Abstract
Glioblastoma multiforme (GBM) is the most fatal form of human brain cancer. Although temozolomide (TMZ), an oral alkylating chemotherapeutic agent, improves the survival rate, the prognosis of patients with GBM remains poor. Naturally occurring carbazole alkaloids isolated from curry leaves (Murraya koenigii Spreng.) have been shown to possess a wide range of anticancer properties. However, the effects of carbazole derivatives on glioblastoma cells remain poorly understood. In the present study, anti-glioblastoma profiles of a series of synthetic carbazole derivatives were evaluated in vitro. The most promising derivative in this series was BC3EE2,9B, which showed significant anti-proliferative effects in GBM8401 and GBM8901 cells. BC3EE2,9B also triggered cell-cycle arrest, most prominently at the G1 stage, and suppressed glioblastoma cell invasion and migration. Furthermore, BC3EE2,9B induced autophagy-mediated cell death and synergistically sensitized GBM cells to TMZ cytotoxicity. The possible mechanism underlying BC3EE2,9B-induced autophagy may involve activation of adenosine monophosphate-activated protein kinase and the attenuation of the Akt and mammalian target of the rapamycin downstream signaling pathway. Taken together, the present results provide molecular evidence for the mode of action governing the ability of BC3EE2,9B to sensitize drug-resistant glioblastoma cells to the chemotherapeutic agent TMZ.
Keywords: carbazole, glioblastoma, temozolomide, autophagy, Akt
Introduction
Glioblastoma multiforme (GBM) (World Health Organization grade IV) is the most fatal form of malignant brain cancer in humans and accounts for 12–15% of all intracranial tumors and 50–60% of all primary brain tumors (1,2). Treatment usually involves radiotherapy combined with temozolomide (TMZ), a DNA-alkylating agent that mispairs nucleotide bases during DNA replication (3). Although TMZ has been shown to increase the two-year survival rate by suppressing the proliferation of glioblastoma cells and upregulation of apoptotic pathways, the overall prognosis of patients with GBM remains poor (4). To date, >75% of patients treated with TMZ succumb within 2 years due to recurrent GBM (5). The development of new combination therapies that increase the sensitivity of TMZ may improve the survival rate of patients with GBM.
Autophagy is a fundamental cellular process responsible for the bulk degradation of cytoplasmic components through an autophagosomal-lysosomal pathway (6). It is generally associated with cell survival or a protective response to stress or inflammation; however, sometimes it promotes cell death depending on specific circumstances (7). Autophagic cell death is regarded as an alternative tumor-suppressing mechanism in chemotherapy-resistant cancers, such as glioblastoma (8–10). The autophagic signaling pathway, comprising PI3K, Akt and mammalian target of rapamycin (mTOR), is an important signaling network involved in cell proliferation, survival and tumorigenesis, and is becoming an important target for treatment of several types of cancer (11). mTOR, a downstream effector of Akt, has a well-known critical role in suppressing autophagy by activating the downstream molecule p70S6 kinase (p70S6K). The role of autophagy in oncogenesis and anticancer therapy is contradictory (12). Although certain studies suggest that autophagy, rather than apoptosis, is associated with TMZ-induced chemoresistance in GBM (13), other studies have shown that TMZ is an effective tumor suppressor by inducing autophagic cell death in tumor cells, particularly in glioblastoma (14).
Carbazole alkaloids, obtained from natural sources, such as the oleoresin of curry leaves (Murraya koenigii Spreng.) (15), and their derivatives from synthesized sources, are well known for their various pharmacological activities, including anti-proliferation (16), anti-angiogenesis and anti-inflammation (17) activities, their ability to inhibit DNA topoisomerase (18), and their ability to sensitize cancer cells to anticancer drugs (19). Previous studies have demonstrated the selective antitumor activity of carbazole in several human cancer cell lines, including lung, colon, liver and leukemia (20). However, the effects of carbazole derivatives on glioblastoma cells remain poorly understood. In our recent study, we proposed a mechanism by which carbazole derivatives effectively trigger cell cycle arrest and programmed cell death in breast cancer cells (20). Carbazole derivatives have also been shown to increase autophagolysosomal membrane permeability, which may resensitize drug-resistant cancer cells to chemotherapeutic agents (21). Therefore, the sensitizing effects of carbazole derivatives may be mediated by the activation of the autophagy signaling pathway.
The aim of the present study was to determine the anti-glioblastoma effects of synthetic carbazole derivatives. In total, 35 different carbazole derivatives were synthesized and their ability to inhibit the proliferation of GBM8401 and GBM8901 cells was measured. Of the derivatives analyzed, bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B) exhibited the most promising anti-proliferation and autophagic-induced cell death activities. Exposure of GBM8901 and GBM8401 cells to BC3EE2,9B induced autophagy-mediated cell death through suppression of the Akt-mTOR signaling pathway and the cells were synergistically sensitized to TMZ cytotoxicity. The results of the study provide a rationale for the mechanism of action through which BC3EE2,9B, a carbazole derivative, sensitizes drug-resistant glioblastoma cells to the chemotherapeutic agent TMZ.
