Abstract
Objective
Hippocampal insults have been observed in multiple sclerosis (MS) patients. Fibroblast growth factor-2 (FGF2) induces neurogenesis in the hippocampus and en- hances the proliferation, migration and differentiation of oligodendrocyte progenitor cells (OPCs). In the current study, we have investigated the effect of FGF2 on the processes of gliotoxin induced demyelination and subsequent remyelination in the hippocampus.
Materials and Methods
In this experimental study adult male Sprague-Dawley rats re- ceived either saline or lysolecithin (LPC) injections to the right hippocampi. Animals re- ceived intraperitoneal (i.p.) injections of FGF2 (5 ng/g) on days 0, 5, 12 and 26 post-LPC. Expressions of myelin basic protein (Mbp) as a marker of myelination, Olig2 as a marker of OPC proliferation, Nestin as a marker of neural progenitor cells, and glial fibrillary acidic protein (Gfap) as a marker of reactive astrocytes were investigated in the right hippocampi by reverse transcriptase-polymerase chain reaction (RT-PCR).
Results
There was reduced Mbp expression at seven days after LPC injection, in- creased expressions of Olig2 and Nestin, and the level of Gfap did not change. FGF2 treatment reversed the expression level of Mbp to the control, significantly enhanced the levels of Olig2 and Nestin, but did not change the level of Gfap. At day-28 post- LPC, the expression level of Mbp was higher than the control in LPC-treated animals that received FGF2. The levels of Olig2, Nestin and Gfap were at the control level in the non-treated LPC group but significantly higher in the FGF2-treated LPC group.
Conclusion
FGF2 enhanced hippocampal myelination and potentiated the recruitment of OPCs and neural stem cells (NSCs) to the lesion area. Long-term application of FGF2 might also enhance astrogliosis in the lesion site.
Keywords: FGF2, Hippocampus, Neural Stem Cells
Introduction
The hippocampus, which has an essential role in learning and memory, is sensitive to different insults including the demyelination that occurs as a result of multiple sclerosis (MS) (1). The extension of demyelination into the hippocampal formation seems to be responsible for some cognitive disorders such as long-term memory impairment reported in patients who suffer from MS (2). The myelinated fibers of the perforant path are the main afferent source of the hippocampus and apparently hippocampal demyelination can affect their performance. The hippocampus contains neural stem cells (NSCs) in its sub-granular zone which are able to differentiate into neurons and glial cells; therefore it is an appropriate location for studying demyelination and the subsequent endogenous remyelination process (3). Direct injection of demyelinating gliotoxins into the white matter leads to demyelination and the consequent remyelination process (4,5). Our previous study has shown that gliotoxins could induce distinct demyelination and remyelination phases in the hippocampus as determined by histological, electrophysiological and molecular analyses (6). Therefore gliotoxin induced-hippocampal demyelination provides an adequate experimental model for evaluating the protective effect of different potential therapies on the myelinating cells or for evaluating their effects on the efficacy of remyelinating mechanisms, especially when the new therapies are expected to target the cognitive aspects of MS.
Multipotent NSCs, as the functional reservoir for brain tissue repair, renew themselves and differentiate to new neurons or glial cells according to the special phenotype that the niche dictates (7,8). Appropriate environmental signals such as growth factors and chemicals that modify signal transduction pathways can contribute to efficient proliferation, migration and final differentiation of NSCs (9). Growth factors brain-derived neurotrophic factor (BDNF), fibroblast growth factor-2 (FGF2) and epidermal growth factor (EGF) are known as the mediators of adult neurogenesis (10). FGF2 regulates the responses of the oligodendrocyte lineage, which include proliferation and migration, followed by final differentiation of precursors into oligodendrocytes (11). In vivo and in vitro studies have suggested different effects of FGF2 on remyelination based on the administration pattern of FGF2. Continuous FGF2 administration (twice daily for 3 days) has been shown to cause loss of myelin-forming oligodendrocytes and myelin in the adult central nervous system (CNS) while transient exposure to FGF2 (single dose) increased myelination in an in vivo study (12,13). When demyelination was induced in the optic chiasm of mice, FGF2 could potentiate remyelination (14).
