Abstract
Objective
Resveratrol, a phytoalexin, has a wide range of desirable biological actions. Despite a growing body of evidence indicating that resveratrol induces changes in neu- ronal function, little effort, if any, has been made to investigate the cellular effect of res- veratrol treatment on intrinsic neuronal properties.
Materials and Methods
This experimental study was performed to examine the acute effects of resveratrol (100 µM) on the intrinsic evoked responses of rat Cornu Ammonis (CA1) pyramidal neurons in brain slices, using whole cell patch clamp re- cording under current clamp conditions.
Results
Findings showed that resveratrol treatment caused dramatic changes in evoked responses of pyramidal neurons. Its treatment induced a significant (P<0.05) increase in the after hyperpolarization amplitude of the first evoked action potential. Resveratrol-treated cells displayed a significantly broader action potential (AP) when compared with either control or vehicle-treated groups. In addition, the mean instantaneous firing frequency between the first two action potentials was significantly lower in resveratrol-treated neurons. It also caused a significant reduction in the time to maximum decay of AP. The rheobase current and the utilization time were both significantly greater following resveratrol treatment. Neurons exhibited a significantly depolarized voltage threshold when exposed to resveratrol.
Conclusion
Results provide direct electrophysiological evidence for the inhibitory effects of resveratrol on pyramidal neurons, at least in part, by reducing the evoked neural activity.
Keywords: Resveratrol, Electrophysiology, Action Potential, Neurons, Whole Cell Patch Clamp
Introduction
In recent years, many naturally occurring compounds consumed by human populations have gained considerable attention for the treatment of neurodegenerative diseases. In this context, resveratrol (3, 5, 4-trihydroxy-trans-stilbene), a phytoalexin produced by a variety of plant species including grapes, mulberries, cranberries and peanuts has been shown to exert a wide range of actions, including neuroprotective effects (1,4). In addition, it has been reported that resveratrol may have a potential role in the control of heart disease, atherosclerosis, arthritis, anti-aging, and autoimmune disorders (5,7).
It has also been reported that it may reduce excite toxicity via an inhibitory effect on postsynaptic glutamatergic transmission and by enhancing glutamate uptake in astrocytes following oxidative stress (8,10). Improvement of cognitive function by resveratrol treatment has been attributed to increasing insulin-like growth factor-I (IGF-I) production and promoting angiogenesis and neurogenesis in the hippocampal astrocytes through stimulation of sensory neurons in the gastrointestinal tract. It could also delay the onset of neurodegenerative disease and prevent learning impairment in transgenic Alzheimer’s disease models (11,14).
In addition, resveratrol has a large attenuation effect on the increased spontaneous excitatory post synaptic currents (sEPSCs) in hippocampal pyramidal neurons (15). Chronic treatment with resveratrol could intensively inhibit the release of extracellular glutamate and aspartate during ischemia/reperfusion. Therefore, the neuroprotective effects of resveratrol may be partly due to its ability to attenuate extracellular excitotoxic glutamate and aspartate accumulation (16). The inhibitory effect of resveratrol on voltage-activated K +currents in rat hippocampal neurons has also been suggested to be useful in treating ischemic brain injury (17). The antinociceptive action of resveratrol in the formalin test has been found to be mediated by opening the Ca 2+activated-K +channels (18). In rat dorsal root ganglion, resveratrol suppressed Na +currents in a concentration-dependent manner (19). Its application can also reduce the frequency of seizures and improve the pathological damage to hippocampal Cornu Ammonis (CA) CA1 and CA3 pyramidal neurons after kainic acid-induced temporal lobe seizures (20).
These observations provide evidence that resveratrol can be applicable in protecting some animals suffering from different types of neurological disorders. However, despite extensive studies on the biological effects of resveratrol, little is known about its cellular mechanisms, including the actual electrophysiological actions on intrinsic neuronal evoked activity.
Considering the proved neuroprotective properties of resveratrol, therefore the main aim of this study was to determine the effects of resveratrol on evoked electrophysiological responses of CA1 pyramidal neurons.