Materials and methods
Chemical reagents
TMZ, 4′,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (München, Germany). Antibodies to β-actin (#sc-47778), anti-adenosine monophosphate-activated protein kinase (AMPK; #sc-25792), anti-p-AMPK (#sc-33524), and anti-p-mTOR (#sc-101738) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies to anti-p-Akt (#05-736) and anti-Akt (#05-591) were obtained from Millipore (Bedford, MA, USA). Anti-LC3 antibodies (#NB100-2220) were purchased from Novus Biologicals (Littleton, CO, USA). Primary antibodies were used at a dilution of 1:1,000 in 0.1% Tween-20 and secondary antibodies were used at 1:5,000 dilutions. Acridine orange was obtained from Life Technologies (Rockville, MD, USA). Carbazole derivatives were synthesized in the laboratory of the Graduate Institute of Pharmaceutical Chemistry, College of Pharmacy, China Medical University (Taichung, Taiwan), under the supervision of Dr L.J. Huang. Carbazole derivatives were synthesized and obtained by the laboratory of Dr L.J. Huang.
Cell cultures and cytotoxicity assays
GBM8901 and GBM8401 cells were maintained in RPMI-1640, and SK-N-MC and Detroit 551 cells were maintained in minimum essential medium (MEM), and were routinely tested for mycoplasma or any other bacterial contamination. All the cultures were supplemented with 10% fetal bovine serum (FBS), penicillin (50 IU/ml) and streptomycin (50 µg/ml), and cells were grown at 37°C in a humidified 5% CO2 atmosphere. Following treatment, cells were treated with MTT (1 mg/ml) and incubated for 2 h at 37°C. Cell viability was measured by the modified MTT assay. Absorbance of the converted dye was measured at a wavelength of 550 nm with a 96-well microplate reader.
Flow cytometry assay
For the cell cycle analysis, cells were cultured in 10-cm culture dishes and seeded at 1×106 cells/dish. Subsequently, cell pellets were fixed with methanol at −20°C overnight. Following fixation, cell pellets were incubated at 37°C for 30 min with 0.5% Triton X-100 in phosphate-buffered saline (PBS) and 0.5 µg/ml RNase A. Cells were exposed to 1 ml of PI solution (50 µg/ml) for 30 min on ice. The nuclei were analyzed in an FACScan laser flow cytometer (Becton-Dickinson, San Jose, CA, USA). Data were acquired and analyzed using WinMDI 2.8 software (Scripps Research Insititute, La Jolla, CA, USA).
Cell migration and invasion assay
The cell migration and invasion assays were performed in a 24-well Boyden chamber with an 8-µm pore size polycarbonate membrane (Corning, Inc., Corning, NY, USA). For the migration assay, 1×105 cells in 200 µl of serum-free medium were added to the upper compartment of the chamber; the lower compartment was filled with 600 µl of RPMI-1640 supplemented with 10% FBS. After incubation at 37°C for 24 h, cells remaining in the upper chamber were removed using swabs. The cells on the lower surface of the membrane were fixed with methanol and stained with 0.1% crystal violet. Images were captured and the cells were counted using a light microscope. The invasion assay was performed using the same procedure, except that the membrane was coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) to form a matrix barrier and 3×104 GBM8901 cells were added to the upper compartment of the chamber.
TUNEL assay
GBM8901 cells were seeded in 24-well plates, exposed for 48 h to microglial-conditioned medium, incubated for 48 h with either protein samples or a control solution, fixed in 4% paraformaldehyde for 30 min at 37°C and subsequently treated with DNase for 15 min. The TUNEL assay (Invitrogen, Carlsbad, CA, USA) was performed according to the manufacturer's instructions.
Immunofluorescence and acridine-orange staining assay
For the immunofluorescence assay, cells were cultured on coated slides and treated for 24 h. Following treatment, cells were fixed with 2% buffered paraformaldehyde, permeabilized in 0.25% Triton X-100 for 5 min at 4°C, and gently agitated in the presence of anti-LC3 at 4°C overnight. Subsequently, the slides were incubated with an fluorescein isothiocyanate-labeled secondary antibody, depending on the origin of the primary antibody, and 4′,6-diamidino-2-phenylindole. For the acridine-orange assay, cells were cultured on coated slides and treated for 24 h. Following treatment, cells were exposed to acridine orange solution (10 µg/ml) in PBS for 5 min and green fluorescence was detected using a fluorescence microscope (DP80/BX53; Olympus, Tokyo, Japan).
Western blot analysis
GBM8901 cells were harvested and homogenized in lysis buffer [50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, 0.5 mM phenylmethylsulfonyl fluoride and 0.5 mM dithiothreitol] for 30 min at 4°C. Equal amounts of total cellular proteins (50 µg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride membranes (Millipore) and probed using primary antibodies, followed by horseradish peroxidase-conjugated secondary antibodies. The immunocomplexes were visualized using an enhanced chemiluminescence kit (Millipore).
Plasmid transfection
DN-AKT and CE-AKT plasmids were obtained from Santa Cruz Biotechnology, Inc. Cells were transfected with transfection reagent Lipofectamine™ 2000 and incubated for 6 h. RPMI-1640 was used instead of Opti-MEM.
