Abstract
Objective
The incidence of heart valve disease is increasing worldwide and the number of heart valve replacements is expected to increase in the future. By mimicking the main tissue structures and properties of heart valve, tissue engineering offers new options for the replacements. Applying an appropriate scaffold in fabricating tissue-engineered heart valves (TEHVs) is of importance since it affects the secretion of the main extracellular matrix (ECM) components, collagen 1 and elastin, which are crucial in providing the proper mechanical properties of TEHVs.
Materials and Methods
Using real-time polymerase chain reaction (PCR) in this experi- mental study, the relative expression levels of COLLAGEN 1 and ELASTIN were obtained for three samples of each examined sheep mitral valvular interstitial cells (MVICs)-seeded onto electrospun poly (glycerol sebacate) (PGS)-poly (ε-caprolactone) (PCL) microfibrous, gelatin and hyaluronic acid based hydrogel-only and composite (PGS-PCL/hydrogel) scaffolds. This composite has been shown to create a synthetic three-dimensional (3D) microenvironment with appropriate mechanical and biological properties for MVICs.
Results
Cell viability and metabolic activity were similar among all scaffold types. Our results showed that the level of relative expression of COLLAGEN 1 and ELASTIN genes was higher in the encapsulated composite scaffolds compared to PGS-PCL-only and hydrogel-only scaffolds with the difference being statistically significant (P<0.05).
Conclusion
The encapsulated composite scaffolds are more conducive to ECM secretion over the PGS-PCL-only and hydrogel-only scaffolds. This composite scaffold can serve as a model scaffold for heart valve tissue engineering.
Keywords: Tissue Engineering, Heart Valve, ELASTIN, COLLAGEN I, Real-Time PCR
Introduction
Heart valve disease is a progressive disorder, with patients requiring heart valve replacement estimated to increase significantly in the future (1- 3). Until 2011, in the USA, the overall prevalence of heart valve disease had been 2.5% with a relatively wide age variation in the afflicted patients (4) and about 100,000 valve replacement surgeries were performed annually (5). There are currently two types of valves which are commonly used, namely biological valves (including autografts, xenografts and homografts) and mechanical valves which are built from synthetic biocompatible materials. Although these replacements improve survival rate of patients, none of them can fully restore native valve function; biological valves are prone to fibrosis, degeneration, calcification and immunogenic reactions while mechanical valves may cause hemorrhage and thromboembolism (6). Furthermore, existing mechanical and biological prostheses lack growth, repair and remodeling capabilities (1, 2) and they are thus not suitable for congenital heart defects and pediatric patients whose heart valves are growing (7). Tissue engineering methods can be used to overcome these limitations (8). Heart valve tissue engineering offers new possibilities to produce artificial heart valves which lack unwanted biological and mechanical properties. However, there are some problems with previously fabricated scaffolds for tissue engineered heart valves (TEHVs) (9) such as plastic deformation (10), high stiffness (11), non-anisotropic properties (12), low stability and unsuturability (13). Three common approaches of bioscaffold formation include decellularised tissues, synthetic scaffold, and preseeded composites (14), among which synthetic scaffolds are suggested to be the most suitable material for valve scaffold formation (1).
A hybrid microfibrous poly (glycerol sebacate)– poly(ε-caprolactone) (PGS-PCL) synthetic scaffold has been previously fabricated that could mimic the mechanical properties in the human valve (10). However, migration of cells out of the structure due to the high porosity of PGS-PCL and difficulties relating to the production of fully cellularized three-dimensional (3D) structures caused by the cell tendency to attach only on the surface of the scaffold are two limitations of using PGSPCL solely in TEHV (10). In our recent study, we were able to overcome these limitations by integrating PGS-PCL within a hybrid hydrogel made from methacrylated hyaluronic acid (HAMA) and methacrylated gelatin (GelMA). By creating this composite scaffold, we combined the advantages of both extracellular matrix (ECM)-mimicking hydrogels and elastomeric PGS-PCL scaffolds to imitate the cellular environment and mechanical properties of native heart valve tissue (15). All native heart valves (aortic, pulmonary, mitral and tricuspid) consist of two cell types, the valvular endothelial cells (VECs) and the valvular interstial cells (VICs). The VICs, the most prevalent cell type in native heart valves, are located throughout all layers and maintains the valvular ECM (VECM) structure of heart. The VECM has three distinct layers including the fibrosa, ventricularis and spongiosa (6, 15, 16). The predominant ECM substance in the fibrosa layer is collagen fibers, ventricularis is comprised of elastin and collagen sheets and the spongiosa, the layer between the fibrosa and the ventricularis, contains glycosaminoglycans (GAGs) and loose collagen (6, 17).
Collagen is inelastic and contributes significantly to the biomechanical strength of the heart valve tissue (18) while previous study on the role of elastin in the valve leaflet have shown that elastin maintains a specific collagen fiber configuration and return the fibers to this state, once external forces are released (19). Mol et al. (2) has shown that increasing the amount of collagen, results in improved mechanical properties of the engineered tissues.