Materials and Methods
This experimental study was performed in accordance with the Ethical guidelines of Shahid Beheshti University of Medical Sciences and efforts were made to minimize animal suffering and the number of animals used. A total of 13 young adult male Wistar rats ( 4-6 weeks old were used and divided into 3 groups: control (n=4 and 10 cells), vehicle [dimethyl sulfoxide (DMSO)]-treated (n=4 and 8 cells) and resveratrol-treated (n=5 and 12 cells). They were kept at a room temperature of 23 ± 3˚C with a 12:12 hour light/dark cycle. Rats were given free access to rodent chow and tap water.
Hippocampal slice preparation and whole cell patch clamp recording
Whole cell patch clamp recording was performed as previously described (21,22). Briefly, animals were anesthetized via inhalation of ether. They were then decapitated and their brains rapidly removed and placed in ice-cold artificial-spinal fluid (ACSF) containing (in mM) 206 sucrose, 2.8 KCl, 1 CaCl 2, 1 MgCl 2, 2 MgSO 4, 1.25 NaH 2PO 4, 26 NaHCO 3, 10 D-glucose, and equilibrated to a pH=7.4 (with 95% O2and 5% CO 2); the osmolarity was adjusted to 295 mOsm. All salts were purchased from Sigma (UK). The hippocampus then was dissected out of the brain, and 300 µm thick transverse slices were obtained using a vibrating microtome (752 M, Campden Instruments Ltd., UK) before being transferred to an incubating chamber containing oxygenated ACSF (in mM): 2 MgSO 4, 2.8 KCl, 2 CaCl 2, 124 NaCl, 1.25 NaH2 PO 4, 26 NaHCO 3, 10 D-glucose, pH=7.4, 295 mOsm, for at least 30 minutes at 32-35˚C. After incubation, slices were kept at room temperature (23-25˚C) until individually transferred into the recording chamber.
Intracellular whole-cell current-clamp recordings
Hippocampal slices were transferred to a submerged recording chamber on the stage of an upright microscope (Olympus, BX 51WI, Japan). CA1 pyramidal neurons were then visualized with a 60×water immersion objective using Nomarskitype differential interference contrast imaging with infrared illumination. Images were captured with a CCDcamera (Hmamatsu, ORSA, Japan). The slices were continuously superfused with normal Resveratrol At tenuates Neuronal Exci tabili ty oxygenated ACSF at room temperature (23-25˚C). Whole cell patch clamp recordings were obtained from hippocampal CA1 pyramidal neurons using Multiclamp 700 B amplifier, Digidata 1320 A/D and pClamp 9.2 software (Axon Instruments, Molecular Devices Co., Sunnyvale, CA). Electrophysiological responses were filtered at 5 kHz and sampled at 10 kHz and stored on a personal computer for offline analysis. Patch pipettes were pulled with a vertical microelectrode puller (PC10, Narishige, Tokyo, Japan) and had resistance of 4-7 MΩ when filled with internal solution containing (in mM): 135 potassium methylsulfate (KMeSO 4), 10 KCl, 10 Hepes, 1 MgCl 2, 2 Na 2ATP, and 0.4 Na 2GTP. The pH of the internal solution was set to 7.3 by KOH and osmolarity was adjusted to 290 mOsm. All salts were purchased from Sigma, UK.
To investigate the effect of resveratrol on the electrically evoked responses, action potentials were elicited applying depolarizing current pulses (520 mseconds) ranging from 100-500 pA in 100 pA increments, in the presence of synaptic channel blockers (100 µM picrotoxin and 1 mM kynurenic acid, Sigma, UK). Alterations in evoked excitability was also examined using a depolarizing ramp current (880 mseconds) with a slope of 3.1 pA/ms from 0 pA to 220 pA.
The following electrophysiological parameters were measured and assessed. The after hyperpolarization potential (AHP) amplitude was measured as the difference between the spike threshold and the minimum voltage following the AP peak. Action potential half width was measured at half amplitude of AP. Instantaneous firing frequency (1/interspike interval) was calculated from a train of AP evoked by pulses of 625 mseconds duration. Rheobase current and utilization time were defined as the minimum current threshold required to evoke AP and the first spike latency during a ramp depolarizing current, respectively.
Drug application
Resveratrol (Tocris, Bristol, UK) was dissolved in DMSO, to prepare a stock solution and was diluted to final concentration of 100 µM (9,15,19). The final concentration of DMSO was less than 0.1%. The recording chamber with a volume of ~1.5 ml was continuously superfused with oxygenated ACSF at 1.5-2 ml/minutes. Following the 8 minutes perfusion with ACSF containing resveratrol whole cell patch clamp recordings were done under current clamp conditions. The entire recording period for each cell lasted approximately 25 minutes.