Statistical analysis
Statistical comparisons of differences between groups were conducted using the Student's t-test. P≤0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed with the statistical package SPSS for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA).
Results
Anti-proliferative activity of carbazole derivatives in GBM8901 and GBM8401 cells
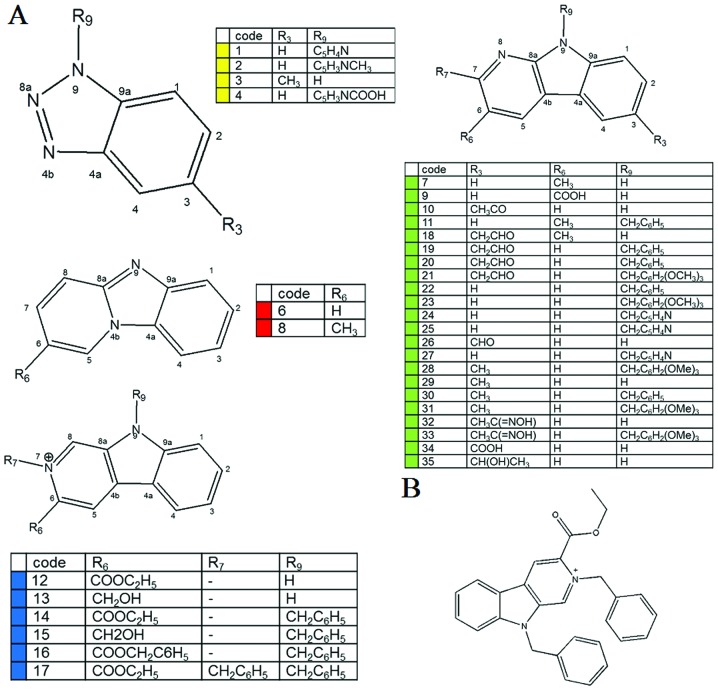
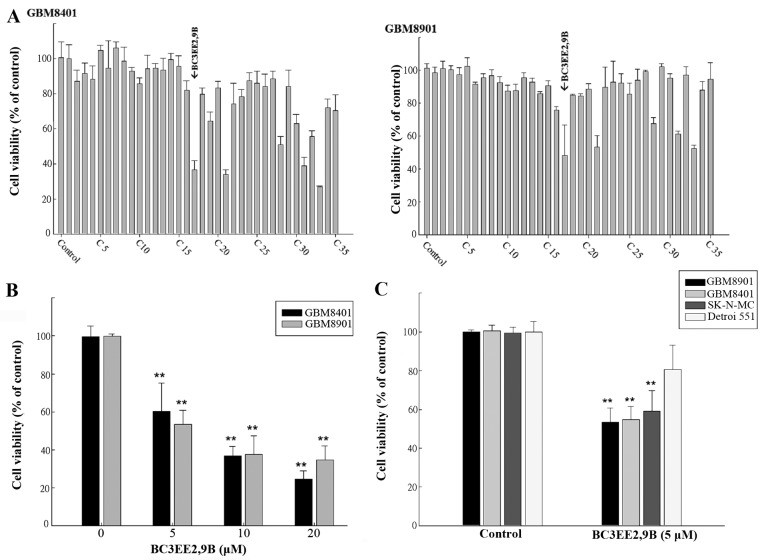
To date, only a few carbazole derivatives have been shown to have antitumor activity (23). In the present study, 35 carbazole derivatives were designed and synthesized, and the anti-proliferation activity of each derivative was tested in two human glioblastoma multiforme cell lines, GBM8901 and GBM8401 (Fig. 1A). Five of the synthetic carbazole derivatives (nos. 17, 21, 28, 31 and 33) significantly reduced the growth of glioblastoma cells at a concentration of 10 µM (Fig. 2A). Of those derivatives, BC3EE2,9B (no. 17) (Fig. 1B) had the most significant anti-proliferative effect. Viability, as a percent of control, of the GBM8901 cells was 48.13±18.6% and that of GBM8401 cells was 36.74±4.95% following exposure to BC3EE2,9B (Fig. 2A). Therefore, BC3EE2,9B was used as the primary carbazole derivative in the following experiments. To further determine the inhibitory effect of BC3EE2,9B on glioblastoma cells, a dose-range experiment was performed by treating cells with various concentrations of BC3EE2,9B for 24 h. The inhibitory effects of BC3EE2,9B were significant at concentrations ranging from 5 to 20 µM in GBM8401 (IC50=7 µM) and GBM8901 (IC50=5 µM) cells (Fig. 2B). To establish whether BC3EE2,9B is toxic to malignant tumor cells, various cell lines, including the human neuroblastoma cell line SK-N-MC and the normal skin cell line Detroit 551, were treated with 5 µM BC3EE2,9B for 24 h. BC3EE2,9B treatments significantly inhibited growth of GBM8901, GBM8401 and SK-N-MC cells, however, Detroit 551 cells at 5 µM were not significantly inhibited (Fig. 2C). These results show that BC3EE2,9B suppresses the proliferation of glioblastoma cells but not healthy cells.
Figure 1.
Chemical structures of the (A) 35 selected carbazole derivatives and (B) bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B).
