Abstract
Objective
Older adults with anxiety disorders are burdened by impairment in neurocognition, which may be mediated by elevated circulating cortisol levels. In a randomized controlled trial of acute serotonin-reuptake inhibitor treatment for late-life anxiety disorder, we examined whether change in salivary cortisol concentrations during treatment predicted improvements in measures of memory and executive function.
Methods
We examined 60 adults aged 60 and older, who took part in a 12-week trial of escitalopram vs. placebo for Generalized Anxiety Disorder. All subjects had pre- and post-treatment assessments that included monitoring of peak and total daily cortisol and a comprehensive neuropsychological evaluation.
Results
Salivary cortisol changes during treatment showed significant associations with changes in immediate and delayed memory, but no association with executive tasks (measures of working memory and set-shifting). Analyses suggested that a decrease in cortisol due to serotonin-reuptake inhibitor treatment was responsible for the memory changes: memory improvement was seen with cortisol reduction among patients receiving escitalopram, but not among patients receiving placebo.
Conclusion
Serotonin-reuptake inhibitor-induced alteration in circulating cortisol during treatment of Generalized Anxiety Disorder predicted changes in immediate and delayed memory. This finding suggests a novel treatment strategy in late-life anxiety disorders: targeting HPA axis dysfunction to improve memory.
Keywords: anxiety, elderly, cortisol, stress, memory, antidepressant, treatment
Introduction
Preserving cognitive function is a key goal of treatment of older adults. Elders with anxiety symptoms, or anxiety disorders such as Generalized Anxiety Disorder (GAD), have poorer cognitive function than healthy elderly comparisons (Beaudreau and O’Hara, 2008). The most consistent neuropsychological finding in late-life anxiety is poorer memory (Wetherell et al., 2002, Mantella et al., 2008, Butters et al., In Press, Potvin et al., 2010), although not all studies have found this relationship (Bierman et al., 2005). Neuropsychological impairments in mental disorders are important in older adults, given the association of such impairments with disability and risk for dementia (Alexopoulos et al., 2002, Middleton and Yaffe, 2009, Gallagher et al., 2011). Therefore, some treatment research has examined cognitive function as a treatment target in late-life mental disorders such as depression (Diaconescu et al., 2010, Naismith et al., 2010, Reynolds et al., 2011). To date no research has focused on improving cognition in late-life anxiety disorders, in part because little is known about its etiology (Lenze and Wetherell, 2009).
Older adults with mental disorders such as anxiety disorders have significantly higher salivary cortisol levels than nonanxious comparisons, putatively demonstrating that late-life anxiety disorders lead to dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis (Beluche et al., 2008, Chaudieu et al., 2008, Mantella et al., 2008). In turn, elevated cortisol in older adults is associated with poorer cognitive status, particularly memory (Beluche et al., 2010, Comijs et al., 2010, Lee et al., 2007, Li et al., 2006, Lupien et al., 1994). This association could be due to the effects of increased cortisol exposure on hippocampal function (McEwen, 1998, Rothman and Mattson, 2010), as the hippocampus has a high concentration of glucocorticoid receptors (Kim and Diamond, 2002). Stress-level plasma concentrations of glucocorticoids have been demonstrated to reversibly worsen memory performance (Newcomer et al., 1999, Het et al., 2005). In addition, there is increasing evidence that chronic stress effects on the hippocampus may be reversible (McEwen, 2008). Thus, in late-life mental disorders marked by hyperactive cortisol response to stress such as anxiety disorders and depression, treatments that reduce plasma cortisol might improve memory.
We have previously reported that elevated cortisol in older adults with GAD is reduced during acute treatment with the serotonin-reuptake inhibitor escitalopram, which represented either a direct serotonergic medication effect or a result of treatment-induced improvement in anxiety symptoms (Lenze et al., 2010). In this report we examined the relationship between neuropsychological change and salivary cortisol change in late-life GAD patients. We hypothesized that changes in cortisol with treatment would predict changes in neuropsychological function, particularly memory.
Methods
The study was a 12-week, double-blind, randomized controlled trial comparing the serotonin-reuptake inhibitor escitalopram and placebo (Lenze et al., 2009). Subjects were age 60 and older, with a principal diagnosis of GAD (according to the Structured Diagnostic Interview for DSM-IV axis I diagnoses [SCID])(First et al., 1996) and a score of ≥17 on the Hamilton Anxiety Scale (Hamilton, 1959). The University of Pittsburgh Institutional Review Board approved the study. Recruitment sources included primary care sites, specialty mental health practices and advertisements. Comorbid unipolar depression and other anxiety disorders were allowed; exclusion criteria included lifetime psychosis or bipolar disorder, dementia, medical instability, exogenous steroid use (including inhaled steroids), and antidepressant or anxiolytic coprescription (with the exception of continuing low-dose benzodiazepines if already in use for at least two months). Subjects were randomized to 10mg of escitalopram (increased to 20mg after four weeks if tolerated and as needed) or placebo.