Cell selection criteria
Patched CA1 pyramidal neurons were accepted for study if they had a patch seal resistance of greater than 1GΩ, a series resistance less than 20% of membrane resistance and a resting membrane potential between -55 to -65 mV.
Data analysis
The results obtained were expressed as mean ± standard error of mean (SEM). Statistical significance was assessed by one-way ANOVA followed by Tukey’s post hoc test, using SPSS software (version 16). Values of P≤0.05 were considered to be significant.
Results
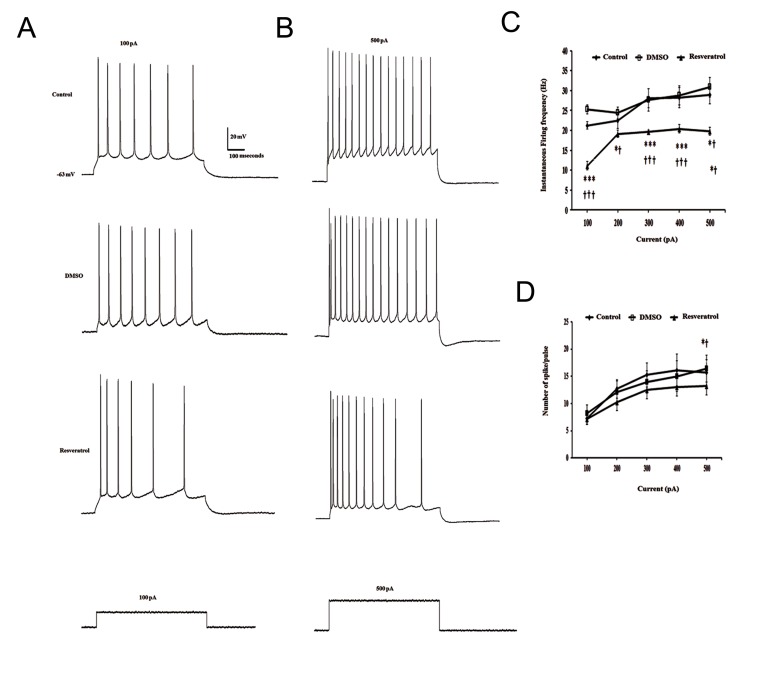
The effects of resveratrol (100 μM) on intrinsic evoked excitability were assessed after blocking synaptic activities. Under current clamp conditions, depolarization of CA1 pyramidal neurons by injecting a series of current pulses with increasing amplitude led to a significant decrease in the instantaneous firing frequency in resveratrol-treated neurons when compared to either control or DMSO-treated neurons [F(2,15)=55.82, P<0.001; F(2,18)=8.41, P<0.05; F(2,18)=28.74, P<0.001; F(2,18)=17.36, P<0.001; F(2,14)=10.75, P<0.01, in response to 100 to 500 pA depolarizing current steps, respectively, Fig.1A-C]. In the presence of resveratrol, the number of evoked AP was also significantly decreased in response to a 500 pA depolarizing current injection compared to control and vehicle treated groups ( One way ANOVA, F(2,20)=3.68, P<0.05, Fig.1D).