Figure 2.
Carbazole derivatives have cytotoxic effects on glioblastoma cells. (A) The cytotoxic potential of the 35 carbazole derivatives. Glioblastoma multiforme (GBM)8401 and GBM8901 cells were incubated with 10 µM of carbazole derivatives for 24 h. Cytotoxicity was measured using the MTT assay, and the values are represented as means ± standard error of the mean of triplicate experiments. (B) Bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B) exerted cytotoxicity in GBM8401 and GBM8901 cells in a dose-dependent manner. (C) BC3EE2,9B exerted cytotoxicity in cancer cells with only a limited effect in Dettori 551 human normal skin fibroblasts at a concentration of 5 µM. **P<0.01 compared with the non-treated control groups.
Combination of BC3EE2,9B and TMZ synergistically increases GBM8901 cell death
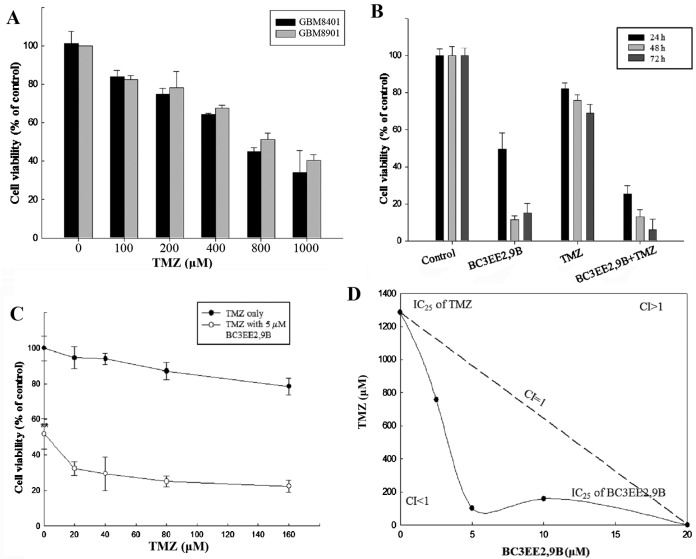
Studies have shown that treatment of glioblastoma cells with TMZ is associated with concentration-limiting toxicity. In our previous experiment, BC3EE2,9B was relatively nontoxic to normal cells. The present study aimed to improve the cytotoxic efficacy of TMZ by reducing its concentration in the presence of BC3EE2,9B. First, a dose-range experiment was performed by treating glioblastoma cells with various concentrations of TMZ alone. A high concentration of TMZ inhibited the proliferation of GBM8901 (IC50, ~800 µM) and GBM8401 (IC50, ~600 µM) cells after 24 h treatment (Fig. 3A). This indicates that TMZ at a high concentration inhibited growth on glioblastoma cells, particularly the GBM8901 cell line. Therefore, the GBM8901 cell line was used in the following experiments. Our previous data showed that BC3EE2,9B exhibits inhibitory activity against glioblastoma cells; therefore, we hypothesized that combining this compound with TMZ will increase the anti-proliferative effect. As shown in Fig. 3B, BC3EE2,9B (5 µM) combined with TMZ (100 µM) significantly inhibited GBM8901 cell growth for ≤72 h in a dose-dependent manner (Fig. 3C). To further assess whether the two compounds have synergistic benefits on growth inhibition of glioblastoma cells, an isobologram curve was plotted (Fig. 3D). The data demonstrated a strong synergistic effect (confidence interval <1) among three different ratios (TMZ:BC3EE2,9B, 750:2.5, 100:5 and 200:10, respectively) at 5 µM BC3EE2,9B combined with 100 µM TMZ. These results show that GBM8901 cells were more sensitive to combined treatment with BC3EE2,9B and TMZ.
Figure 3.
Synergistic activity of bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B) and temozolomide (TMZ) in glioblastoma cells. (A) The cytotoxicity effects in glioblastoma multiforme (GBM)8401 and GBM8901 cells of various concentrations of TMZ after 24 h treatment. (B) BC3EE2,9B combined with TMZ significantly inhibited GBM8901 cancer cell viability in a time-and dose-dependent manner. (C) BC3EE2,9B combined with doses ≤160 µM of TMZ. Viability (as a percentage of controls) of cancer cells treated with BC3EE2,9B combined with TMZ was ~20% lower compared with the viability of cancer cells treated with either BC3EE2,9B or TMZ alone. (D) The synergistic anticancer effect of the two agents was clearly demonstrated by isobologram analysis shown for the IC25 doses. Bars depict mean ± standard error of the mean of at least three experiments. A statistically significant difference from the control group, *P<0.05 and **P<0.01, respectively.