Neuropsychological assessment included pre- and post- treatment testing with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)(Randolph, 1998). The present analysis focused on the two RBANS memory indices: immediate memory and delayed memory. The test battery also included two measures of executive functioning: the Letter-Number Sequencing task which tests working memory, and the Delis-Kaplan Executive Function Scale (DKEFS) sorting task (Delis et al., 2001), which tests ability to shift set (similar to the Wisconsin Card Sorting Task). Details of this assessment have been published previously; these four tests were chosen for the present analysis because they are the tests on which late-life GAD subjects performed significantly worse than non-GAD comparisons (Butters et al., In Press). Cortisol was measured by sampling salivary cortisol for two consecutive days at waking, waking plus 30 minutes, noon, 4pm, 8pm, and bedtime and taking the average value at each time. Details of the cortisol sampling have been published previously (Mantella et al., 2008). Neuropsychological assessment and cortisol measures were conducted at baseline prior to starting medication, and both were repeated at the end of the 12-week double-blind phase. The RBANS and DKEFS sorting are designed to be repeated; forms A and B were administered in a counterbalanced manner.
We examined the relationship between cortisol change and neuropsychological change in 60 GAD subjects for whom both pre and post treatment cortisol and neuropsychological data were available. Generalized Estimating Equations analyses (GEE) were used to test the hypothesis that neuropsychological change was inversely correlated with cortisol change; e.g., that decrease in cortisol during the clinical trial was associated with increase in neuropsychological functioning. GEE analysis is a regression analysis which models correlated data from longitudinal evaluations, making it appropriate for this type of hypothesis. In testing this hypothesis, we limited our examination of neuropsychological measures to the immediate memory and delayed memory indices from the RBANS, the Letter-Number Sequencing task, and the number of correct sorts on the DKEFS sorting task. Our two cortisol variables were total cortisol (an area under the curve measure using all six timepoints) and peak cortisol (30 minutes after waking). For significant GEE results, we further examined the scope of neuropsychological changes, contrasting those with reduced cortisol vs. increased or unchanged cortisol over the course of treatment.
Path analysis methods were used to assess relationships between variables of interest, including cortisol change, neuropsychological change, and treatment assignment. Additional data for the path analyses included clinical symptoms using Hamilton Anxiety Rating Scale (Hamilton, 1959) from which we examined baseline and change values, and total medical (nonpsychiatric) comorbidity rated by the Cumulative Illness Rating Scale for Geriatrics (Miller et al., 1992). Because we additionally sought to explore all of the determinants of both neuropsychological change and cortisol change, all paths between baseline variables and change variables were examined, with nonsignificant paths sequentially removed to arrive at a final model shown in figure 2. A two-tailed alpha of 0.10 was used as the cutoff for retention of path coefficients because of the small sample size.
Figure 2. Path analysis of mechanisms of memory change.
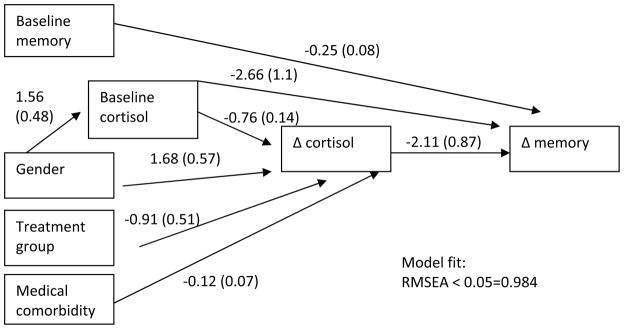
Note: Gender is coded 0=female, 1=male, treatment group is coded 0=placebo, 1=escitalopram.
Results
Table 1 shows the sample’s demographic and clinical data at baseline, and Table 2 shows changes in the primary variables of interest (cortisol: peak [30 minutes after waking] cortisol, total [area under the curve] cortisol; neuropsychological: immediate and delayed memory indices from the RBANS, Letter-Number Sequencing, DKEFS sorting task). Of the 60 subjects, 28 were randomized to escitalopram and 32 to placebo.
Table 1.