Myelin basic protein (Mbp), a component of myelin proteins essential for myelin compaction and stability (15), is frequently used as an index of myelination (14,17). The decreased Mbp expression reflects the reduced number of myelinating oligodendrocytes. Changes in the expression of Olig2 show the relative number of activated oligodendrocyte progenitor cells (OPCs) at the site of demyelination (18). In several studies, Nestin (intermediate filament protein VI) is used as a biological marker to determine NSCs (19). Glial fibrillary acidic protein (Gfap) is expressed by reactive astrocytes at the lesion sites. Although both harmful and beneficial activities have been attributed to these cells, recent studies show roles for reactive astrocytes in limiting inflammation and protecting neurons and oligodendrocytes in lesions (20).
The current study investigated the effects of FGF2 on the responses of myelinating cells, OPCs and reactive astrocytes in rat hippocampi following local injections of lysolecithin (LPC) at days 7 and 28 post-demyelination induction.
Materials and Methods
Animals
For this experimental study 24 adult male Sprague-Dawley rats were obtained from Razi Institute, Karaj, Iran. Rats weighed 280-320 g and were maintained 4 per cage under a 12 hour light/ dark cycle in a room with controlled temperature (24 ± 2˚C). Food and water were available ad-libitum. All research and animal care procedures were performed according to International Guidelines on the Use of Laboratory Animals and were approved by the Ethical Committee for Animal Research at Tarbiat Modares University.
Surgery procedure and treatment
Animals received saline (control group) or LPC to induce hippocampal demyelination. For induction of demyelination, rats were anaesthetized with intraperitoneal (i.p.) injections of combined ketamine (10 mg/kg) and xylazine (2 mg/kg) after which they each received a 1.5 µl injection of 1% LPC dissolved in sterile normal saline. Injections were performed using a 30-gauge needle attached to a 5-µL Hamilton syringe into the right hippocampus (coordinates: A, 5 mm; L-3 mm; V, 2.5 mm below the dura). Animals were divided into three groups: control, non-treated LPC, and FGF2treated LPC. Animals in the FGF2-treated group received i.p. injections of 5 ng/g FGF2 (Royan, Iran) (21) once/day before the injection of LPC and on days 5, 12 and 26 post-LPC injection.
Gene expression study
Based on our previous studies in rats (6,14), we have determined that demyelination is the most active process within the first week and can be FGF2 and Hipocampal Demyelinat ion observed at day 7. Therefore day 7 is considered adequate for assessing the effect of treatments on demyelination. Up to day 28 (week 4), the major phenomenon is remyelination; therefore day 28 appears to be adequate for assessing the effect of treatments on the remyelination process.
For the gene expression study, rats were killed while under CO 2-induced light anesthesia by rapid decapitation at days 7 or 28 post-LPC injection; the right hippocampi were removed and immediately frozen in liquid nitrogen (n=6-8 for each group/ data point). For dissection of the hippocampus, the rat brain was cut along the longitudinal fissure by a surgical blade and then the regions posterior to the lambda were removed. The cerebral hemisphere was then placed medial side up for removing the diencephalon and exposing the medial side of the hippocampus. The final dissection of tissue was performed with a needle-tip.
We measured expressions of the Mbp, Olig2, Nestin and Gfap genes by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) as previously described (22). Primer sequences used for the amplification of Mbp, Olig2, Nestin and Gfap cDNA were designed on the basis of sequences in the Gene Bank. Primer sequences, the length of PCR products, amplification cycles, annealing temperature, and the amount of cDNA template used for each reaction are presented in table 1. The PCR products were resolved on agarose gel, visualized and photographed using a gel documentation apparatus, and finally quantified with band densitometry.
Statistical analysis
Results are expressed as mean ± standard error of mean (SEM). For gene expression analysis, the values of the band density were measured using band densitometry by Image J software. Values were subsequently normalized to Gapdh band density at the same sample. Then, the normalized values were averaged for each group and compared statistically. Statistical assessments were performed using one-way analysis of variance followed by LSD post-hoc. We considered P values <0.05 to be statistically significant.