Effects of BC3EE2,9B on GBM8901 cell morphology, cell cycle and migration/invasion
Microscopic analysis revealed that GBM8901 cells exposed to 5 µM BC3EE2,9B for 24 h showed more evidence of cell shrinkage and detachment from culture plates, as well as more cytoplasmic vacuoles compared with the untreated cells (Fig. 4A). By contrast, no clear morphological changes were observed in cells treated with 100 µM of TMZ. These results suggest that BC3EE2,9B induces cell death in glioblastoma. To further analyze the nature of cell death, flow cytometric analysis was performed in GBM8901 cells following exposure to BC3EE2,9B with or without TMZ for 24 h (Fig. 4B). The results showed that 5 µM BC3EE2,9B combined with 100 µM TMZ slightly induced G1 arrest (8.65%); however, no significant sub-G1 hypoploid cell population was observed, indicating that apoptosis may not be the mechanism governing cell death. As motility and metastatic spread are well-known hallmarks of all malignant tumors, particularly glioblastoma, wound healing assays were performed to verify the effects of BC3EE2,9B on migration and invasion of GBM8901 glioblastoma cells. Cells were allowed to grow to confluence, and were scratched to create a cleared area within the monolayer. Following treatments, the movement of glioblastoma cells was imaged and quantified by measuring the migration distance at different time points and comparing it with the front area at time zero. Even a low concentration of BC3EE2,9B (2.5 µM) combined with TMZ treatment significantly inhibited cell migration at 72 h (68.81±5.71%) (Fig. 4C). Subsequently, a Transwell assay was employed to further measure the effects of BC3EE2,9B on the migratory and invasive capacities of glioblastoma cells. BC3EE2,9B (2.5 µM) alone and combined with TMZ (100 µM) markedly suppressed the migration of GBM8901 cells (Fig. 4D). As cell migration is believed to be a fundamental step in tumor invasion, the effect of chrysin on the invasive capacity of GBM8901 cells was examined. Treatment of Matrigel-coated wells with BC3EE2,9B (2.5 µM) or combined with TMZ (100 µM) significantly reduced cell invasion. In particular, the number of invasive GBM8901 cells in cultures exposed to BC3EE2,9B and TMZ decreased by 91.98±1.07% relative to the number of cells treated with TMZ alone. These findings suggest that low levels of BC3EE2,9B combined with TMZ effectively stimulate cell death via a non-apoptotic mechanism.
Figure 4.
Inhibitory effect of bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B) and temozolomide (TMZ) on glioblastoma multiforme (GBM)8901 cancer cell morphology, cell cycle and migration and invasion ability. (A) Microscopic assay showed that BC3EE2,9B causes cell shrinkage, detachment from culture plates and a significant number of cytoplasmic vacuoles in GBM8901 cells. (B) Flow cytometry with propidium iodide (PI) staining. No significant sub-G1 hypoploid cell populations were observed in cells treated with BC3EE2,9B and TMZ. Migration and invasion ability, as measured by (C) wound assay or (D) Boyden chamber assay. Data depict mean ± standard error of the mean of at least three experiments. A statistically significant difference from control group, **P<0.01. Scale bar represents 100 µM.
BC3EE2,9B combined with TMZ induces autophagy, but not apoptosis, in GBM8901 cells
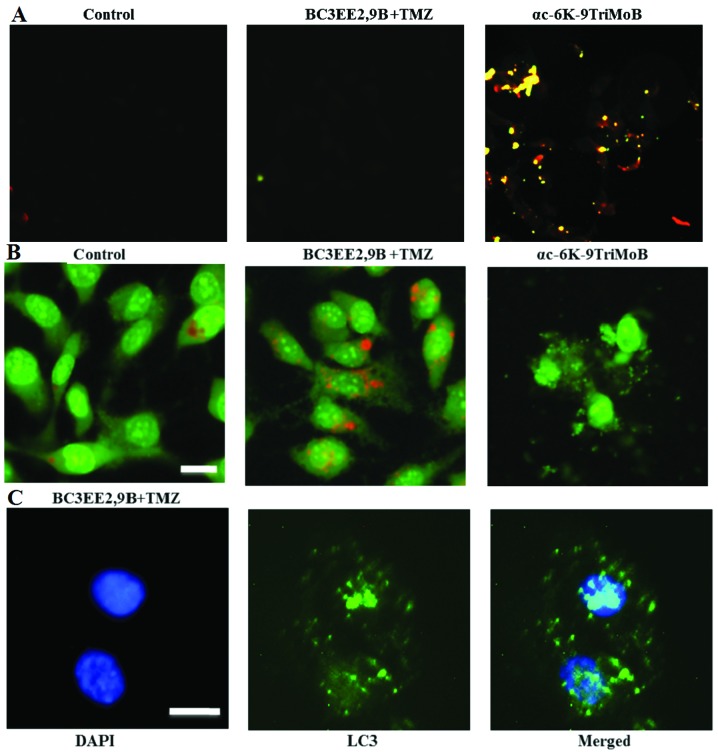
Our previous results indicated that treatment of GBM8901 cells with BC3EE2,9B results in cell death via a non-apoptotic mechanism. Therefore, the TUNEL assay was used to confirm these finding (Fig. 5A). No TUNEL-positive cells were detected after 24 h of BC3EE2,9B treatment. Notably however, another carbazole derivative, αc-6K-9TriMoB (no. 21), significantly induced apoptosis. These findings confirmed that cell death due to BC3EE2,9B combined with TMZ is not governed by an apoptotic pathway. A previous study reported that TMZ can induce autophagic cell death in malignant glioblastoma cells (14). Therefore, whether BC3EE2,9B combined with TMZ treatment also induces autophagic cell death was examined. Cells showed an increase in orange fluorescence, indicating the accumulation of acridine orange in the acidic compartments of TMZ-treated cells after exposure to BC3EE2,9B for 24 h (Fig. 5B). However, treatment with αc-6K-9TriMoB resulted in significantly less orange fluorescence. Furthermore, cells treated with combination therapy showed the presence of LC3-II aggregation, a specific marker associated with autophagosome formation (Fig. 5C). These results indicate that the combination of BC3EE2,9B with TMZ induces autophagy, but not apoptosis, in GBM8901 cells.