About 34 genes are associated with collagen formation and COL1 gene encodes the most abundant collagen of the human body. Elastin, the predominant element of elastic fibers, has a crucial role in integrity and dynamicity of tissue and paracrine signaling (20). Elastin is the main protein of ECM placed in the arterial wall and can contribute its dry weight up to 50% (6). The protein product of the ELASTIN gene is synthesized by vascular smooth muscle cells and secreted as a tropoelastin monomer that is soluble, non-glycosylated and highly hydrophobic. Tropoelastin is crosslinked after post-translational modifications and classified into elastin polymers. These polymers create concentric rings of elastic sheet around the medial layer of arteries. In humans, elastin is encoded by the ELN gene (21).
To reach an ideal TEHV with the capability of mimicking the native heart valve ECM, the relative quantity of collagen and elastin should be optimal in the TEHV. Collagen, elastin and proteoglycans account for ~60, ~10 and ~20% dry weight of the native heart valves respectively (22, 23). The normal valve has 74% type I, 24% type III and 2% type V collagen while these amounts are altered in myxomatous valves (24). Elastin disruption can produce smooth muscle sub-endothelial proliferation and thus may lead to obstructive arterial disease in mouse models (20, 25). In terms of creating a TEHV, it has been shown that the amount of VICs’ collagen production within collagen gels can be increased by adding glycosaminoglycans (26). Also, HA is an important material in fabricating TEHVs which promotes elastin production and secretion in VICs (26, 27). Changes in the quantity and structure of collagen and elastin directly alter the mechanical and functional features of TEHVs (28).
In this study, using the real-time polymerase chain reaction (PCR) technique, we compared the expression level of COLLAGEN and ELASTIN genes between VICs-seeded scaffolds constructed by either PGS-PCL or hydrogel and the newly fabricated composite scaffold consisting of both PGSPCL and hydrogel components (15).
Materials and Methods
Scaffold preparation
Synthesis of methacrylated hyaluronic acid and methacrylated gelatin
HA sodium salt (Mn: 7.52×105 kDa, Lifecore Biomedical, Chaska, MN) was dissolved in deionized water and HAMA solutions were prepared in a final concentration of 1% w/v. Methacrylic anhydride (Sigma-Aldrich, St. Louis, WI) was added dropwise (2 ml per 200 ml) while the pH was kept at 8.0. The solution was placed on ice for several hours and for the pH to remain at 8, it was vital to be in 15 minutes time-steps. The solution was permitted to move around by stirring overnight in a cold room (4˚C) and was then dialyzed for two days in distilled water (dI H2O) with multiple solution changes using 12-14 kDa Molecular Weight Cut-Off’s (MWCO) dialysis tubes. The solutions were lyophilized for 1 week and the the final product was kept at -80˚ C.
According to the aforementioned protocol, porcine skin type A gelatin (Bloom index 300, Sigma, Madison, WI) was methacrylated (19, 29). In brief, the mixture of gelatin 10% (w/v) with Dulbecco’s phosphate-buffered saline (DPBS, Invitrogen, Grand Island, NY) was incubated at 60˚C and stirred until totally dissolved. It was vital to add methacrylic anhydride (0.8 ml/g, 8 ml) at a rate of 0.5 mL/minutes into the gelatin solution (10 g). The emulsion was agitated at 60˚C and permitted to react for 1 hour. To prevent any further reaction, warm 2x DPBS (40˚C) was added to the solution. The solution was then dialyzed for one week in distilled water using MWCO dialysis tubes (12- 14 kDa) with some solution changes. After 1 week, the freeze-drying of the solution resulted in a white porous foam that had to be preserved at -80˚C.
Formation of poly (glycerol sebacate)-poly (ε-caprolactone) microfibrous scaffold
Through using a standard electrospinning set-up, the PGS-PCL microfibrous scaffolds were developed. Since aluminum foil is a deficient collector of stable fibers, covering a copper wire with a non-adhesive special tape with a glass slide on top was used as a collector. PGS (MW 12000, gift from Langer Laboratory, Department of Chemical Engineering, MIT) and PCL (MW70000-90000, Sigma-Aldrich, Madison, WI) were dissolved at a 2:1 ratio in anhydrous chloroform-ethanol (Sigma- Aldrich, WI, USA, 9:1 v/v) and were electrospun at 12.5 KV. The total polymer concentration was 33%. As it was hard to electrospin the highly viscous 33% w/v PCL solution, pure PCL scaffolds were electrospun by using 16% w/v polymer solution (10).
Fibers were formed at constant flow rate of 2 ml/hours by keeping the distance between the 21-gauge needle and the collector at 18 cm. Each sample was fabricated in 20 minutes. To remove any remaining solvent, the mentioned scaffolds were dried overnight in a vacuum desiccator.