Results
Effect of fibroblast growth factor-2 on Mbp expression
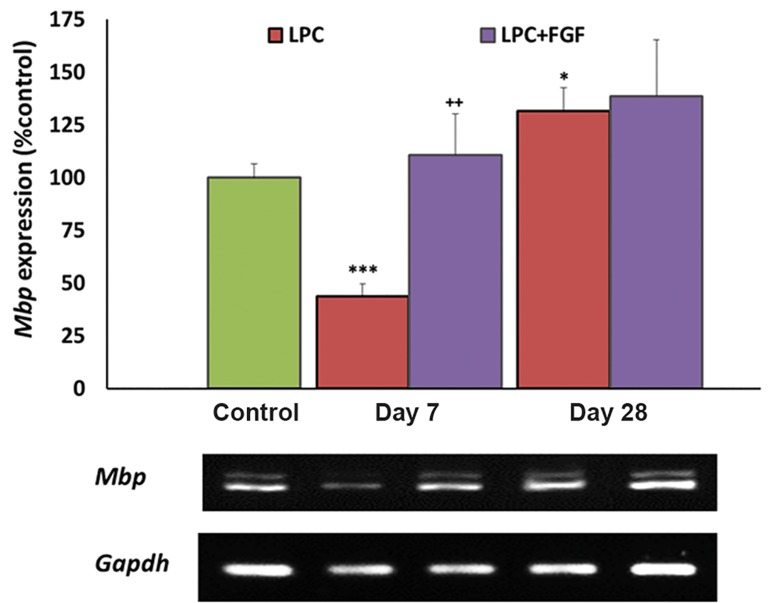
The expression level of Mbp was measured as an index of myelination by myelinating oligodendrocytes. At seven days after LPC injection, there was a significant decrease in the level of Mbp (P<0.001). Although in the FGF2-treated group there was no significant change in the expression of Mbp when compared to the control, however the expression was significantly higher than the LPCtreated group. At day 28, there was no significant difference between FGF2-treated and non-treated groups, while the expression level was more than the control group (P<0.05, Fig .1).
Fig.1.
Evaluation of the effect of fibroblast growth factor-2 (FGF2) on myelin basic protein (Mbp) gene expression by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) following local injection of lysolecithin (LPC). We observed increased Mbp gene expression at day 7 in the FGF2-treated group. Bars: Mean ± standard error of mean (SEM), n≥5, *; P<0.05,***; P<0.001 compared to the control group and ++; P<0.01 compared to the non-treated group at day 7 as evaluated by the LSD test.
Table 1.
Primer sequences and characteristics of the polymerase chain reaction (PCR) reactions used for amplifications of myelin basic protein (Mbp), Olig2, Nestin and glial fibrillary acidic protein (Gfap) cDNAs
| Genes | Primer sequence | Product size (kb) | Number of cycles | T annealing (˚C) | cDNA (μl) |
|---|---|---|---|---|---|
| Mbp | F: CACAGAAGAGACCCTCACAG | 337/415 | 26 | 56 | 2 |
| R: CCCCAGCTAAATCTGCTGAG | |||||
| Olig2 | F: ACACCACCACGTGTCGGCTA | 450 | 30 | 56 | 3 |
| R: CTGCGTCTCTTCTAAGCCCAGA | |||||
| Nestin | F: GGAGTGTCGCTTAGAGGT | 483 | 30 | 58.4 | 2 |
| R: GGAGTGTCGCTTAGAGGT | |||||
| Gfap | F: CTCGTGTGGATCTGGAGAGGAA | 572 | 26 | 56 | 2 |
| R: GCCCTCCAGCAATTTCCTGTAG | |||||
| Gapdh | F: AAACCCATCACCATCTTCCA | 198 | 24 | 56 | 1 |