Baseline demographic and clinical variables in the sample (N=60)
| Variable | N (%) or mean (SD) | |||
|---|---|---|---|---|
| Full sample (N=60) | Placebo group (N=32) | Escitalopram group (N=28) | p | |
| Gender | ||||
| Male | n=23 (38%) | n=13 (41%) | n=10 (36%) | 0.70 |
| Female | n=37 (62%) | n=19 (59%) | n=18 (64%) | |
| Race | ||||
| Black | n=8 (13%) | n=3 (9%) | n=5 (18%) | 0.45 |
| White | n=52 (87%) | n=29 (91%) | n=23 (82%) | |
| Current Major Depressive Disorder | ||||
| Yes | n=8 (13%) | n=5 (16%) | n=3 (11%) | 0.71 |
| No | n=52 (87%) | n=27 (84%) | n=25 (89%) | |
| Taking Benzodiazepines | ||||
| Yes | n=7 (12%) | n=3 (9%) | n=4 (14%) | 0.70 |
| No | n=53 (88%) | n=29 (91%) | n=24 (86%) | |
| Age | 71.95 (SD 7.75) | 71.88 (SD 8.15) | 72.04 (SD 7.41) | 0.94 |
| Age of onset | 45.72 (SD 27.57) | 40.91 (SD 28.71) | 51.21 (SD 25.61) | 0.25 |
| Duration, months | 303.09 (SD 319.71) | 372.93 (SD 345.67) | 223.28 (SD 271.63) | 0.16 |
| Education, years | 13.98 (SD 2.35) | 14.16 (SD 2.60) | 13.79 (SD 2.06) | 0.55 |
| Cumulative Illness Rating Scale score | 8.47 (SD 4.00) | 8.16 (SD 4.11) | 8.82 (SD 3.92) | 0.53 |
| RBANS Total Score | 96.17 (SD 14.98) | 96.06 (SD 16.46) | 96.29 (SD 13.38) | 0.95 |
| Penn State Worry Questionnaire | 52.64 (SD 12.73) | 52.34 (SD 13.85) | 53.00 (SD 11.51) | 0.85 |
| Hamilton Depression Scale | 10.60 (SD 3.17) | 11.13 (SD 2.99) | 10.00 (SD 3.32) | 0.17 |
| Hamilton Anxiety Scale | 21.64 (SD 3.83) | 21.53 (SD 3.63) | 21.76 (SD 4.11) | 0.82 |
| Mini-Mental State Exam | 28.43 (SD 1.51) | 28.28 (SD 1.46) | 28.61 (SD 1.57) | 0.41 |
Note: p-values are from exact test for proportions or t-test (or non-parametric test) for continuous variables. Cortisol values are in ng/ml (to convert to nmol/l, multiply by 2.76)
RBANS: Repeatable Battery for the Assessment of Neuropsychological Status
Table 2.
Baseline and week 12 values for cortisol and neuropsychological outcome variables in the entire sample (n=60)
| WEEK 0 MEAN (SD) | WEEK 12 MEAN (SD) | t* | p | |
|---|---|---|---|---|
| Immediate Memory | 99.10 (14.66) | 102.08 (15.55) | 2.10 | 0.04 |
| Delayed Memory | 95.30 (15.79) | 99.18 (13.21) | 2.51 | 0.01 |
| Sorting Task | 7.90 (2.92) | 8.20 (3.17) | 1.16 | 0.25 |
| Letter Number Sequencing | 9.40 (2.35) | 9.47 (2.37) | 0.28 | 0.78 |
| Total cortisol | 30.27 (12.84) | 29.92 (14.48) | −0.20 | 0.84 |
| Peak cortisol | 5.28 (1.97) | 4.97 (2.35) | −0.98 | 0.33 |
paired t-tests compared pre and post treatment scores (df=59 for all)
Table 3 shows associations of cortisol changes with neuropsychological changes. The strongest effect was for total cortisol change with delayed memory, with similar effects for peak cortisol changes with delayed memory and for peak cortisol but not total cortisol with immediate memory. In each of the significant associations, the direction of the correlation was in the hypothesized direction; e.g., reduction in cortisol during treatment was associated with improvement in memory. In contrast, neither working memory nor set shifting, which are both considered executive functions, changed in concert with cortisol.
Table 3.
Association between cortisol changes and neuropsychological changes in the sample.
| Neuropsychological variable | Entire group (N=60) β* (SE) estimate, p |
Escitalopram Subgroup (N=28) β* (SE) estimate, p |
Placebo Subgroup (N=32) β* (SE) estimate, p |
|---|---|---|---|
| Memory total (Immediate + Delayed Memory) | |||
| Total cortisol | −0.33 (.11), p=0.003 | −0.42 (.18), p=0.02 | −0.22 (.13), p=0.10 |
| Peak cortisol | −2.36 (.76), p=0.002 | −3.42 (1.17), p=0.003 | −.77 (.85), p=0.38 |
| RBANS Immediate Memory Index | |||
| Total cortisol | −0.13 (.08), p=0.11 | −0.16 (.11), p=0.17 | −0.09 (.11), p=0.43 |
| Peak cortisol | −1.024 (.43), p=0.02 | −1.44 (.64), p=0.02 | −.43 (.53), p=0.42 |
| RBANS Delayed Memory Index | |||
| Total cortisol | −0.25 (.06), p=0.0001 | −0.27 (.10), p=0.009 | −0.22 (.08), p=0.006 |
| Peak cortisol | −1.53 (.48), p=0.001 | −2.04 (.68), p=0.003 | −0.88 (.67), p=0.19 |
| Working memory (Letter Number Sequencing) | |||
| Total cortisol | 0.004 (.01), p=0.7428 | n/a | n/a |
| Peak cortisol | 0.023 (.07), p=0.7506 | n/a | n/a |
| Executive function (DKEFS Sorting task) | |||
| Total cortisol | −0.002 (.02), p=0.8920 | n/a | n/a |
| Peak cortisol | 0.11 (.09), p=0.2510 | n/a | n/a |
β estimates are from generalized estimating equation models. Adjustment for treatment group (escitalopram vs. placebo) did not change the results.
Note: DKEFS: Delis-Kaplan Executive Functioning Scale; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status. Total cortisol: area under the curve estimate of 6 time points (wake, wake+30, noon, 4pm, 8pm, bedtime), Peak cortisol: wake+30 value.