Hydrogel preparation
2.5% GelMA macromer and 0.5% HAMA macromer (as a hydrogel precursor solution) were mixed with culture media. The photoinitiator 2-hydroxy-1-(4-(hydroxyethoxy) phenyl)-2-methyl- 1-propanone (Irgacure 2959, BASF, Ludwigshafen, Germany) was added to a final concentration of 0.1% (v/w) for crosslinking the solution and it was exposed afterwards to ultraviolet (UV) light (ƛ=408 nm, 45 seconds at 2.6 mW/cm2). In order to control and make the composite scaffolds, the resulting hydrogel (20 μl initial solution volume, ~6 mm diameter, ~ 0.5 mm thick) was used.
Fabrication of composite hydrogel/microfibrous poly (glycerol sebacate)–poly(ε-caprolactone) scaffolds
Composite of hydrogel/microfibrous PGS-PCL scaffolds was fabricated as previously described (15). Briefly, PGS-PCL sheets were used to create 6 mm diameter scaffolds. The PGS-PCL scaffolds were placed in contact with the precursor hydrogel solution. Soon after the whole solution was absorbed by the scaffold, UV light (ƛ =408 nm, 2.6 mW/cm2, 45 seconds) was used for crosslinking to get the resulting composite.
Molecular and cellular experiments
Scanning electron microscopy (SEM)
To characterize the size of pores of the scaffold and the fiber morphology, scanning electron microscope (SEM) images were taken with a field emission SEM (FE-SEM, JSM 6700, JEOL, Peabody, MA). Images were taken from both surface of the hydrogel-only, the composite scaffold and the PGS-PCL-only scaffold. The samples were fixed in 2.5% gluteraldehyde, and were frozen using liquid nitrogen and then stored at -80˚C. The samples were lyophilized before sputter coating, with an iron coater of palladium and platinum. The SEM was equilibrated at 40 mA for 40 seconds.
Mitral valvular interstitial cells (MVICs)
Cell culture
Sheep mitral VICs (MVICs, gift from Dr. Bischoff, Children’s Hospital, Boston, MA) with passage number between 3 and 4 were kept in endothelial basal medium (EBM-2, Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, Saint Louis, MO) and 1% penicillin-streptomycin (Gibco, Langley, OK) at 37˚C and 5% CO2. Cell culture was done on gelatin-coated flasks where cells were passaged weekly with media changed every other day.
Encapsulation of mitral valvular interstitial cells within hydrogels (GelMA/HAMA) and composite scaffolds
MVICs were trypsinized and re-suspended in the hydrogel precursor solution (2.5% GelMa/0.5% HAMA) having 1% (w/v) of photoinitiator (Irgacure 2959, BASF, Ludwigshafen, Germany, concentration: 6×106 cells/ml). As mentioned before, both the hydrogel and composite scaffolds were made. The composites and the hydrogel precursor solution with cells were exposed to 2.6 mW/cm2 UV light (408 nm, 45 seconds) and were then incubated in medium (EBM2, 21 days) under standard culture conditions. In order to remove the remaining photoinitiator, the medium was changed every 30 minutes for the first two hours. The medium was then changed every other day.
Seeding mitral valvular interstitial cells on poly (glycerol sebacate)–poly (ε-caprolactone scaffolds
PGS-PCL scaffolds were immersed in ethanol (70%, 2 hours) to be disinfected prior to seeding the cells. The scaffolds were then exposed to UV light and were washed with DPBS. The cells were seeded onto scaffolds (0.6 cm×0.6 cm) at a concentration of 2×104 cells/scaffold. To seed the cells onto scaffolds, 20 μl of cell suspension was added to scaffolds in 48-well plates which were then incubated (1 hour) for the cells to attach. Every other day the medium was changed.
Cell viability and proliferation
Cell viability
The cell viability of scaffolds was evaluated with the use of a live-dead assay (calcein-AM/ ethidiumhomodimer; Invitrogen, Carlsbad, CA) per manufacturer’s protocol. Cell viability studies were performed by seeding MVICs (6×106 cells/ ml) with each scaffold and incubating them for 21 days. Media of the samples were changed every other day. It was fundamental to determine the viability of the cells for each type of sample particularly on days 1 and 21 of culture. To determine the percent viability, Image J software was used.
Cell proliferation
The metabolic activity of cells on each scaffold or hydrogel from 1 to 21 days of culture was assessed by using the Alamar Blue (AB) assay in accordance with manufacturer’s protocol (Invitrogen, CA, USA). Cells were seeded onto scaffolds or were encapsulated within plain hydrogels or composite scaffolds (n=3/group). In order to avoid counting cells that had adhered to the plate surface during seeding, the cell-seeded scaffolds were transferred to new wells. While two cell-free scaffolds/ hydrogels were used as negative controls, one well which contained only medium was used to measure the background signal. AB (10% v/v) was then added to each well and samples were incubated for 2 hours at 37˚C. Afterwards, the solution from each well was transferred to wells in a 96-well plate (100 μl, n=3/group) and the fluores cence was measured at 540 nm. The 21-day measurements were normalized to the corresponding measurements from day 1.
RNA extraction and cDNA preparation
Three samples of each VICs-seeded scaffold (PGS-PCL-only, hydrogel-only and the composite scaffolds) was examined to assess their capability to express COLLAGEN and ELASTIN genes. Total RNA was extracted from each studied sample using an RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription PCR (RT-PCR) was performed with a RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific, PA, USA). DNAse I (Invitrogen) digestion of RNA samples (0.5 μg) was performed prior to reverse transcription.