| R: GTGGTTCACACCCATCACAA | |||||
Effect of fibroblast growth factor-2 on Olig2 expression
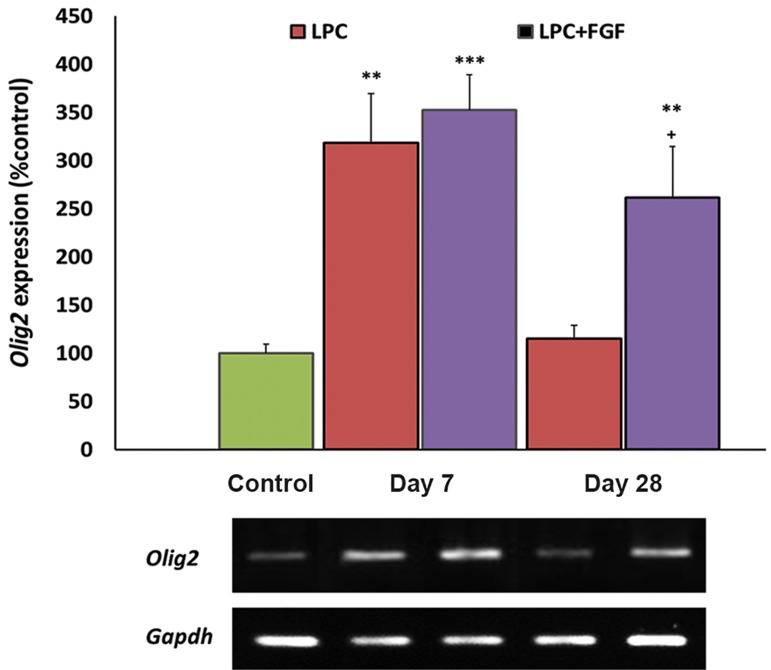
Olig2 expression level was measured as an index of the presence oligodendrocyte precursors. Following local injection of LPC into the hippocampus, we observed a significantly increased expression level of Olig2 at day 7 (P<0.01) that returned to the control level at day 28 post-LPC injection. When the LPC injected animals were treated with FGF2, the expression levels of Olig2 significantly increased at both 7 (P<0.001) and 28 days (P<0.01) post-LPC. When compared with non-treated animals, FGF2 treated animals showed higher levels of Olig2 expression at day 28 post-LPC (P<0.05, Fig .2).
Fig.2.
Evaluation of the effect of fibroblast growth factor-2 (FGF2) on Olig2 gene expression by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) following local injection of lysolecithin (LPC). FGF2 increased the expression of Olig2 at day 28 compared to the non-treated group. Bars: Mean ± standard error of mean (SEM), n≥5, **; P<0.01, ***; P<0.001 compared to the control group and +; P<0.051 compared to the non-treated group at day 28 as evaluated by the LSD test.
Effect of fibroblast growth factor-2 on Nestin expression
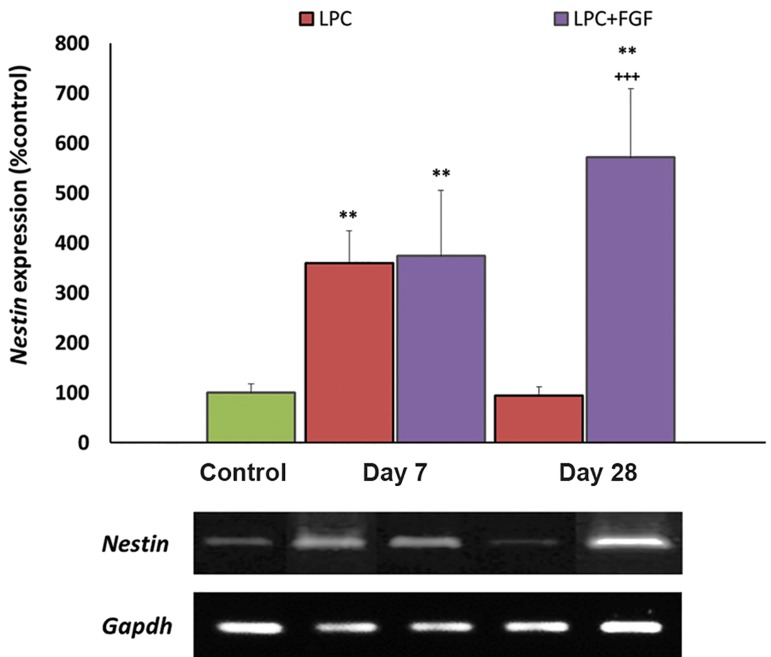
Nestin gene expression was measured to monitor the recruitment of NSCs within the hippocampal lesions. Both FGF2-treated and non-treated groups showed increased expressions of Nestin within the lesion area at 7 days post-LPC injection (both P<0.05). At 28 days post-LPC, the expression level of Nestin returned to the control level in the non-treated group, but it remained higher than the control group in FGF2-treated animals. At day 28, the gene expression level of Nestin in the FGF2-treated group was significantly higher than the non-treated group (P<0.001, Fig .3).