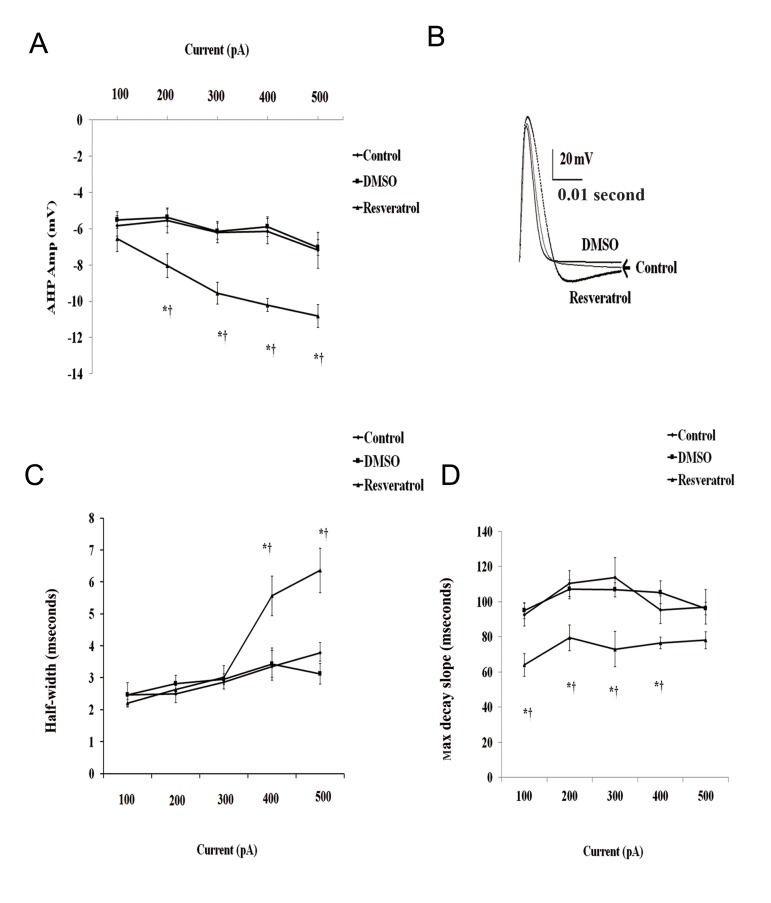
In addition, comparison of the evoked action potentials before and after application of resveratrol revealed a significantly larger AHP amplitude ( One way ANOVA, F(2,17)=16.34, F(2,18)=18.98), F(2,19)=34.57 and F(2,18)=24.93, in response to 200, 300, 400 and 500 pA, respectively, P<0.001, Fig.2A, B). Resveratrol treatment also caused a significant prolongation of action potential duration at 50% repolarization in response to higher depolarizing current injections ( One way ANOVA, F(2,16)=5.86 and F(2,14)=10.47, in response to 400 pA and 500 pA current injections, respectively, Fig.2B, C).
535 After the application of 100 μM resveratrol to the bath, depolarizing current injections resulted in a significant reduction in the time to max decayslope of AP ( One way ANOVA, F(2,11)=82.67, F(2,13)=38.78, F(2,13)=62.28, F(2,13)=66.55, F(2,11)=34.68, P<0.001, Fig.2C).
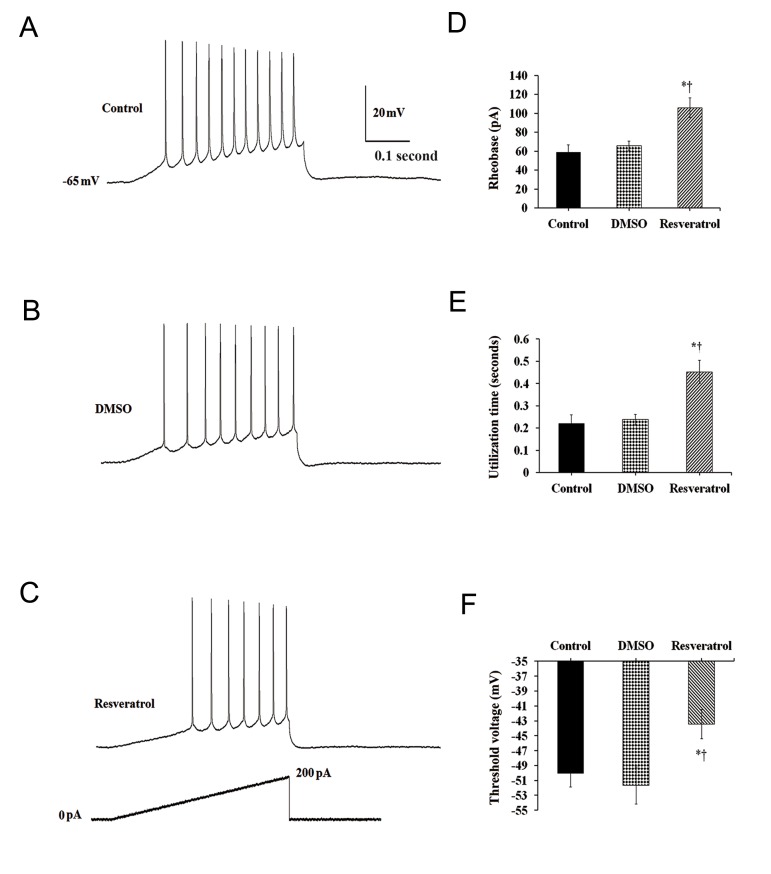
To further assess alteration of intrinsic neuronal excitability after resveratrol application, rheobase (threshold current), action potential voltage threshold and utilization time were measured in response to a depolarizing current ramp from 0 pA to 220 pA lasting 880 mseconds. (Fig .3A).