Real time polymerase chain reaction
Real-time PCR assay was replicated three times for each sample and the difference of the threshold cycle (Ct) values between the replicates was no more than 0.5. The average Ct was used for statistical analysis. All reactions were performed using Fast SYBR Green PCR Master Mix with the default settings on an ABI Biosystems Step One Plus Real-Time PCR Machine following: denaturation at 95˚C for 5 minutes, and 40 cycles of 95˚C for 35 seconds and 60˚C for 1 minute. Relative expression levels were determined from collected data as threshold cycle numbers. Table 1 shows the sequence of the designed primers used.
Table 1.
Primer sequences of studied genes and the reference gene
| Gene | Primer sequencing |
|---|---|
| COL 1 | F: CCACTGGTCCCCAAATCTAA |
| R: GCTTCTTTGGCAGTCTGAGG | |
| ElASTIN | F: CAGCCAAATACGGTGAAACA |
| R: AACACCAGGGACTCCAACAC | |
| GAPDH | F: ATGCTGGTGCTGAGTACGTG |
| R: GGCATTGCTGACAATCTTGA | |
Data analysis
The real-time PCR technique requires that the amplification efficiencies of each studied gene to be close to 100% to allow comparative analysis of the results. The amplification efficiency (E values) obtained for all studied genes were more than 98%. Relative gene expressions were obtained using the ΔCt method of relative quantification based on the fact that the loci under study (as well as their internal control gene) are amplified with the same efficiency in each sample. The relative gene expression was calculated as 2-ΔΔCt, where ΔCt = Ct Target-Ct reference. The GAPDH gene served as an internal control.
Statistical analysis
One-way ANOVA analysis was performed followed by post-hoc comparison in SPSS (V.15, SPSS Inc., Chicago, IL). A P value ≤0.05 was considered statistically significant. Data are reported as mean ± standard deviation (SD).
Results
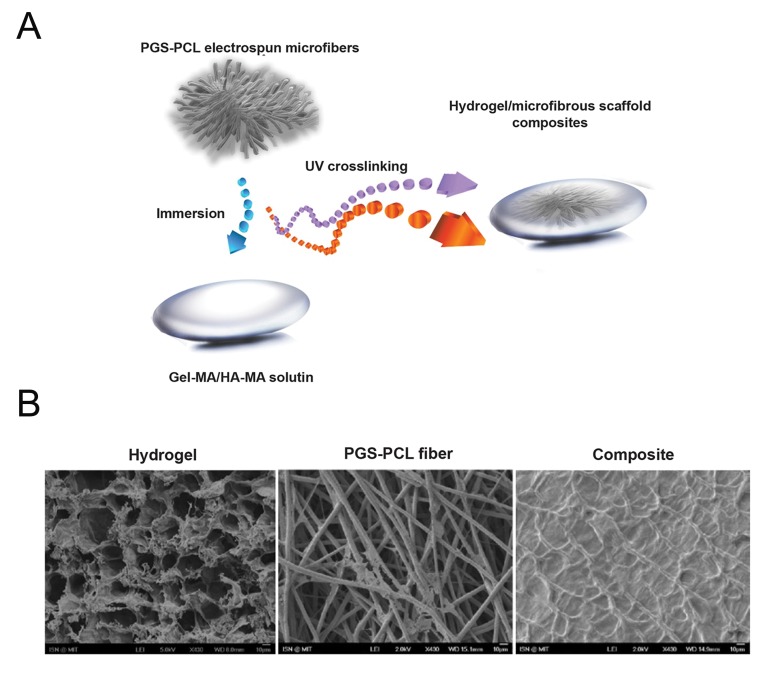
The composite structures were synthesized using an immersion technique (15). This composite was combined by adding an ECM-like microenvironment within and around the microfibers with the addition of the hydrogel (Fig .1A). Because of the utilization of hydrophobic polymers, fiber/hydrogel composite constructs were prepared by simultaneous electrospinning of the microfibers and electrospraying of the hydrogel (28). The advantage of this method was due to the ability of the PGS-PCL microfibers in absorbing the hydrogel precursor solution. The composite was manufactured after electrospinning and cells were directly encapsulated within the composite during the hydrogel crosslinking step. The PGS component of the microfibers was necessary to simplify this complete interface because the GelMA/HAMA gel solution was unable to penetrate the PCL-only fibers (15). The presence of PGS renders PGSPCL microfibrous scaffolds more hydrophilic thus enabling their further modification (10). Additionally, the gel solution was absorbed more quickly when the scaffold was submerged in the gel solution compared to directly adding it to the scaffold structure (Fig .1A). SEM images of the composites show that the scaffold microfibers were encapsulated within a layer of hydrogel (Fig .1B).
Fig.1.
Fabrication of the fiber reinforced hydrogel (2.5% GelMA+0.5% HAMA) scaffolds.