Table 3 also indicates that the relationship between changes in cortisol and changes in memory (examined using GEE) was affected by treatment condition (escitalopram vs. placebo). Specifically, the relationship between peak cortisol change and both immediate and delayed memory change was stronger within escitalopram-treated subjects than placebo-treated subjects.
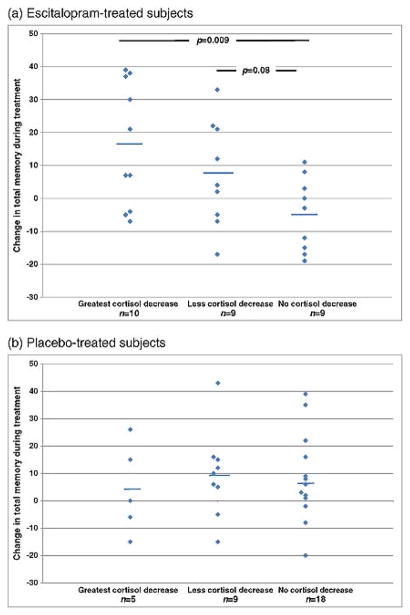
Next, we examined the scope of these memory improvements by contrasting those with reduced cortisol vs. increased or unchanged cortisol during treatment. As with the GEE, we examined a total memory score by combining the immediate and delayed memory index scores from the RBANS, and we examined the escitalopram-treated (N=28) and placebo-treated (N=32) subjects separately. Each treatment group was subdivided by how much cortisol reduced during treatment. As Figure 1 shows, memory improvements were most substantial in the escitalopram-treated patients who had the greatest reduction in peak cortisol during treatment. Conversely, among placebo-treated patients there was no relationship between cortisol change and memory change. We did not find this relationship if total cortisol was used instead of peak cortisol (data not shown).
Figure 1. Improvement in memory is associated with cortisol reduction in escitalopram-treated but not placebo-treated subjects.
a. Escitalopram-treated patients (n=28)
b. Placebo-treated patients (n=32)
ANOVAs for group differences in memory change: escitalopram, F=4.2 (df=2, 25), p=0.03; placebo, F=0.2 (df=2, 29), p=0.8.
The three subgroups by cortisol change are: greatest drop (highest quartile of cortisol reduction), range −6.6–−1.9; less drop (cortisol reduced but not highest quartile), range −1.7–−0.1; no drop, range 0.0 - +4.5. Solid lines show means. Significant or trend p-values for post-hoc t-tests are displayed.
Examining the results shown in Figure 1, the memory improvement in concert with cortisol reduction during escitalopram treatment was accounted for by eight subjects with the greatest memory improvement in the escitalopram sample. These subjects improved in delayed memory from mean 89 to mean 106.1, and in immediate memory from 97.6 to 110.6; for reference, the standard deviation of each memory scale in the RBANS is 15. Because these eight subjects may have comprised a treatment-relevant subgroup, we examined their data further (Supplementary table 1). Compared to the rest of the escitalopram sample, these 8 subjects had a greater reduction in cortisol as well as shorter duration of GAD and greater reduction in Ham-A during treatment.
Finally, we carried out a path analysis to examine the potential mechanisms underlying change in total memory (see Figure 2). We tested the direct effect of peak cortisol change on memory change, and we also tested effects of baseline demographic (age, gender, race, education), and clinical (Hamilton Anxiety score, cortisol level, neuropsychological function, treatment group assignment, and medical burden) values and of changes in clinical values (direct effect of Hamilton Anxiety score change and indirect effects of treatment group and of clinical change via cortisol changes). Model fit was good: root mean square error of approximation (RMSEA) was 0.000 (likelihood RMSEA < 0.05 = 0.984, 90% confidence interval of RMSEA estimate = 0.000-0.000). As figure 2 shows, we found an inverse association of cortisol change with memory change, consistent with the GEE analyses in Table 3, but found no other relationships to account for this association. Because the path analysis did not find either a direct or indirect relationship of anxiety symptom change with memory change using the Hamilton Anxiety Scale, we also substituted other measures of anxiety treatment response used in the study: Clinical Global Impressions-Improvement scale (dichotomizing by much to very much improvement vs. minimal to no improvement), and Penn State Worry Questionnaire score change. Neither of these changed the results.
The path analysis also revealed the following relationships: (a) men had higher baseline cortisol; (b) women, those with higher baseline cortisol, those with higher medical comorbidity, and those randomized to escitalopram experienced a greater drop in cortisol; (c) higher baseline memory and higher baseline cortisol were both associated with negative change (worsening) in memory.
Discussion
In this sample of 60 older adults with GAD in a randomized placebo-controlled trial of escitalopram, we found that cortisol changes during treatment predicted changes in immediate and delayed episodic memory. We found no relationship of cortisol change with change in executive function measures.
The relationship between peak cortisol change and memory change seen in escitalopram-treated subjects but for the most part not seen placebo-treated subjects. Further, we found a dose-response effect in which greater levels of peak cortisol reduction are associated with greater improvements in memory, in the escitalopram group; no such effect is found in the placebo group. This is consistent with our previous observation that cortisol reduction was significantly greater with escitalopram treatment (Lenze et al., 2010). A path analysis suggested a direct effect of cortisol changes on memory changes.
