynamic response to growth hormone-releasing factor or growth hormone. Nonetheless, data from a recent population pharmacokinetic analysis indicate that race does not affect tesamorelin pharmacokinetics in HIV-infected patients and healthy subjects [45]. No predictors of response were identified when data were examined at the earlier 3-month time point.
A level of VAT <140 cm2 has been associated with lower risk of adverse health outcomes [23, 36, 37]. In the current analysis, we identified a variable response to treatment depending on baseline VAT above a certain threshold, as well as by different BMI categories. The most robust response appears to be in subjects with VAT above 140 cm2 but below 200 cm2, as well as those in the overweight range for BMI measures. However, individuals with VAT >200 cm2 still lost a significant amount of VAT. Stanforth et al. [46] showed that a VAT-prediction model derived from the HERITAGE Family Study had decreasing accuracy with increasing abdominal visceral fat. It is possible that our tools to accurately identify VAT may be limited. For example, a CT scan at L4–L5 may not measure VAT with the same accuracy in the obese or highest VAT group who may have a more variable distribution of VAT, and hence the reduction with treatment may not be captured as precisely at the same vertebral level [47–49]. Our results demonstrating a decrease to level of VAT <140 cm2 in normal weight subjects (BMI 18.5 to <25 kg/m2) with VAT ≥140 cm2 at baseline may have important clinical implications as a significant proportion of HIV-infected patients may present with sarcopenic obesity [50], which could be due to a reduced GHRH secretion, resulting in decreased GH synthesis, and subsequently, lower hepatic production of IGF-1 [51]. Obesity, loss of muscle mass, and decreased IGF-1 levels are independently associated with disability and frailty in elderly subjects [52–54]. Data from the Study of Fat Redistribution and Metabolic Change in HIV infection (FRAM) showed that decreased limb muscle mass and increased VAT are associated with all-cause 5-year mortality in HIV-infected patients [27].
It is unclear if a reduction of VAT to below a critical threshold is necessary or if a clinical benefit can be achieved with any amount of VAT reduction, especially as this has not been studied in the HIV-infected population. Among overweight and obese individuals in the general population, the prevalence of hypertension, impaired fasting glucose levels, and MetS has been shown to increase linearly and significantly across increasing VAT quartiles [55–56]. The relationship between visceral adiposity and cardiovascular risk should be considered as a continuum [57–58], with an increased risk of metabolic abnormalities and cardiovascular endpoints generally occurring with VAT levels above the range of 130–150 cm2 [36, 37, 59]. In patients with HIV, increased VAT has been associated with atherogenic lipid profiles,elevated FRS, and increased epicardial and liver fat [60–62]. Furthermore, VAT was independently associated with prevalent cardiovascular diseases in a study involving HIV-infected men [63]. More recently, Freitas et al. [64] reported a positive correlation beween VAT and carotid intima media thickness in HIV-infected patients with lipodystrophy. Tesamorelin has been shown to improve lipid profile by reducing triglyceride levels, without clinically meaningful changes in glucose parameters. While there was a statistically significant difference in HbA1C elevation between the tesamorelin and placebo arms, no clinically significant changes in glycemic measures have been observed [30, 39]. Nonetheless, all patients treated with tesamorelin should be monitored periodically for changes in glucose metabolism. Administration of tesamorelin to HIV-infected patients with excess abdominal fat was recently shown to be associated with a significant decrease in liver fat. Interestingly, the reduction in liver fat was significantly associated with the reduction in VAT [65].
A recent exploratory analysis of the same Phase 3 data from over 800 patients found that the metabolic benefits of tesamorelin are limited to HIV-infected patients who respond to tesamorelin with a reduction in VAT [32]. Decreased VAT during tesamorelin therapy was associated with improvements in triglycerides, adiponectin levels, and preservation of glucose homeostasis. These benefits were not seen in individuals who do not respond to tesamorelin with a reduction in VAT. A recent study in HIV-negative patients showed a correlation between VAT reduction and the number of obesity-related risk factors with central adiposity [66]. However, this study involved all Asian patients, who accounted for only 5% of our study population. The current analyses differ from those published previously in that absolute change in VAT was examined to identify predictive factors. The rationale to select absolute change in VAT rather than percent change in VAT was based on the Phase 3 data showing that below a certain baseline VAT, patients had little, if any, VAT reduction with tesamorelin, despite the fact that all subjects had excessive accumulation of abdominal fat at baseline, as defined by pre-specified entry criteria [39]. This indicates that the actual measured VAT bears some value in determining how a patient will respond to tesamorelin.