Figure 5.
Combination of bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B) and temozolomide (TMZ) induced autophagy, but not apoptosis, in glioblastoma multiforme (GBM)8901 cells. (A) Cell apoptosis was measured by the TUNEL assay and fluorescence microscopy. GBM8901 cells were treated with αc-6K-9TriMoB or TMZ combined with BC3EE2,9B for 24 h. (B) Fluorescence microscopy analysis of GBM8901 cells treated with TMZ combined with BC3EE2,9B for 24 h, followed by acridine orange staining. Formation of acridine orange-accumulating autophagic vacuoles (orange-red fluorescence) in cells treated with BC3EE2,9B and TMZ. (C) Cells stained with 4′,6-diamidino-2-phenylindole (DAPI) and green fluorescent anti-LC3-II. The images show that cells treated with TMZ combined with BC3EE2,9B have significant LC3-II aggregation.
BC3EE2,9B combined with TMZ enhances autophagy through inactivation of the Akt-mTOR pathway in GBM8901 cells
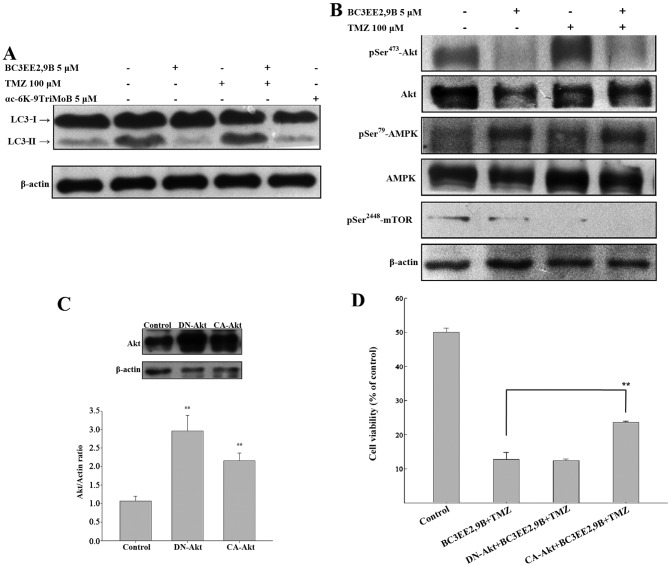
The detection of LC3-I to LC3-II conversion is a useful and sensitive marker of autophagy (22). Western immunoblotting was performed to probe for LC3. BC3EE2,9B combined with TMZ resulted in a significant increase in the LC3-II level after 24 h (Fig. 6A); however, αc-6K-9TriMoB treatment did not lead to an increase in LC3-II level. It is well-known that inhibition of Akt and its downstream target mTOR initiates autophagy. As autophagy is negatively regulated by the PI3K-Akt-mTOR pathway, interference with this pathway promotes autophagy. To elucidate whether BC3EE2,9B enhances autophagy through inactivation of the Akt-mTOR pathway, western blot assays using specific antibodies were performed. BC3EE2,9B combined with TMZ resulted in a significant decrease in Akt and mTOR phosphorylation, as well as a significant increase in AMPK phosphorylation after 24 h (Fig. 6B), suggesting that BC3EE2,9B/TMZ-induced autophagy may involve activation of AMPK and the attenuation of the Akt and mTOR downstream signaling pathway. To further confirm whether the inactivation of the Akt pathway is essential for combination treatment-induced autophagy, constitutively active Akt (CA-Akt) or dominant negative Akt (DN-Akt) plasmids were transfected into GBM8901 cells. The two plasmids were successfully transfected and expressed in glioblastoma cells (Fig. 6C). The cells were subsequently treated with BC3EE2,9B combined with TMZ to observe the treatment effect on cell viability. The results showed that the DN-Akt significantly protected against cytotoxicity of BC3EE2,9B combined with TMZ treatments (21.76%) (Fig. 6D). These results indicate that BC3EE2,9B combined with TMZ induces autophagic cell death via the AKT-mTOR pathway in GBM8901 cells.
Figure 6.
Induction of autophagy in glioblastoma multiforme (GBM)8901 cells treated with bis(carbazole-2,9N-benzyl)-3-ethyl ethanoate (BC3EE2,9B) combined with temozolomide (TMZ) is governed by the Akt-mammalian target of rapamycin (mTOR) pathway. (A) Monitoring of LC3-I to LC3-II conversion in western blot analysis shows autophagy induction. BC3EE2,9B combined with TMZ resulted in a significant increase in the levels of LC3-II after 24 h. (B) Western blot analysis showed p-Akt/Akt, p-AMPK/AMPK and p-mTOR expression in GBM8901 cells treated with BC3EE2,9B alone or treated with BC3EE2,9B in combination with TMZ (100 µM). (C) Constitutively active Akt (CA-Akt) or dominant negative Akt (DN-Akt) plasmids were transiently transfected into GBM8901 cells and confirmed by western blot analysis. (D) Overexpression of CA-Akt but DN-Akt was noted in GBM8901 cells treated with BC3EE2,9B combined with TMZ.