Fig.3.
Evaluation of the effect of fibroblast growth factor-2 (FGF2) on Nestin gene expression by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) following local injection of lysolecithin (LPC). An increased expression of Nestin was observed at day 28 in the FGF2-treated group. Bars: Mean ± standard error of mean (SEM), n≥5, **; P<0.01 compared to the control group and +++; P<0.001 compared to the non-treated group at day 28 as evaluated by the LSD test.
Effect of fibroblast growth factor-2 on Gfap expression
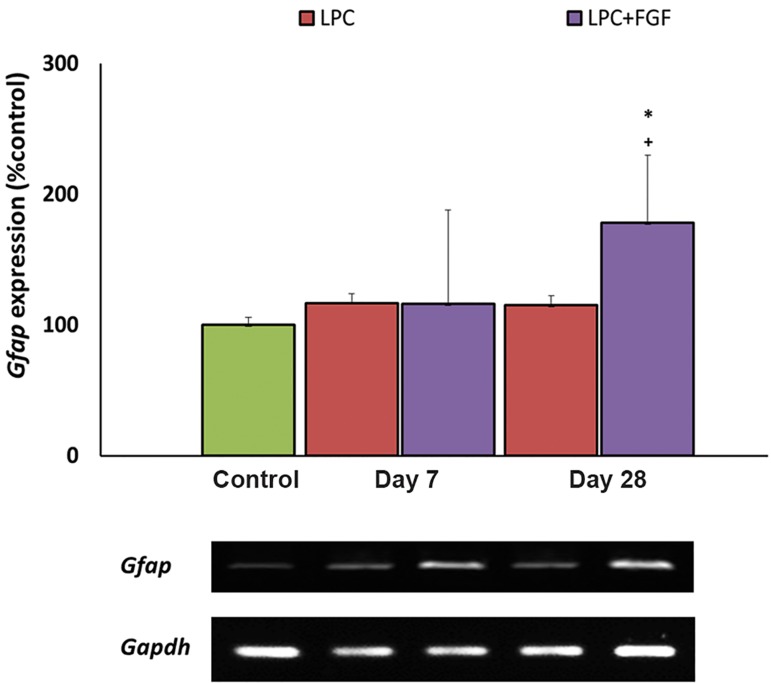
We monitored the Gfap expression level in order to measure for a possible effect of FGF2 on astrocyte reactivation within the lesion area Gfap. At 7 days post-LPC injection, none of the treated and non-treated groups of animals showed changes in the level of Gfap, while at 28 days post-lesion, the expression level of Gfap was significantly higher in the FGF2-treated group when compared with the control and nontreated animals (both P<0.05, Fig .4).
Fig.4.
Evaluation of the effect of fibroblast growth factor-2 (FGF2) on the expression level of glial fibrillary acidic protein (Gfap) by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) following local injection of lysolecithin (LPC). An increased expression of Gfap was observed at day 28 in the FGF2- treated group. Bars: mean ± standard error of mean (SEM), n≥5, *; P<0.01 compared to the control group and +; P<0.05 compared to the non-treated group at day 28 as evaluated by the LSD test.