The analyses presented here are pooled post-hoc analyses of two studies that were not originally designed to collect data specifically for calculations of the three composite risk scores (FRS, MetS-NCEP, and MetS-IDF). Therefore, it was necessary to derive some of the data elements needed for the composite risk scores and to conduct data review of some of the textual data collected to extract the needed data. Additional study limitations relate to the study population and ART regimens in this analysis. As the study population consisted largely of white male patients, our findings may not apply to other ethnicities or to women. Furthermore, most patients received older, less metabolically neutral ART regimens. Lastly, study eligibility criteria required all patients to have anthropometric parameters consistent with abdominal obesity for enrollment. Thus, it remains uncertain if only treated HIV patients with the excess VAT towards the higher end of the spectrum are those most likely to benefit from tesamorelin therapy.
In conclusion, patients with baseline MetS-NCEP, elevated triglycerides, or of white race are most likely to experience reductions in VAT after 6 months of therapy with tesamorelin. Furthermore, while overweight rather than obese individuals and those with VAT below 200 cm2 at baseline were most likely to achieve a reduction in VAT below the 140 cm2 threshold, it is currently unknown whether a clinical benefit can be achieved with any amount of VAT reduction.
Acknowledgments
PROMETRIKA, LCC (Cambridge, MA) (MS) provided statistical analyses support. Medical writing support was provided by Caroline Hoang at ACUMED (New York, NY) and Siân Marshall for ACUMED (UK).
Data Availability
All relevant data are within the paper.
Funding Statement
Theratechnologies, Inc. sponsored the two Phase 3 studies. Exploratory analyses were sponsored and conducted by EMD Serono, Inc. EMD Serono, Inc., Theratechnologies Inc. and PROMETRIKA provided support in the form of salaries for authors AM, BH, JCM and MS respectively, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2012;8: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cofrancesco J Jr, Freedland E, McComsey G. Treatment options for HIV-associated central fat accumulation. AIDS Patient Care STDS. 2009;23: 5–18. 10.1089/apc.2008.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morse CG, Kovacs JA. Metabolic and skeletal complications of HIV infection: the price of success. JAMA. 2006;296: 844–854. [DOI] [PubMed] [Google Scholar]
- 4. Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205 (Suppl 3): S383–S390. 10.1093/infdis/jis205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duran S, Saves M, Spire B, Cailleton V, Sobel A, Carrieri P, et al. Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS. 2001;15: 2441–2444. [DOI] [PubMed] [Google Scholar]
- 6. Blashill AJ, Gordon JR, Safren SA. Appearance concerns and psychological distress among HIV-infected individuals with injection drug use histories: prospective analyses. AIDS Patient Care STDS. 2012;26: 557–561. 10.1089/apc.2012.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duracinsky M, Lalanne C, Le Cœur S, Herrman S, Berzins B, Armstrong AR, et al. Psychometric validation of the PROQOL-HIV questionnaire, a new health-related quality of life instrument-specific to HIV disease. J Acquir Immune Defic Syndr. 2012;59: 506–515. 10.1097/QAI.0b013e31824be3f2 [DOI] [PubMed] [Google Scholar]
- 8. Glass TR, Battegay M, Cavassini M, De Geest S, Furrer H, Vernazza PL, et al. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2010;54: 197–203. 10.1097/QAI.0b013e3181ca48bf [DOI] [PubMed] [Google Scholar]
- 9. Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients' perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45: 334–341. [DOI] [PubMed] [Google Scholar]
- 10. Protopopescu C, Raffi F, Roux P, Reynes J, Dellamonica P, Spire B, et al. Factors associated with non-adherence to long-term highly active antiretroviral therapy: a 10 year follow-up analysis with correction for the bias induced by missing data. J Antimicrob Chemother. 2009;64: 599–606. 10.1093/jac/dkp232 [DOI] [PubMed] [Google Scholar]
- 11. Barbaro G, Barbarini G. Highly active antiretroviral therapy-associated metabolic syndrome and cardiovascular risk. Chemotherapy. 2006;52: 161–165. [DOI] [PubMed] [Google Scholar]
- 12. Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 13. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005; 352: 48–62. [DOI] [PubMed] [Google Scholar]
- 14. Hadigan C, Meigs JB, Corcoran C, Rietschell P, Piecuch S, Basgoz N, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001; 32: 130–139. [DOI] [PubMed] [Google Scholar]