These results have implications for treatment development in the aging population. Cognitive impairment in older adults is a substantial public health problem, and modifiable mechanisms underlying it are of great interest. As older adults with stress-related mental disorders such as GAD and depression have elevated cortisol, our finding suggests that treatments that target cortisol elevations may produce improvements in cognition, particularly in hippocampal-based cognition (i.e., episodic memory).
Our results are consistent with prior research demonstrating that hippocampal-mediated cognition in older adults is adversely affected by HPA axis dysregulation resulting in cortisol excess (Lee et al., 2007, Li et al., 2006, Lupien et al., 1994, (Peavy et al., 2007, Comijs et al., 2010). Chronic stress and elevated glucocorticoid effects on hippocampus include synaptic changes – dendritic retraction, synapse loss – that can be reversible (Conrad, 2008, Tata and Anderson, 2010). Some preclinical and clinical research suggests that hippocampal volume loss and neuronal loss in stress paradigms are reversible with antidepressant traetment (Neumeister et al., 2005, Sheline et al., 2003, Vythilingam et al., 2004, Jayatissa et al., 2008). The serotonin system is tightly linked to the HPA axis in older adults (O’Hara et al., 2007), which may explain why we only saw this effect in those receiving serotonin-reuptake inhibitor treatment; other treatments that reduce the impact of HPA axis dysfunction might usefully be tested for their ability to improve cognition via this cortisol-mediated pathway (Hinkelmann et al., 2009).
However, we have not found that escitalopram treatment in late-life GAD provides a significant improvement in memory in the aggregate (Butters et al., In Press). Instead, the present study found substantial memory improvements in a subgroup (8/28, or 29%) of the escitalopram group. This subgroup was notable for having a treatment-related reduction in circulating cortisol, as well as poorer memory performance and higher cortisol at baseline compared to the overall sample. Conversely, some subjects had a net decrease in memory with escitalopram treatment – generally, those whose cortisol did not decrease during treatment, as seen in Figure 1. Serotonin-reuptake inhibitors such as escitalopram produce broad CNS effects including impaired attention (Fava et al., 2006, Dumont et al., 2005, Drueke et al., 2009), particularly in older adults who are more susceptible to neurocognitive side effects of psychotropics (Pollock, 2005). Therefore, future research in this area should examine treatments, pharmacologic (Young et al., 2004) or psychotherapeutic (Peavy, 2008) that more specifically block cortisol’s CNS effects. Such research should also examine whether biological and neuropsychological data can usefully subtype elderly persons with anxiety disorders, in order to match treatments that could improve cognition to those who would benefit most.
There are several caveats to this finding. First, cognitive impairments in late-life GAD include executive functions as well as memory (Butters et al., In Press, Mantella et al., 2007), but we found a relationship between cortisol reduction and memory only. It remains unclear whether other measures of executive function are sensitive to cortisol reductions in this population. Second, HPA axis dysfunction with elevated circulating cortisol levels may also cause chronic or irreversible cognitive decline with hippocampal atrophy (Sheline et al., 1996, Peavy et al., 2009, Gerritsen et al., 2009), although evidence is mixed regarding this mechanism of memory decline in elderly persons (Comijs et al., 2010). GAD tends to have a chronic course absent treatment (Lenze et al., 2005, Le Roux et al., 2005, Stanley et al., 2003), so if chronic neurotoxicity is a mechanism of cognitive impairment in late-life mental disorders, a lengthy duration of treatment would be necessary to determine whether targeting cortisol may remediate cognition by this mechanism. Third, there are likely many pathways from stress and aging to cognitive impairment (Butters et al., 2008) and we did not include other biomarker measures such as neuroimaging of the hippocampus. Many causes of cognitive impairment in late-life anxiety disorders, such as underlying neurodegenerative disease due to Alzheimer’s disease pathology, may involve the HPA axis but not be remediable (Csernansky et al., 2006, Lind et al., 2007). Finally, it is unclear whether this finding would generalize to all anxiety disorders and related mental disorders (e.g., depression). For example, Post-Traumatic Stress Disorder may have a different neuroendocrine picture (Yehuda et al., 2005, Yehuda et al., 2007), while in depression both elevated and reduced cortisol has been reported (Beluche et al., 2008, Bremmer et al., 2007, Fiocco et al., 2006, O’Brien et al., 2004, Penninx et al., 2007). These caveats suggest that future research should clarify how much neurocognitive impairment could be improved specifically by targeting cortisol and in whom.
Two other findings of mention: memory at baseline was inversely associated with change in memory (i.e., those with high memory at the start of treatment were more likely to have a decrease in memory during the study). This most likely represents a regression to the mean. Less clear is the finding that cortisol level at baseline is inversely associated with change in memory (i.e., those with high cortisol at baseline were less likely to have an improvement in memory). The finding is counterintuitive and reasons for it are unclear; perhaps such subjects had greater levels of neurodegenerative disease that could not be remediated. Both of these variables should be explored in future research.