Fig.3.
Effect of resveratrol on neuronal excitability.
A. Representative voltage responses to a depolarizing current ramp in control, B. Dimethyl sulfoxide (DMSO) -treated and C. Resveratroltreated groups, D. Histograms summarizing the effect of resveratrol on the rheobase current), utilization (latency to the first spike) and E. Voltage threshold compared to control and vehicle-treated neurons. *; Represents significant difference between control and resveratroltreated groups (P<0.05) and †; Donates significant difference DMSO and resveratrol-treated groups (P<0.05).
The average rheobase of CA1 pyramidal neurons was significantly higher after resveratrol treatment when compared to control and vehicletreated groups ( One way ANOVA, F(2,18)= 8.56, P<0.01, Fig.3B).
Addition of resveratrol to the ACSF led to a significant increase in the utilization time (0.451 ± 0.05 seconds) compared to control (0.220 ± 0.03 seconds) and DMSO (0.238 ± 0.023 seconds) groups (One way ANOVA, F(2,20)=9.62, P<0.01, Fig.3C). The average AP voltage threshold was -43.45 ± 1.96 mV (P<0.05), after resveratrol application when compared to control (50.07 ± 1.79 mV) and DMSO-treated (51.67 ± 2.48 mV) groups, respectively (One way ANOVA, F(2,19)=4.58, Fig.3D). There were no significant difference in the measured electrophysiological parameters between control and vehicle-treated neurons.
Fig.1.
Suppressive effect of resveratrol on the evoked responses of CA1 pyramidal neurons representative membrane voltage responses to depolarizing current pulses of A. 100 pA, B. 500 pA before and after resveratrol treatment, C. The effect of resveratrol on the intrinsic firing frequency and D. The number of evoked spike per pulse.
CA; Cornu ammonis and DMSO; Dimethyl sulfoxide .
Fig.2.
Effect of resveratrol on evoked action potential parameters.
A. Histograms show the mean amplitude of after hyperpolarization potential (AHP). Superimposed traces of first evoked AP in response to 500 pA depolarizing current pulse recorded from control, dimethyl sulfoxide (DMSO)-treated and resveratrol treated neurons is shown in B. C. The mean half-width of action potential (AP) and D. The average time to maximum decay slope. *; Represents significant difference between control and resveratrol-treated groups (P<0.05) and †; Donates significant difference DMSO and resveratrol-treated groups (P<0.05).
Discussion
This study set out with the aim of assessing the acute electrophysiological effects of resveratrol on CA1 hippocampal pyramidal neurons. Here, the intrinsic evoked firing properties of CA1 pyramidal neurons were investigated for the first time after blocking fast synaptic inputs. Several studies have provided evidence that resveratrol exerts neuroprotective activities (23-25). However, very little is known about its effects on evoked electrophysiological properties.
The findings of the present study revealed that extracellular application of resveratrol profoundly affected the evoked electrophysiological response properties of CA1 pyramidal neurons.
Resveratrol treatment produced a broader action potential width and larger AHP amplitude which was associated with a lower neuronal excitability. The enhancement of the AHP amplitude could be due to the larger Ca2+ entry allowed by the wider AP (26). It has been shown that the AHP is an intrinsic property of hippocampal pyramidal neurons and is caused by the contribution of Ca2+-dependent K+ current. There has been an increasing accumulation of evidence suggesting that resveratrol modulates the activities of several types of ion channels, such as stimulation of Ca2+- activated K (KCa2+) channels in vascular endothelial cells, inhibition of L-type Ca2+ currents in ventricular myocytes (15, 27), and inhibition of Na+ channel currents in rat dorsal root ganglion neurons (19). It has been shown that the stimulatory effect of resveratrol on KCa2+ was associated with membrane hyperpolarization (28), which thereby may cause enhancement of AHP amplitude. Here, the large AHP amplitude observed in resveratroltreated neurons could be due to the activation of KCa2+ which was accompanied with a significant decrease in firing frequency.