- 15. Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205(Suppl 3):S355–S361. 10.1093/infdis/jis195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29: 932–940. 10.1093/eurheartj/ehn018 [DOI] [PubMed] [Google Scholar]
- 17. Ghandehari H, Le V, Kamal-Bahl S, Bassin SL, Wong ND. Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int J Obes (Lond). 2009;33: 239–248. [DOI] [PubMed] [Google Scholar]
- 18. Hu G, Tuomilehto J, Silventoinen K, Sarti C, Männistö S, Jousilahti P, et al. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 19. Nanchahal K, Morris JN, Sullivan LM, Wilson PW. Coronary heart disease risk in men and the epidemic of overweight and obesity. Int J Obes (Lond). 2005;29: 317–323. [DOI] [PubMed] [Google Scholar]
- 20. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359: 2105–2120. 10.1056/NEJMoa0801891 [DOI] [PubMed] [Google Scholar]
- 21. Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL, et al. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity (Silver Spring). 2009;17: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 22. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 23. Katzmarzyk PT, Mire E, Bouchard C. Abdominal obesity and mortality: The Pennington Center Longitudinal Study. Nutr Diabetes. 2012;2: e42 10.1038/nutd.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Currier J, Scherzer R, Bacchetti P, Heymsfield S, Lee D, Sidney S, et al. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008;48: 35–43. 10.1097/QAI.0b013e318164227f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lake JE, Wohl D, Scherzer R, Grunfeld C, Sidney S, Currier JS. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23: 929–938. 10.1080/09540121.2010.543885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S, Bacchetti P, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48: 44–52. 10.1097/QAI.0b013e31816d9ba1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scherzer R, Heymsfield SB, Lee D, Powderly G, Tien PC, Bacchetti P, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25: 1405–1414. 10.1097/QAD.0b013e32834884e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guaraldi G, Zona S, Orlando G, Carli F, Ligabue G, Fiocchi F, et al. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2012;28: 935–941. 10.1007/s10554-011-9898-y [DOI] [PubMed] [Google Scholar]
- 29. Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24: 243–253. 10.1097/QAD.0b013e328333ea9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falutz J, Mamputu JC, Potvin D, Moyle G, Soulban G, Mamputu JC, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled Phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95: 4291–4304. 10.1210/jc.2010-0490 [DOI] [PubMed] [Google Scholar]
- 31. Stanley TL, Falutz J, Mamputu JC, Soulban G, Potvin D, Grinsponn SK. Effects of tesamorelin on inflammatory markers in HIV patients with excess abdominal fat: relationship with visceral adipose reduction. AIDS. 2011;25: 1281–1288. 10.1097/QAD.0b013e328347f3f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanley TL, Falutz J, Marsolais C, Morin, Soulban G, Mamputu JC, et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis. 2012;54: 1642–1651. 10.1093/cid/cis251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23: 469–480. [DOI] [PubMed] [Google Scholar]
- 34. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 35. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97: 1837–1847. [DOI] [PubMed] [Google Scholar]
- 36. Despres JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev. 1993;6: 137–159. 10.1079/NRR19930010 [DOI] [PubMed] [Google Scholar]
- 37. Hunter GR, Snyder SW, Kekes-Szabo T, Nicholson C, Berland L. Intra-abdominal adipose tissue values associated with risk of possessing elevated blood lipids and blood pressure. Obes Res. 1994;2: 563–568. [DOI] [PubMed] [Google Scholar]
- 38. Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Després JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64: 685–693. [DOI] [PubMed] [Google Scholar]
- 39. Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357: 2359–2370. [DOI] [PubMed] [Google Scholar]
- 40. Falutz J, Allas S, Mamputu JC, Potvin D, Kotler D, Somero M, et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS. 2008;22: 1719–1728. 10.1097/QAD.0b013e32830a5058 [DOI] [PubMed] [Google Scholar]
- 41. Falutz J, Potvin D, Mamputu JC, Assaad H, Zoltowska M, Michaud SE, et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr. 2010;53: 311–322. 10.1097/QAI.0b013e3181cbdaff [DOI] [PubMed] [Google Scholar]
- 42. Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53: 2087–2094. [DOI] [PubMed] [Google Scholar]
- 43. Falutz J. Management of fat accumulation in patients with HIV infection. Curr HIV/AIDS Rep. 2011;8: 200–208. 10.1007/s11904-011-0087-3 [DOI] [PubMed] [Google Scholar]