Limitations of our study include a small sample size which could have led to negative results due to lack of power. The study suggests but cannot definitely show that memory improvements are directly due to cortisol reduction, rather than a result of improved anxiety; likely this question could only be answered in a clinical population by a direct pharmacological manipulation of cortisol or its receptors. Late-life anxiety disorder patients are a heterogeneous group with many factors that could affect cognitive outcomes, and we were unable to measure all of these factors. In particular, we did not have brain imaging to characterize brain disease. Finally, salivary cortisol is a peripherally-collected biomarker of HPA axis dysfunction and the study lacked more direct markers of the putative mechanism (e.g., glucocorticoid receptor number/function, hippocampal neuroimaging); and the neuropsychological battery is an imperfect snapshot of real world cognitive functioning.
These limitations may reduce precision of findings but do not detract from positive results in the study, which in summary demonstrate a role for cortisol reduction during late-life anxiety treatment to improve neurocognition (particularly in hippocampal-mediated domains). Preserving cognitive function is a critical goal in treatment of stress-related disorders such as anxiety disorders in elderly persons; therefore, these findings hold importance for future treatment development in the field that could target the HPA axis.
Supplementary Material
Acknowledgments
R01 MH070547 (Lenze PI); K23 MH086686 (Andreescu PI); P30 MH071944 and the UPMC Endowment for Geriatric Psychiatry (Reynolds PI); R01 MH072947 (Butters PI). Forest Laboratories provided medication and placebo for this study.
Footnotes
Disclosure statement:
Dr. Lenze discloses that he has received research funding from Forest Laboratories, as well as research support in the form of medication from Pfizer and Bristol-Myer Squibb. He has been a consultant for Fox Learning Systems.
Dr. Mantella is an employee of Abbott Labs.
Dr. Reynolds discloses that he has received research funding from Forest Laboratories and Lilly, as well as research support in the form of medication from Pfizer and Bristol-Myer Squibb.
Dr. Newcomer has received research grant support from NIMH, Sydney R. Baer Jr Foundaiton, Bristol-Myers Squibb, and Pfizer. He has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Biovail, Obecure, Janssen Pharmaceuticals, H. Lundbeck, Pfizer and Sepracor/Sunovion. He has been a consultant to litigation. He has been a member of Data Safety Monitoring Boards for Dainippon Sumitomo Pharma America, Inc., Schering-Plough/MERCK, and Vivus, Inc. He has received royalties from Jones & Bartlett Publishers for a metabolic screening form.
Dr. Butters as been a consultant for Fox Learning Systems. She also has provided neuropsychological assessment services for clinical trials conducted by Medtronic and Northstar Neuroscience.
References
- ALEXOPOULOS GS, KIOSSES DN, KLIMSTRA S, KALAYAM B, BRUCE ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- BEAUDREAU SA, O’HARA R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16:790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- BELUCHE I, CARRIERE I, RITCHIE K, ANCELIN ML. A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychol Med. 2010;40:1039–49. doi: 10.1017/S0033291709991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELUCHE I, CHAUDIEU I, NORTON J, CARRIERE I, BOULENGER JP, RITCHIE K, ANCELIN ML. Persistence of abnormal cortisol levels in elderly persons after recovery from major depression. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.10.011. [DOI] [PubMed] [Google Scholar]
- BIERMAN EJ, COMIJS HC, JONKER C, BEEKMAN AT. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005;13:686–93. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- BREMMER MA, DEEG DJ, BEEKMAN AT, PENNINX BW, LIPS P, HOOGENDIJK WJ. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry. 2007;62:479–86. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- BUTTERS MA, BHALLA RK, ANDREESCU C, WETHERELL JL, MANTELLA RC, BEGLEY AE, LENZE EJ. Changes in neuropsychological functioning following treatment of Generalized Anxiety Disorder. British Journal of Psychiatry. doi: 10.1192/bjp.bp.110.090217. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERS MA, YOUNG JB, LOPEZ O, AIZENSTEIN HJ, MULSANT BH, REYNOLDS CF, 3RD, DEKOSKY ST, BECKER JT. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–57. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUDIEU I, BELUCHE I, NORTON J, BOULENGER JP, RITCHIE K, ANCELIN ML. Abnormal reactions to environmental stress in elderly persons with anxiety disorders: evidence from a population study of diurnal cortisol changes. J Affect Disord. 2008;106:307–13. doi: 10.1016/j.jad.2007.07.025. [DOI] [PubMed] [Google Scholar]
- COMIJS HC, GERRITSEN L, PENNINX BW, BREMMER MA, DEEG DJ, GEERLINGS MI. The association between serum cortisol and cognitive decline in older persons. Am J Geriatr Psychiatry. 2010;18:42–50. doi: 10.1097/JGP.0b013e3181b970ae. [DOI] [PubMed] [Google Scholar]