A. Schematics of the design and fabrication. Immersing the electrospun fibers inside the hydrogel precursor solution (to which cells can be added), and then crosslinking via light exposure to form a cell-laden hydrogel with electrospun fibers for reinforcement [adapted from figure 1 (15)] and B. Surface SEM images of hydrogel, PGS-PCL fiber and composite scaffolds showing the integration of the hydrogel with the electrospun microfiber.
PGS; Poly (glycerol sebacate), PCL; Poly (ε-caprolactone), UV; Ultraviolet, GelMA; Methacrylated gelatin, HAMA: Methacrylated hyaluronic acid and SEM; Scanning electron microscopy.
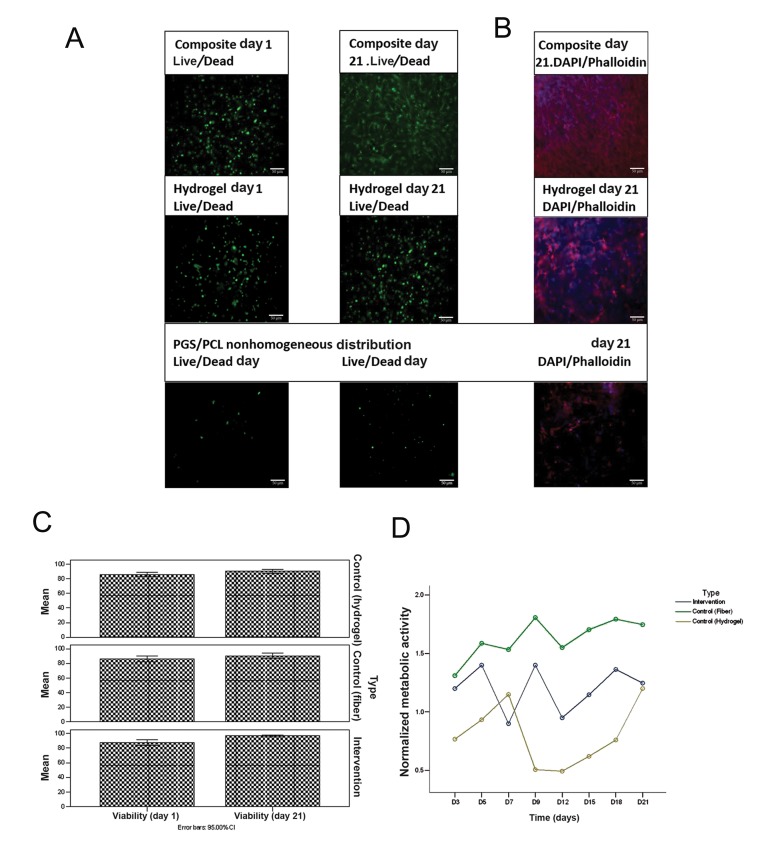
The initial encapsulation process maintained a high level of cell viability (≥90%). At day 21, the viability of MVICs was still above 90% for all three scaffolds (Table 2), accompanied by a significant increase in the cell number (Fig .2A). The metabolic activity of seeded cells was used as an indirect method to estimate cell number. The cells within the composite scaffolds showed higher metabolic activity compared to those on the scaffold only or within the hydrogel samples at all time points (Table 3). The metabolic activity showed distinct peaks at different time points for the samples. This may be caused by cells getting a quiescent status when a defined cellular density is reached (Fig . 2B).
Table 2.
| Type | Viability (day 1) | Viability (day 2) | |
|---|---|---|---|
| Copmosite | Mean | 87.67 | 96.67 |
| Std. deviation | 1.528 | 0.577 | |
| Control (fiber) | Mean | 86.33 | 90.33 |
| Std. deviation | 1.528 | 1.528 | |
| Control (hydrogel) | Mean | 86.00 | 90.00 |
| Std. deviation | 1.000 | 1.000 | |
MVICs; Mitral valvular interstitial cells.
Fig.2.
The behavior of cells encapsulated in composite scaffolds compared with cells seeded on PGS-PCL fibers and/or encapsulated in GelMA/HAMA hydrogel alone.
A. Viability of the encapsulated or seeded cells as observed through Live/Dead staining of the MVICs on days 1 and 21, B. DAPI/phalloidin staining on day 21 showing cell spreading and distribution, C. Quantification of the Live/Dead images by Image J (n=3) and D. Metabolic activity determined using Alamar Blue (n=3, P<0.05).
PGS; Poly (glycerol sebacate), PCL; Poly (ε-caprolactone), GelMA; Methacrylated gelatin, HAMa; Methacrylated hyaluronic acid and DAPI; 4, 6-diamidino-2-phenylindole.
Table 3.