Discussion
GBM is one of the most treated refractory tumors. Standard therapy for GBM includes surgical resection, focal radiotherapy and treatment with the alkylating agent TMZ. However, this therapeutic approach results in only a modest increase in survival of patients with the disease. Novel therapeutic approaches aimed at improving the efficacy of TMZ are, therefore, urgently required. The present study identified that treatment with the carbazole derivative, BC3EE2,9B, resulted in a marked decrease in cell viability, migration and invasion in GBM8901 glioblastoma cells, but not in Detroit 551 normal cells. Furthermore, combination treatment with BC3EE2,9B and TMZ inhibited glioblastoma growth to a much higher extent compared with the treatment with TMZ or BC3EE2,9B alone. In addition, co-administration of BC3EE2,9B and TMZ significantly enhanced autophagic death, most likely through inhibition of the Akt-mTOR signaling pathway. This supports the idea that BC3EE2,9B synergistically sensitizes glioblastoma cells to TMZ-induced cytotoxicity. Acquired drug resistance is associated with TMZ treatment in GBM patients. The results indicate that combination therapy, comprising a toxic chemotherapeutic agent (TMZ) with a nontoxic naturally occurring compound that has anticancer properties (BC3EE2,9B) could be a new strategy to overcome this problem.
Glioblastoma cells frequently carry mutations in the PTEN tumor-suppressor gene, a gene that inhibits the continuous activation of Akt (3), making them relatively resistant to TMZ treatment (4). As mutations in glioblastoma cells often inactivate the apoptotic pathway, they are likely to be more sensitive to autophagy as an alternative response to therapeutic agents (23). Furthermore, agents that induce autophagy may result in fewer side effects than those that induce apoptosis, as apoptotic bodies are removed by phagocytic cells, thus preventing an inflammatory response (9,10). Carbazole derivatives have been shown to act as inhibitors of DNA topoisomerase and exert their cytotoxic effects in replicating cells by interfering with DNA repair and inducing DNA strand breaks (24). O6-methylguanine-DNA methyltransferase (MGMT), a DNA repair protein that removes O6-methylguanine adducts from damaged DNA, is the most important determinant of TMZ resistance in patients with GBM. As a result, proficient DNA repair activities promote glioblastoma cell survival, leading to TMZ resistance and poor clinical outcome. These observations support the present findings that although glioblastoma cells treated with TMZ alone failed to show significant autophagy, TMZ combined with BC3EE2,9B treatment inhibited Akt-mTOR signaling enhanced sensitivity of these cells by increasing the extent of autophagy and appeared to synergistically promote cell death. The autophagic effect of carbazole derivatives provides insight into the anticancer activities of this compound, particularly in TMZ-resistant and apoptosis-resistant glioblastoma cells.
AMPK is a sensor of energy status. Although it is best known for its effects on metabolism, AMPK is involved in numerous important pathways that govern a variety of physiological activities including cell growth, survival, migration and cell-cycle regulation (25). Recent studies have shown that the activation of the AMPK pathway is associated with autophagy in cancer cells (26). In the present study, BC3EE2,9B treatment decreased the phosphorylation of Akt and activated AMPK. In fact, various active compounds isolated from natural products are effective in inducing autophagic cell death in apoptosis-resistance cells. For example, ursolic acid has been shown to induce cell death and modulate autophagy in apoptosis-resistant colorectal cancer cells (27). Additionally, coibamide A, a potent anti-proliferative cyclic depsipeptide isolated from a marine cyanobacterium, has been shown to induce autophagic cell death in apoptosis-resistant glioblastoma cells (28). Therefore, a number of autophagy-inducing carbazole compounds that can activate autophagic cell death independent of the apoptotic process were synthesized in the present study. These results point to a potential therapeutic role of alkaloids in apoptosis-resistant cancers, such as TMZ-treated glioblastoma cells.
In the present study, BC3EE2,9B, a carbazole derivative, was demonstrated to inhibit glioblastoma cell migration, invasion and growth. The data also indicate that BC3EE2,9B induced autophagy-mediated cell death and synergistically sensitized GBM cells to TMZ cytotoxicity. The possible mechanism underlying BC3EE2,9B-induced autophagy may involve activation of AMPK and the attenuation of the Akt and mTOR downstream signaling pathway. Taken together, the data provide molecular evidence for the mode of action governing the ability of BC3EE2,9B to sensitize drug-resistant glioblastoma cells to the chemotherapeutic agent TMZ.