Discussion
this study, we measured the extent of LPC-induced local demyelination of the hippocampus by monitoring the expression level of Mbp. A decreased level of Mbp at day 7 and its reversal to a level higher than the control at day 28 confirmed the occurrence of previously reported demyelination and consequent remyelination by endogenous progenitors (22). The higher level of Mbp expression at day 28 was likely due to a higher level of Mbp expression by newly generated remyelinating cells in the lesion area. FGF2 increased the level of Mbp at day 7 post-LPC injection. This observation might be due to the protection of oligodendrocytes against LPC-induced lesions or accelerated myelin repair via endogenous progenitors (OPCs and NSCs). While both possibilities were plausible, the increased expressions of Olig2 and Nestin implied that the increased myelin repair was at least partially responsible for higher myelination at day 7 in FGF2-treated animals. Based on a previous report, treatment with FGF2 in animals suffering from experimental autoimmune encephalomyelitis (EAE) reduced the extent of demyelination (23). Another study showed a dual effect of FGF2 on oligodendrocytes in the adult CNS. On the one hand FGF2 caused the destruction of adult oligodendrocytes and loss of myelin, but on the other hand it caused production of new immature oligodendrocyte cells (24). These reports also supported the possibility of increased myelin repair by FGF2 administration, as it was detected by increased Mbp expression at day 7.
OPCs and immature oligodendrocytes express Olig2 (25), therefore the recruitment of OPCs into the lesion area increases the level of Olig2. At day 7 post-LPC injection both FGF2-treated and nontreated groups were shown to have higher levels of Olig2 which fortified the migration of OPCs into the lesion area. This finding concurred with a previous report concerning the migration of endogenous OPCs into demyelinated lesions in adult brains (26). While at day 28, the number of OPCs appeared to return to control levels in non-treated animals, this was mainly due to the differentiation of OPCs to myelinating cells; in FGF2-treated rats an increased number of OPCs in the lesion area was observed. This observation might be attributed to more extensive recruitment of OPCs by administration of FGF2. The same response was observed for Nestin expressing cells. NSCs appeared to migrate toward the lesion area at day 7 and differentiate to myelinating cells at day 28. Again FGF2 increased Nestin expression at day 28 which might imply more extensive recruitment of NSCs in FGF2-treated animals. This finding was supported by previous data in the demyelinated optic nerve where the treatment with FGF2 increased myelin repair and progenitor cell migration (14). Another study also showed that Olig2 gene expression increased in response to FGF2 (27). Since FGF2 has been shown to increase the recruitment of NSCs and OPCs into the lesion area, accelerated myelin repair and the production of myelinating cells from progenitor cells might explain this discrepancy. As it was detected by higher expression of Mbp at day 7, it appeared that some progenitors differentiated into myelinating cells in the FGF2-treated group. Hence, we might consider higher total numbers of differentiated and non-differentiated cells in the FGF2-treated group at day 7.
A previous study showed that a single injection of FGF2 could increase proliferation, recruitment, and migration of subventricular zone (SVZ) NSCs to a demyelinated area of the corpus callosum (21). Interestingly, in other experiments, continuous administration of FGF2 (twice a day, for 3 days) caused loss of myelin-forming oligodendrocytes and myelin loss in the adult CNS, whereas single administration of FGF2 increased myelination in an in vivo study (12,13). In the current study we injected FGF2 once per day on days 0, 5, 12 and 26. Although we repeated the injection of FGF2, this administration was non-continuous and we expected myelin repair by the formation of new myelinating oligodendrocytes. As a result, it seemed that non-continuous FGF2 administration caused progenitor cell proliferation and migration, after which these progenitors differentiated into myelinating cells.
Gfap expression represents reactivated astrocytes at the lesion site. FGF2 administration had no effect on the level of Gfap expression at day 7 but it increased Gfap levels at day 28. Since both harmful and beneficial roles have been reported for the reactive astrocytes (20), more studies are required to elucidate the actual effect of Gfap expressing cells on myelin repair. Increased expression of Gfap at day 28 suggested that we should be concerned about its long-term administration.
Conclusion
Administration of FGF2 during demyelination and remyelination phases of LPC can potentiate myelination, probably by promoting the proliferation of NSCs and OPCs, and their migration toward demyelinating lesions.
Acknowledgments
This study was financially supported as a Master of Science thesis by a grant from Tarbiat Modares University Research Affairs. The authors are thankful to Royan Institute for providing FGF2 as a gift. The Authors declare no conflict of interest.