Metabolic activity determined using Alamar Blue (n=3)
| Type | NMA (day 3) | NMA (day 5) | NMA (day 7) | NMA (day 9) | NMA (day 12) | NMA (day 15) | NMA (day 18) | NMA (day 21) | |
|---|---|---|---|---|---|---|---|---|---|
| Composite | Mean | 1.200 | 1.400 | 0.900 | 1.400 | 0.950 | 1.147 | 1.363 | 1.247 |
| Std. deviation | 0.0500 | 0.0500 | 0.0500 | 0.0500 | 0.0500 | 0.0252 | 0.0709 | 0.0351 | |
| Control (fiber) | Mean | 1.310 | 1.587 | 1.533 | 1.807 | 1.550 | 1.703 | 1.793 | 1.747 |
| Std. deviation | 0.0854 | 0.0709 | 0.0351 | 0.0702 | 0.0300 | 0.611 | 0.0902 | 0.0252 | |
| Control (hydrogel) | Mean | 0.767 | 0.933 | 1.150 | 0.507 | 0.493 | 0.620 | 0.760 | 1.200 |
| Std. deviation | 0.0208 | 0.0289 | 0.0400 | 0.0321 | 0.0306 | 0.0200 | 0.5973 | 0.2000 | |
NMA; Normalized metabolic activity.
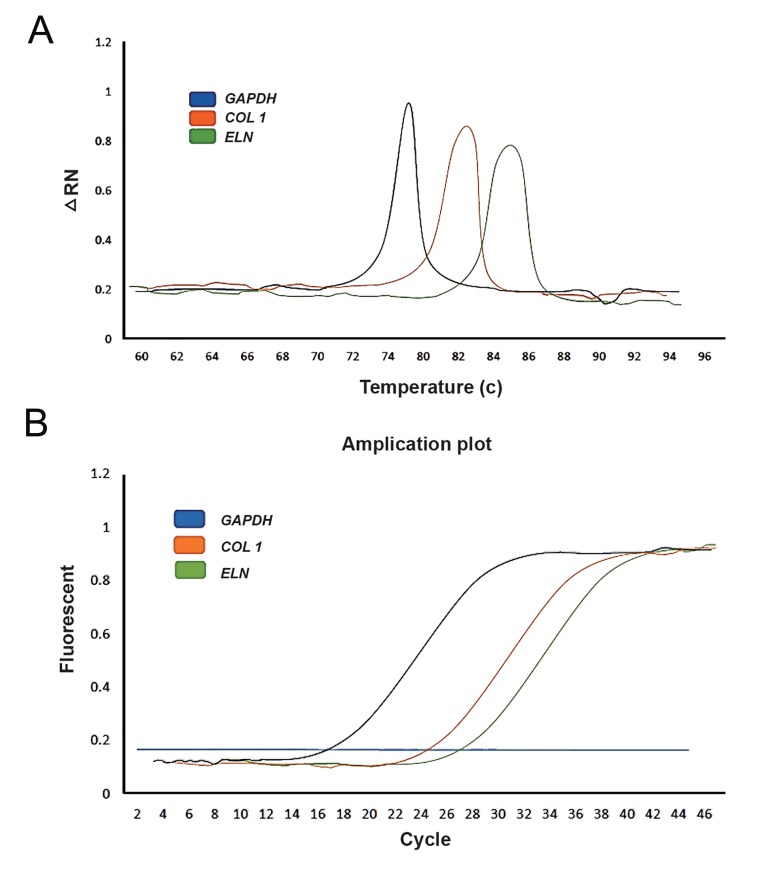
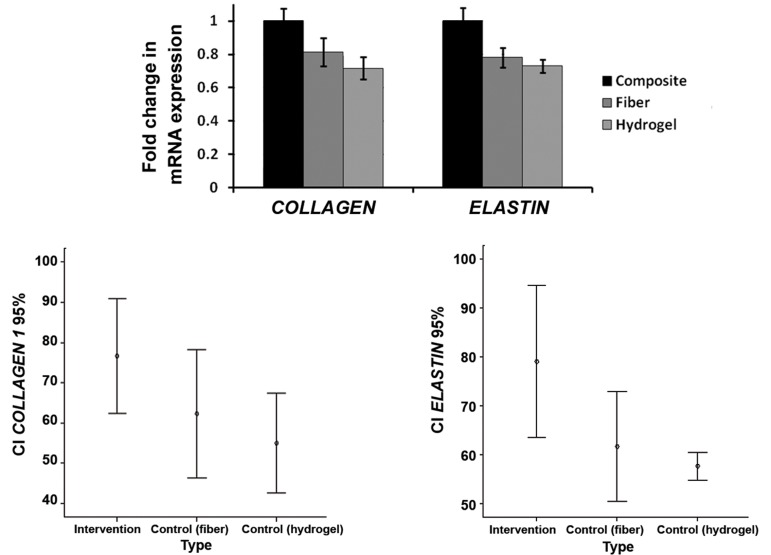
Our statistical analysis showed that the level of COLLAGEN 1 gene expression was higher in the VICs-seeded composite scaffold compared with PGS-PCL-only and hydrogel-only scaffolds (Fig .3) and the difference was statistically significant (P=0.010). The most and the least COLLAGEN 1 gene expression percent were related to the composite (76.67 ± 5.8%) and hydrogel-only (55 ± 5%) scaffolds respectively. The difference of ELASTIN gene expression level between the composite, PGS-PCL-only and hydrogel-only scaffolds was also statistically highly significant (P=0.003) with the highest level in the composite scaffold (79 ± 6.2%) and the lowest level in the hydrogel-only scaffold (57.67 ± 3%). The COLLAGEN 1 gene expression of the hydrogel-only and PGS-PCL-only scaffolds was decreased 0.21 and 0.19 fold in comparison to composite scaffold respectively. The corresponding values for ELASTIN were 0.27 and 0.22 fold respectively. The COLLAGEN-ELASTIN genes expression ratio in the composite scaffold was 1 (Fig .4).