The suppressive effect of resveratrol on neuronal excitability observed in the present study is consistent with those of Li et al. (16, 29) who found that resveratrol inhibited the spontaneous discharge and ischemia-induced glutamate release in the rat hippocampal CA1 region. The present result is also consistent with Li et al. (28) who found resveratrol can significantly reduce epileptiform discharges induced by L-glutamate and reverse the increased discharges induced by Bay K8644 which strongly suggests the inhibitory effects of resveratrol on voltage-gated L-type calcium channels. Suppression of voltage-dependent Ca2+ channel activity in rat cerebrocortical nerve terminals as a potential mechanism underlying the inhibition of glutamate release by resveratrol has also been reported (30). This also could be the reason for decreasing the neuronal excitability caused by resveratrol.
Acute exposures of CA1 pyramidal neurons to resveratrol also resulted in a significant increase in the rheobase current and utilization time and a significant depolarized threshold voltage, which all contribute to the decreased neuronal excitability. Several factors including action potential threshold and waveform may affect neuronal excitability (31). Modulation of voltage-gated Na+ channels regulates the depolarizing rising phase of action potential and firing frequency (32, 33). One possible explanation for the higher rheobase (threshold) current and utilization time, and depolarized threshold voltage following resveratrol treatment could be due to a significant increase in the AHP amplitude, which in turn may lead to fewer Na+ channels available for activation. The mechanism for the inhibitory effect of resveratrol on Na+ channels reported by Kim et al. (19) could also be the reason for the higher rheobase (threshold) current which reflects the lower excitability seen in neurons treated with resveratrol in the present work.
Conclusion
The present results further characterized the electrophysiological consequences of the suppressive effects of resveratrol on intrinsic neuronal excitability and electrical responsiveness in CA1 pyramidal neurons. This suppressive action of resveratrol on intrinsic neuronal activity may be one of the underlying mechanisms for its neuroprotective effect against brain diseases which are associated with neuronal hyperexcitability.
Acknowledgments
The present work was partially financially supported by a grant from Neuroscience Research Center and The Research Deputy of Shahid Beheshti Medical School. This work is a part of the Ph.D. thesis of Meftahi at The Department of Physiology, Medical School, Shahid Beheshti Medical Sciences University. The authors declare no conflict of interest.
References
- 1.Gong QH, Li F, Jin F, Shi JS. Resveratrol attenuate neuroinflamationmediated cognitive deficits in rats. J Health Sci. 2010;56(6):655–663. [Google Scholar]
- 2.Sale S, Verschoyle RD, Boocock D, Jones DJ, Wilsher N, Ruparelia KC, et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3, 4, 5, 4΄-tetramethoxystilbene. Br J Cancer. 2004;90(3):736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JA, Lee H, Lim YK, Koh Y, Choi JH, Park EM. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J Neuroimmunol. 2010;227(1-2):93–100. doi: 10.1016/j.jneuroim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41(2-3):375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian C, Ma J, Zhang P, Luo A, Wang C, Ren Z, et al. Resveratrol attenuates the Na(+)-dependent intracellular Ca(2+) overload by inhibiting H(2)O(2)-induced increase in late sodium current in ventricular myocytes. PLoS One. 2012;7(12):e51358–e51358. doi: 10.1371/journal.pone.0051358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26(9):1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida Y, Shioi T, Izumi T. Resveratrol ameliorates experimental autoimmune myocarditis. Circ J. 2007;71(3):397–404. doi: 10.1253/circj.71.397. [DOI] [PubMed] [Google Scholar]
- 8.de Almeida LM, Pineiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, et al. Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol. 2007;27(5):661–668. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111(1):41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 10.Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in thehippocampus. J Nutr Biochem. 2011;22(12):1150–1159. doi: 10.1016/j.jnutbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Lee KW, Lee HJ. Protective effects of piceatannol against beta-amyloid-induced neuronal cell death. Ann N Y Acad Sci. 2007;1095:473–482. doi: 10.1196/annals.1397.051. [DOI] [PubMed] [Google Scholar]