References
- 1.Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(Pt 4):1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 2.Paulesu E, Perani D, Fazio F, Comi G, Pozzilli C, Martinelli V, et al. Functional basis of memory impairment in multiple sclerosis: a[18F]FDG PET Study. Neuroimage. 1996;4(2):87–96. doi: 10.1006/nimg.1996.0032. [DOI] [PubMed] [Google Scholar]
- 3.Rietze R, Poulin P, Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol. 2000;424(3):397–408. [PubMed] [Google Scholar]
- 4.Pavelko KD, van Engelen BG, Rodriguez M. Acceleration in the rate of CNS remyelination in lysolecithin-induced demyelination. J Neurosci. 1998;18(7):2498–2505. doi: 10.1523/JNEUROSCI.18-07-02498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25(3):216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Azin M, Goudarzvand M, Mirnajafi-Zadeh J, Javan M. Field potential recording from rat hippocampus provides a functional evaluation method for assessing demyelination and myelin repair. Neurol Res. 2013;35(8):837–843. doi: 10.1179/1743132813Y.0000000221. [DOI] [PubMed] [Google Scholar]
- 7.Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res. 2004;76(2):223–231. doi: 10.1002/jnr.20040. [DOI] [PubMed] [Google Scholar]
- 8.Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131(Pt 10):2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brecknell JE, Fawcett JW. Axonal regeneration. Biol Rev Camb Philos Soc. 1996;71(2):227–255. doi: 10.1111/j.1469-185x.1996.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 10.Watts C, McConkey H, Anderson L, Caldwell M. Anatomical perspectives on adult neural stem cells. J Anat. 2005;207(3):197–208. doi: 10.1111/j.1469-7580.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murtie JC, Zhou YX, Le TQ, Vana AC, Armstrong RC. PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol Dis. 2005;19(1-2):171–182. doi: 10.1016/j.nbd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Magy L, Keita M, Richard L, Piaser M, Vallat JM. Transient exposure to FGF2 enhances myelination in embryonic brain cell cocultures. Exp Neurol. 2003;181(1):17–24. doi: 10.1016/s0014-4886(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 13.Butt AM, Dinsdale J. Fibroblast growth factor 2 induces loss of adult oligodendrocytes and myelin in vivo. Exp Neurol. 2005;192(1):125–133. doi: 10.1016/j.expneurol.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Dehghan S, Javan M, Pourabdolhossein F, MirnajafiZadeh J, Baharvand H. Basic fibroblast growth factor potentiates myelin repair following induction of experimental demyelination in adult mouse optic chiasm and nerves. J Mol Neurosci. 2012;48(1):77–85. doi: 10.1007/s12031-012-9777-6. [DOI] [PubMed] [Google Scholar]
- 15.Messersmith DJ, Murtie JC, Le TQ, Frost EE, Armstrong RC. Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res. 2000;62(2):241–256. doi: 10.1002/1097-4547(20001015)62:2<241::AID-JNR9>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30(2):289–299. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husted C. Structural insight into the role of myelin basic protein in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103(12):4339–4340. doi: 10.1073/pnas.0601002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27(3):247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 20.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 21.Decker L, Picard-Riera N, Lachapelle F, Baron-Van Evercooren A. Growth factor treatment promotes mobilization of young but not aged adult subventricular zone precursors in response to demyelination. J Neurosci Res. 2002;69(6):763–771. doi: 10.1002/jnr.10411. [DOI] [PubMed] [Google Scholar]
- 22.Mozafari S, Sherafat MA, Javan M, Mirnajafi-Zadeh J, Tiraihi T. Visual evoked potentials and MBP gene expression imply endogenous myelin repair in adult rat optic nerve and chiasm following local lysolecithin induced demyelination. Brain Res. 2010;1351:50–56. doi: 10.1016/j.brainres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 24.Fressinaud C, Vallat JM, Labourdette G. Basic fibroblast growth factor down-regulates myelin basic protein gene expression and alters myelin compaction of mature oligodendrocytes in vitro. J Neurosci Res. 1995;40(3):285–293. doi: 10.1002/jnr.490400302. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Hu X, Cai J, Liu B, Peng X, Wegner M, et al. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev Biol. 2007;302(2):683–693. doi: 10.1016/j.ydbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Franklin RJ, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997;50(2):337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, Ivanova A, et al. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18(20):8331–8343. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]