Fig.3.
Real-time PCR assay of COLLAGEN 1, ELASTIN and GAPDH gene expression in the intervention sample. A. Melt curve analysis of three studied genes (Blue; GAPDH, Orange; COLLAGEN 1 and Green; ELASTIN) and B. Amplification plot of the three genes (Blue; GAPDH, Orange; COLLAGEN 1 and Green; ELASTIN).
Fig.4.
Level of COLLAGEN 1 and ELASTIN genes expression in three studied sample types (intervention, fiber and hydrogel). Level of COLLAGEN 1 and ELASTIN genes expression are highest in intervention case in comparison with PGS-PCL fiber-only and Hydrogel-only cases (n=3, P=0.010). PGS; Poly (glycerol sebacate) and PCL; Poly (ε-caprolactone).
Discussion
VICs have a heterogeneous phenotype resulting from the ECM layered structure with fibrous, collagen- rich and glycosaminoglycan-rich regions. This special structure is necessary for heart valves to tolerate significant mechanical forces such as stretch and flexure during diastole and systole respectively (16). Thus, choosing appropriate biomaterials and synthetic scaffolds is a crucial step in fabricating TEHVs especially because they affect the quantity and architecture of ECM components which can directly alter the mechanical, elastic and tensile strength of TEHVs (30).
The main proteins secreted by VICs are collagen (types I, III and IV), laminin and fibronectin (31) among which collagen has a considerable role in maintaining tissue integrity and providing mechanical strength (32). Ramamurthi and Vesely (26) have shown that by adding glycosaminoglycans, the amount of VICs’ collagen production in collagen gels can be increased. Sant et al. (33) investigated the ability of VICs seeded on fibrous PGS-PCL scaffold to secrete fibrous proteins, specifically collagen type I. Recently, Masoumi et al. (9) demonstrated that scaffolds with (2:1) PGSPCL polymer ratios have the highest level of COLLAGEN expression.
Similarly, in the current study, we assessed the ability of collagen type 1 secretion by VICs seeded on PGS-PCL-only scaffold and obtained the level of COLLAGEN gene expression using real-time PCR. The necessary elastic properties in native heart valves are obtained by elastin, another major ECM component. Sant et al. (33) also evaluated elastin secretion by VICs on PGS-PCL fibrous scaffold and did not find any elastin secretion by VICs in their study. Previous studies have shown that HA substrates promote the production of elastin by smooth muscle cells (26, 27). Masters et al. (34) in a study on VICs cultured on tissue culture polystyrene, reported an increase in elastin production and a decrease in collagen production in response to the delivery of HA oligosaccharides. In the current study, the level of ELASTIN gene expression was obtained in the hydrogel-only scaffold which consisted of both HAMA (promoting elastin secretion) and GelMA (promoting cell spreading) (22). We also investigated the expression level of COLLAGENE in the hydrogelonly scaffold. We further assessed the quantity of COLLAGEN and ELASTIN genes expression in our recently fabricated VIC-seeded composite scaffold consisting of both PGS-PCL (with its collagenous layers providing mechanical strength) and the hydrogel component (GelMA/HAMA; providing a 3D glycosaminoglycan rich ECM-like microenvironment). We then compared the obtained quantities with those from PGS-PCL-only and hydrogelonly scaffolds.
Conclusion
Our results demonstrate that the expression level of COLLAGEN and ELASTIN genes was statistically significantly higher in the composite scaffold compared with those of PGS-PCL-only scaffold and hydrogel-only scaffold. The optimal expression levels of ELASTIN and COLLAGEN genes in the VIC-seeded composite scaffold demonstrate the superiority of this composite compared with the PGS-PCL-only and hydrogel-only scaffolds. This composite scaffold can serve as a model scaffold for heart valve tissue engineering which can provide the necessary mechanical, elastic and tensile strength. Furthermore, this composite scaffold has the capability to grow, be repaired and remodeled and may thus be suitable for congenital heart defects.
Acknowledgments
The authors would like to thank Dr. Hamid Gourabi for scientific discussions the project. We also acknowledge Dr. Nihal Engin Vrana and Aslan Fanni for their scientific input. This study was financially supported by the Royan Institute and the following grants from the National Institute of Health (EB007249, DE019024 and HL092836). There is no conflict of interest in this article.