- CONRAD CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSERNANSKY JG, DONG H, FAGAN AM, WANG L, XIONG C, HOLTZMAN DM, MORRIS JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–9. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELIS DC, KAPLAN E, KRAMER JH. The Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- DIACONESCU AO, KRAMER E, HERMANN C, MA Y, DHAWAN V, CHALY T, EIDELBERG D, MCINTOSH AR, SMITH GS. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUEKE B, BAETZ J, BOECKER M, MOELLER O, HIEMKE C, GRUNDER G, GAUGGEL S. Differential effects of escitalopram on attention: a placebo-controlled, double-blind cross-over study. Psychopharmacology (Berl) 2009;207:213–23. doi: 10.1007/s00213-009-1649-6. [DOI] [PubMed] [Google Scholar]
- DUMONT GJ, DE VISSER SJ, COHEN AF, VAN GERVEN JM. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVA M, GRAVES LM, BENAZZI F, SCALIA MJ, IOSIFESCU DV, ALPERT JE, PAPAKOSTAS GI. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J Clin Psychiatry. 2006;67:1754–9. doi: 10.4088/jcp.v67n1113. [DOI] [PubMed] [Google Scholar]
- FIOCCO AJ, WAN N, WEEKES N, PIM H, LUPIEN SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: relation to cognitive functioning. Stress. 2006;9:143–52. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- FIRST MB, SPITZER RL, GIBBON M. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version: Administration Booklet. Washington, DC: 1996. [Google Scholar]
- GALLAGHER D, COEN R, KILROY D, BELINSKI K, BRUCE I, COAKLEY D, WALSH B, CUNNINGHAM C, LAWLOR BA. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2011;26:166–72. doi: 10.1002/gps.2509. [DOI] [PubMed] [Google Scholar]
- GERRITSEN L, COMIJS HC, DEEG DJ, PENNINX BW, GEERLINGS MI. Salivary cortisol, APOE-varepsilon4 allele and cognitive decline in a prospective study of older persons. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.007. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- HET S, RAMLOW G, WOLF OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–84. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- HINKELMANN K, MORITZ S, BOTZENHARDT J, RIEDESEL K, WIEDEMANN K, KELLNER M, OTTE C. Cognitive impairment in major depression: association with salivary cortisol. Biol Psychiatry. 2009;66:879–85. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- JAYATISSA MN, BISGAARD CF, WEST MJ, WIBORG O. The number of granule cells in rat hippocampus is reduced after chronic mild stress and re-established after chronic escitalopram treatment. Neuropharmacology. 2008;54:530–41. doi: 10.1016/j.neuropharm.2007.11.009. [DOI] [PubMed] [Google Scholar]
- KIM JJ, DIAMOND DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- LE ROUX H, GATZ M, WETHERELL JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- LEE BK, GLASS TA, MCATEE MJ, WAND GS, BANDEEN-ROCHE K, BOLLA KI, SCHWARTZ BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64:810–8. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- LENZE EJ, MANTELLA RC, SHI P, GOATE AM, NOWOTNY P, BUTTERS MA, ANDREESCU C, THOMPSON PA, ROLLMAN BL. Elevated Cortisol in Older Adults With Generalized Anxiety Disorder Is Reduced by Treatment: A Placebo-Controlled Evaluation of Escitalopram. Am J Geriatr Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENZE EJ, MULSANT BH, MOHLMAN J, SHEAR MK, DEW MA, SCHULZ R, MILLER MD, TRACEY B, REYNOLDS CF., 3RD Generalized anxiety disorder in late life: lifetime course and comorbidity with major depressive disorder. Am J Geriatr Psychiatry. 2005;13:77–80. doi: 10.1176/appi.ajgp.13.1.77. [DOI] [PubMed] [Google Scholar]
- LENZE EJ, ROLLMAN BL, SHEAR MK, DEW MA, POLLOCK BG, CILIBERTI C, COSTANTINO M, SNYDER S, SHI P, SPITZNAGEL E, ANDREESCU C, BUTTERS MA, REYNOLDS CF. Escitalopram for older adults with Generalized Anxiety Disorder: a placebo-controlled trial. JAMA. 2009;301:296–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENZE EJ, WETHERELL JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry. 2009;24:1–14. doi: 10.1002/gps.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G, CHERRIER MM, TSUANG DW, PETRIE EC, COLASURDO EA, CRAFT S, SCHELLENBERG GD, PESKIND ER, RASKIND MA, WILKINSON CW. Salivary cortisol and memory function in human aging. Neurobiol Aging. 2006;27:1705–14. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- LIND K, EDMAN A, NORDLUND A, OLSSON T, WALLIN A. Increased saliva cortisol awakening response in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;24:389–95. doi: 10.1159/000109938. [DOI] [PubMed] [Google Scholar]
- LUPIEN S, LECOURS AR, LUSSIER I, SCHWARTZ G, NAIR NP, MEANEY MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14:2893–903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTELLA RC, BUTTERS MA, AMICO JA, MAZUMDAR S, ROLLMAN BL, BEGLEY AE, REYNOLDS CF, LENZE EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–81. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTELLA RC, BUTTERS MA, DEW MA, MULSANT BH, BEGLEY AE, TRACEY B, SHEAR MK, REYNOLDS CF, 3RD, LENZE EJ. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:673–9. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- MCEWEN BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- MCEWEN BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLETON LE, YAFFE K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–5. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER MD, PARADIS CF, HOUCK PR, MAZUMDAR S, STACK JA, RIFAI AH, MULSANT B, REYNOLDS CF., 3RD Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- NAISMITH SL, DIAMOND K, CARTER PE, NORRIE LM, REDOBLADO-HODGE MA, LEWIS SJ, HICKIE IB. Enhancing Memory in Late-Life Depression: The Effects of a Combined Psychoeducation and Cognitive Training Program. Am J Geriatr Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181dba587. [DOI] [PubMed] [Google Scholar]