- 13.Vieira de Almeida LM, Pineiro CC, Leite MC, Brolese G, Leal RB, Gottfried C, et al. Protective effects of resveratrol on hydrogen peroxide induced toxicity in primary cortical astrocyte cultures. Neurochem Res. 2008;33(1):8–15. doi: 10.1007/s11064-007-9399-5. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Gupta YK. Chronic treatment with transresveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71(21):2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Schools GP, Lei T, Wang W, Kimelberg HK, Zhou M. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Exp Neurol. 2008;212(1):44–52. doi: 10.1016/j.expneurol.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Yan Z, Yang J, Chen H, Li H, Jiang Y, et al. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochem Int. 2010;56(3):495–500. doi: 10.1016/j.neuint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Gao ZB, Hu GY. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res. 2005;1056(1):68–75. doi: 10.1016/j.brainres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Granados-Soto V, Arguelles CF, Ortiz MI. The peripheralantinociceptive effect of resveratrol is associated with activation of potassium channels. Neuropharmacology. 2002;43(5):917–923. doi: 10.1016/s0028-3908(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim HI, Kim TH, Song JH. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005;1045(1-2):134–141. doi: 10.1016/j.brainres.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res. 2009;34(8):1393–1400. doi: 10.1007/s11064-009-9920-0. [DOI] [PubMed] [Google Scholar]
- 21.Ghotbedin Z, Janahmadi M, Mirnajafi-Zadeh J, Behzadi G, Semnanian S. Electrical low frequency stimulation of the kindling site preserves the electrophysiological properties of the rat hippocampal CA1 pyramidal neurons from the destructive effects of amygdala kindling: the basis for a possiblepromising epilepsy therapy. Brain Stimul. 2013;6(4):515–523. doi: 10.1016/j.brs.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Haghani M, Janahmadi M, Shabani M. Protective effect of cannabinoid CB1 receptor activation against altered intrinsic repetitive firing properties induced by Aβ neurotoxicity. Neurosci Lett. 2012;507(1):33–37. doi: 10.1016/j.neulet.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Rahvar M, Nikseresht M, Shafiee SM, Naghibalhossaini F, Rasti M, Panjehshahin MR, et al. Effect of oral resveratrol on the BDNF gene expression in the hippocampus of the rat brain. Neurochem Res. 2011;36(5):761–765. doi: 10.1007/s11064-010-0396-8. [DOI] [PubMed] [Google Scholar]
- 24.Robb EL, Stuart JA. Trans-resveratrol as a neuroprotectant. Molecules. 2010;15(3):1196–1212. doi: 10.3390/molecules15031196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat. J Neurotrauma. 2010;27(6):1091–1099. doi: 10.1089/neu.2010.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22(5):1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liew R, Stagg MA, MacLeod KT, Collins P. The red wine polyphenol, resveratrol, exerts acute direct actions on guinea-pig ventricular myocytes. Eur J Pharmacol. 2005;519(1-2):1–8. doi: 10.1016/j.ejphar.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Li HF, Chen SA, Wu SN. Evidence for the stimulatory effect of resveratrol on Ca(2+) -activated K+ current in vascular endothelial cells. Cardiovasc Res. 2000;45(4):1035–1045. doi: 10.1016/s0008-6363(99)00397-1. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Wang QS, Chen Y, Wang ZM, Liu Z, Guo SM. Resveratrolinhibits neuronal discharges in rat hippocampal CA1 area. Sheng Li Xue Bao. 2005;57(3):355–360. [PubMed] [Google Scholar]
- 30.Chang Y, Wang SJ. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by resveratrol. Neurochem Int. 2009;54(2):135–141. doi: 10.1016/j.neuint.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Rutecki PA. Neuronal excitability: voltage-dependent currents and synaptic transmission. J Clin Neurophysiol. 1992;9(2):195–211. [PubMed] [Google Scholar]
- 32.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8(6):451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 33.Milescu LS, Yamanishi T, Ptak K, Smith JC. Kinetic properties and functional dynamics of sodium channel during repetitive spiking in a slow pacemaker neuron. J Neurosci. 2010;30(36):12113–12127. doi: 10.1523/JNEUROSCI.0445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]