Acknowledgments
The present study was supported by grants from the Changhua Christian Hospital (no. 103-CCH-IRP-025) and from the Ministry of Science and Technology (no. 101-2320-B-040-015-MY3). Fluorescence microscopy and imaging analysis were performed at the Instrument Center of the Chung Shan Medical University, which is supported by the Ministry of Science and Technology, Ministry of Education and the Chung Shan Medical University.
References
- 1.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States 1977–2000. Cancer. 2004;101:2293–2299. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 2.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: A review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 3.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 4.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 5.Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amistà P, Morandi L, Spagnolli F, Ermani M. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: Correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 6.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen HM, Codogno P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy. 2011;7:457–465. doi: 10.4161/auto.7.5.14226. [DOI] [PubMed] [Google Scholar]
- 8.Lefranc F, Facchini V, Kiss R. Proautophagic drugs: A novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395–1403. doi: 10.1634/theoncologist.12-12-1395. [DOI] [PubMed] [Google Scholar]
- 9.Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009;1793:1516–1523. doi: 10.1016/j.bbamcr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Chen J, Zhao KN. Editorial: Signalling pathways in anti-cancer drug resistance. Curr Med Chem. 2014;21:3007–3008. doi: 10.2174/092986732126140804160443. [DOI] [PubMed] [Google Scholar]
- 12.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng X, Kinsella TJ. A novel role for DNA mismatch repair and the autophagic processing of chemotherapy drugs in human tumor cells. Autophagy. 2007;3:368–370. doi: 10.4161/auto.4205. [DOI] [PubMed] [Google Scholar]
- 14.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 15.Math M, Balasubramaniam P. Curry leaves (Murraya Koenigii spreng) and halitosis. BMJ (South Asia ED) 2003;19:211. [Google Scholar]
- 16.Tsao LT, Lee CY, Huang LJ, Kuo SC, Wang JP. Inhibition of lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages by a synthetic carbazole, LCY-2-CHO. Biochem Pharmacol. 2002;63:1961–1968. doi: 10.1016/S0006-2952(02)01023-7. [DOI] [PubMed] [Google Scholar]
- 17.Arbiser JL, Govindarajan B, Battle TE, Lynch R, Frank DA, Ushio-Fukai M, Perry BN, Stern DF, Bowden GT, Liu A, et al. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J Invest Dermatol. 2006;126:1396–1402. doi: 10.1038/sj.jid.5700276. [DOI] [PubMed] [Google Scholar]
- 18.Hajbi Y, Neagoie C, Biannic B, Chilloux A, Vedrenne E, Baldeyrou B, Bailly C, Mérour JY, Rosca S, Routier S, et al. Synthesis and biological activities of new furo[3,4-b]carbazoles: Potential topoisomerase II inhibitors. Eur J Med Chem. 2010;45:5428–5437. doi: 10.1016/j.ejmech.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Yoon S, Kim JH, Lee YJ, Ahn MY, Choi G, Kim WK, Yang Z, Lee HJ, Moon HR, Kim HS. A novel carbazole derivative, MHY407, sensitizes cancer cells to doxorubicin-, etoposide-, and radiation treatment via DNA damage. Eur J Pharmacol. 2012;697:24–31. doi: 10.1016/j.ejphar.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Liu CH, Lin C, Tsai KJ, Chuang YC, Huang YL, Lee TH, Huang LJ, Chan HC. Biological evaluation of 9-[(6-chloro-pyridin-4-yl)methyl]-9H-carbazole-3-carbinol as an anticancer agent. Oncol Rep. 2013;29:1501–1509. doi: 10.3892/or.2013.2255. [DOI] [PubMed] [Google Scholar]
- 21.Kang CC, Huang WC, Kouh CW, et al. Chemical principles for the design of a novel fluorescent probe with high cancer-targeting selectivity and sensitivity. Integr Biol. 2013;5:1217–1228. doi: 10.1039/c3ib40058a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 23.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 24.Zembower DE, Xie Y, Koohang A, Kuffel MJ, Ames MM, Zhou Y, Mishra R, Mar AA, Flavin MT, Xu ZQ. Methylenedioxy-and ethylenedioxy-fused indolocarbazoles: Potent human topoisomerase I inhibitors and antitumor agents. Anticancer Agents Med Chem. 2012;12:1117–1131. doi: 10.2174/187152012803529628. [DOI] [PubMed] [Google Scholar]
- 25.Lee YK, Park SY, Kim YM, Kim DC, Lee WS, Surh YJ, Park OJ. Suppression of mTOR via Akt-dependent and -independent mechanisms in selenium-treated colon cancer cells: Involvement of AMPKalpha1. Carcinogenesis. 2010;31:1092–1099. doi: 10.1093/carcin/bgq040. [DOI] [PubMed] [Google Scholar]
- 26.Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, Auberger P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 27.Xavier CP, Lima CF, Pedro DF, Wilson JM, Kristiansen K, Pereira-Wilson C. Ursolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cells. J Nutr Biochem. 2013;24:706–712. doi: 10.1016/j.jnutbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Hau AM, Greenwood JA, Löhr CV, Serrill JD, Proteau PJ, Ganley IG, McPhail KL, Ishmael JE. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS One. 2013;8:e65250. doi: 10.1371/journal.pone.0065250. [DOI] [PMC free article] [PubMed] [Google Scholar]