- NEUMEISTER A, WOOD S, BONNE O, NUGENT AC, LUCKENBAUGH DA, YOUNG T, BAIN EE, CHARNEY DS, DREVETS WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–7. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- NEWCOMER JW, SELKE G, MELSON AK, HERSHEY T, CRAFT S, RICHARDS K, ALDERSON AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–33. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- O’BRIEN JT, LLOYD A, MCKEITH I, GHOLKAR A, FERRIER N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–90. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- O’HARA R, SCHRODER CM, MAHADEVAN R, SCHATZBERG AF, LINDLEY S, FOX S, WEINER M, KRAEMER HC, NODA A, LIN X, GRAY HL, HALLMAYER JF. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: association and interaction with cortisol. Mol Psychiatry. 2007;12:544–55. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEAVY GM. The effects of stress and APOE genotype on cognition in older adults. Am J Psychiatry. 2008;165:1376–8. doi: 10.1176/appi.ajp.2008.08081249. [DOI] [PubMed] [Google Scholar]
- PEAVY GM, LANGE KL, SALMON DP, PATTERSON TL, GOLDMAN S, GAMST AC, MILLS PJ, KHANDRIKA S, GALASKO D. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry. 2007;62:472–8. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEAVY GM, SALMON DP, JACOBSON MW, HERVEY A, GAMST AC, WOLFSON T, PATTERSON TL, GOLDMAN S, MILLS PJ, KHANDRIKA S, GALASKO D. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166:1384–91. doi: 10.1176/appi.ajp.2009.09040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINX BW, BEEKMAN AT, BANDINELLI S, CORSI AM, BREMMER M, HOOGENDIJK WJ, GURALNIK JM, FERRUCCI L. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am J Geriatr Psychiatry. 2007;15:522–9. doi: 10.1097/JGP.0b013e318033ed80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK BG. The pharmacokinetic imperative in late-life depression. J Clin Psychopharmacol. 2005;25:S19–23. doi: 10.1097/01.jcp.0000162809.69323.66. [DOI] [PubMed] [Google Scholar]
- POTVIN O, HUDON C, DION M, GRENIER S, PREVILLE M. Anxiety disorders, depressive episodes and cognitive impairment no dementia in community-dwelling older men and women. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2647. [DOI] [PubMed] [Google Scholar]
- RANDOLPH C. Repeatable Battery for the Assessment of Neuropsychological Status Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- REYNOLDS CF, 3RD, BUTTERS MA, LOPEZ O, POLLOCK BG, DEW MA, MULSANT BH, LENZE EJ, HOLM M, ROGERS JC, MAZUMDAR S, HOUCK PR, BEGLEY A, ANDERSON S, KARP JF, MILLER MD, WHYTE EM, STACK J, GILDENGERS A, SZANTO K, BENSASIS, KAUFER DI, KAMBOH MI, DEKOSKY ST. Maintenance Treatment of Depression in Old Age: A Randomized, Double-blind, Placebo-Controlled Evaluation of the Efficacy and Safety of Donepezil Combined With Antidepressant Pharmacotherapy. Arch Gen Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHMAN SM, MATTSON MP. Adverse stress, hippocampal networks, and Alzheimer’s disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELINE YI, GADO MH, KRAEMER HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- SHELINE YI, WANG PW, GADO MH, CSERNANSKY JG, VANNIER MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY MA, BECK JG, NOVY DM, AVERILL PM, SWANN AC, DIEFENBACH GJ, HOPKO DR. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71:309–19. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- TATA DA, ANDERSON BJ. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav. 2010;99:186–93. doi: 10.1016/j.physbeh.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VYTHILINGAM M, VERMETTEN E, ANDERSON GM, LUCKENBAUGH D, ANDERSON ER, SNOW J, STAIB LH, CHARNEY DS, BREMNER JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- WETHERELL JL, REYNOLDS CA, GATZ M, PEDERSEN NL. Anxiety, cognitive performance, and cognitive decline in normal aging. J Gerontol B Psychol Sci Soc Sci. 2002;57:P246–55. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- YEHUDA R, GOLIER JA, KAUFMAN S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry. 2005;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- YEHUDA R, HARVEY PD, BUCHSBAUM M, TISCHLER L, SCHMEIDLER J. Enhanced effects of cortisol administration on episodic and working memory in aging veterans with PTSD. Neuropsychopharmacology. 2007;32:2581–91. doi: 10.1038/sj.npp.1301380. [DOI] [PubMed] [Google Scholar]
- YOUNG AH, GALLAGHER P, WATSON S, DEL-ESTAL D, OWEN BM, FERRIER IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29:1538–45. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.