- 44. Janiszewski PM, Ross R, Despres JP, Lemieux I, Orlando G, Carli F, et al. Hypertriglyceridemia and waist circumference predict cardiovascular risk among HIV patients: a cross-sectional study. PLoS One 2011:6: e25032 10.1371/journal.pone.0025032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. González-Sales M, Barrière O, Trembaly PO, Nekka F, Mamputu JC, Boudreau S, et al. (2014) Population pharmacokinetic analysis of tesamorelin in HIV-infected patients and healthy subjects. Clin Pharmacokinet 2014 October 31 10.1007/s40262-014-0202-x [DOI] [PubMed] [Google Scholar]
- 46. Stanforth PR, Jackson AS, Green JS, Gagnon J, Rankinen T, Desprès JP, et al. Generalized abdominal visceral fat prediction models for black and white adults aged 17–65 y: the HERITAGE Family Study. Int J Obes Relat Metab Disord. 2004;28: 925–932. [DOI] [PubMed] [Google Scholar]
- 47. Ellis KJ, Grund B, Visnegarwala F, Thackeray L, Miller CG, Chesson CE, et al. Visceral and subcutaneous adiposity measurements in adults: influence of measurement site. Obesity (Silver Spring). 2007;15: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 48. Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Regional intra-subject variability in abdominal adiposity limits usefulness of computed tomography. Obes Res. 2002;10: 260–265. [DOI] [PubMed] [Google Scholar]
- 49. Shen W, Punyanitya M, Wang Z, Gallagher D, St Onge MP, Albu J, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004; 80: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc. 2012; 60: 545–549. 10.1111/j.1532-5415.2011.03819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013; 63: 209–215. 10.1097/QAI.0b013e318289bb7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leng SX, Cappo a AR, Andersen RE, Blackman MR, Koenig K, Blair M, et al. Serum levels of insulin-like growth-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16: 153–157. [DOI] [PubMed] [Google Scholar]
- 53. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002; 50: 889–896. [DOI] [PubMed] [Google Scholar]
- 54. Jankowski CM, Gozansky WS, Van Pelt RE, Schenkman ML, Wolfe P, Schwartz RS, et al. Relative contributions of adiposity and muscularity to physical function in community-dwelling older adults. Obesity (Silver Spring). 2008;16: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116: 39–48. [DOI] [PubMed] [Google Scholar]
- 56. Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012; 97: 1517–1525. 10.1210/jc.2011-2550 [DOI] [PubMed] [Google Scholar]
- 57. Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur J Pharmacol. 2008;585: 119–129. 10.1016/j.ejphar.2008.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thomas GN, Ho SY, Lam KS, Janus ED, Hedley AJ, Lam TH. Impact of obesity and body fat distribution on cardiovascular risk factors in Hong Kong Chinese. Obes Res. 2004;12: 1805–1813. [DOI] [PubMed] [Google Scholar]
- 59. Marques MD, Santos RD, Parga JR, Rocha-Filho JA, Quaglia LA, Miname MH, et al. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. Atherosclerosis. 2010;209: 481–486. 10.1016/j.atherosclerosis.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 60. Lo J, Abbara S, Rocha-Filho JA, Shturman L, Wei J, Grinspoon SK. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24: 2127–2130. 10.1097/QAD.0b013e32833c055a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guaraldi G, Scaglioni R, Zona S, Orlando G, Carli F, Ligabue G, et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. AIDS. 2011;25: 1199–1205. 10.1097/QAD.0b013e3283474b9f [DOI] [PubMed] [Google Scholar]
- 62. Sterling RK, Smith PG, Brunt EM. Hepatic steatosis in human immunodeficiency virus: a prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J Clin Gastroenterol. 2013;47: 182–187. 10.1097/MCG.0b013e318264181d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orlando G, Guaraldi G, Zona S, Carli F, Bagni P, Menozzi M, et al. Ectopic fat is linked to prior cardiovascular events in men with HIV. J Acquir Immune Defic Syndr. 2012;59: 494–497. 10.1097/QAI.0b013e31824c8397 [DOI] [PubMed] [Google Scholar]
- 64. Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, Pereira J, et al. Carotid intima media thickness is associated with body fat abnormalities in HIV-infected patients. BMC Infect Dis. 2014; 2014 June 23;14:348 10.1186/1471-2334-14-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312: 380–389. 10.1001/jama.2014.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizayaka Y, Oka R, Okada M, et al. Reduction of Visceral Fat Correlates with the Decrease in the Number of Obesity-Related Cardiovascular Risk Factors in Japanese with Abdominal Obesity (VACATION-J Study). J Atheroscler Thromb. 2012;19: 1006–1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.