References
- 1.Rippel RA, Ghanbari H, Seifalian AM. Tissue-engineered heart valve: future of cardiac surgery. World J Surg. 2012;36(7):1581–1591. doi: 10.1007/s00268-012-1535-y. [DOI] [PubMed] [Google Scholar]
- 2.Mol A, Smits AI, Bouten CV, Baaijens FP. Tissue engineering of heart valves: advances and current challenges. Expert Rev Med Devices. 2009;6(3):259–275. doi: 10.1586/erd.09.12. [DOI] [PubMed] [Google Scholar]
- 3.Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–61. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 4.dArcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B. Valvular heart disease: the next cardiac epidemic. Heart. 2011;97(2):91–93. doi: 10.1136/hrt.2010.205096. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of con genital heart disease. Am Heart J. 2004;147(3):425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Filova E, Straka F, Mirejovsky T, Masin J, Bacakova L. Tissue-engineered heart valves. Physiol Res. 2009;58(Suppl 2):S141–158. doi: 10.33549/physiolres.931919. [DOI] [PubMed] [Google Scholar]
- 7.Brody S, Pandit A. Approaches to heart valve tissue engineering scaffold design. J Biomed Mater Res B Appl Biomater. 2007;83(1):16–43. doi: 10.1002/jbm.b.30763. [DOI] [PubMed] [Google Scholar]
- 8.Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am. 2009;300(5):64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 9.Masoumi N, Larson BL, Annabi N, Kharaziha M, Zamanian B, Shapero KS, et al. Electrospun PGS: PCL microfibers align human valvular interstitial cells and provide tunable scaffold anisotropy. Adv Healthc Mater. 2014;3(6):929–939. doi: 10.1002/adhm.201300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sant S, Hwang CM, Lee SH, Khademhosseini A. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. J Tissue Eng Regen Med. 2011;5(4):283–291. doi: 10.1002/term.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelmayr GC Jr, Sacks MS. Prediction of extracellular matrix stiffness in engineered heart valve tissues based on nonwoven scaffolds. Biomech Model Mechanobiol. 2008;7(4):309–321. doi: 10.1007/s10237-007-0102-1. [DOI] [PubMed] [Google Scholar]
- 12.Robinson PS, Johnson SL, Evans MC, Barocas VH, Tranquillo RT. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cellproduced collagen. Tissue Eng Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 13.Hockaday LA, Kang KH, Colangelo NW, Cheung PY, Duan B, Malone E, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4(3):035005–035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zorlutuna P, Vrana NE, Khademhosseini A. The expanding world of tissue engineering: the building blocks and new applications of tissue engineered constructs. IEEE Rev Biomed Eng. 2013;6:47–62. doi: 10.1109/RBME.2012.2233468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eslami M, Vrana NE, Zorlutuna P, Sant S, Jung S, Masoumi N, et al. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J Biomater Appl. 2014;29(3):399–410. doi: 10.1177/0885328214530589. [DOI] [PubMed] [Google Scholar]
- 16.Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PM, Cass AEG, Yacoub MH. Extracellular matrix scaffolds for tissue engineering heart valves. Prog Pediatr Cardiol. 2006;21(2):219–225. [Google Scholar]
- 18.Balguid A, Rubbens MP, Mol A, Bank RA, Bogers AJ, van Kats JP, et al. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets--relevance for tissue engineering. Tissue Eng. 2007;13(7):1501–1511. doi: 10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- 19.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31(2):115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 20.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393(6682):276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71(1):40–49. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan TC, Pandit A. Living artificial heart valve alternatives: a review. Eur Cell Mater. 2003;6:28–45. doi: 10.22203/ecm.v006a04. [DOI] [PubMed] [Google Scholar]
- 23.Kunzelman KS, Cochran RP, Murphree SS, Ring WS, Verrier ED, Eberhart RC. Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J Heart Valve Dis. 1993;2(2):236–244. [PubMed] [Google Scholar]
- 24.Cole WG, Chan D, Hickey AJ, Wilcken DE. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J. 1984;219(2):451–460. doi: 10.1042/bj2190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinton RB, Adelman-Brown J, Witt S, Krishnamurthy VK, Osinska H, Sakthivel B, et al. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res. 2010;107(4):549–557. doi: 10.1161/CIRCRESAHA.110.221358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramamurthi A, Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials. 2005;26(9):999–1010. doi: 10.1016/j.biomaterials.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Shah DN, Recktenwall-Work SM, Anseth KS. The effect of bioactive hydrogels on the secretion of extracellular matrix molecules by valvular interstitial cells. Biomaterials. 2008;29(13):2060–2072. doi: 10.1016/j.biomaterials.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30(34):6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10(83):20121004–20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flanagan TC, Black A, OBrien M, Smith TJ, Pandit AS. Reference models for mitral valve tissue engineering based on valve cell phenotype and extracellular matrix analysis. Cells Tissues Organs. 2006;183(1):12–23. doi: 10.1159/000094902. [DOI] [PubMed] [Google Scholar]
- 32.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118(18):1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 33.Sant S, Iyer D, Gaharwar AK, Patel A, Khademhosseini A. Effect of biodegradation and de novo matrix synthesis on the mechanical properties of valvular interstitial cellseeded polyglycerol sebacate-polycaprolactone scaffolds. Acta Biomater. 2013;9(4):5963–5973. doi: 10.1016/j.actbio.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26(15):2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]