Abstract
Purpose.
It is well established that lens fiber differentiation depends on an FGF-initiated growth factor signaling cascade. Given that recent studies indicate Wnt-Frizzled/Planar Cell Polarity (Wnt-Fz/PCP) signaling has a role in coordinating the orientation and alignment of fibers, this study set out to investigate the relationship between this pathway and FGF-induced fiber differentiation.
Methods.
Rat lens epithelial explants were cultured with FGF-2. Regulators of Wnt-Fz signaling, secreted frizzled-related protein-1 (Sfrp1), and inhibitor of Wnt production-2 (IWP-2) were applied to assess the role of this pathway in FGF-induced fiber differentiation. A TCF/Lef reporter mouse was used to assess canonical Wnt-Fz/β-catenin signaling.
Results.
FGF-induced fiber differentiation was accompanied by upregulation of Wnt-Fz signaling components, Fz3, Fz6, Dishevelled-2 (Dvl2), and Dishevelled-3. During differentiation, Fz and the centrosome/primary cilium translocated to the apical tip/leading edge of similarly polarized groups of cells. Addition of Sfrp1 or IWP-2 to FGF-treated explants inhibited cell elongation and reduced expression of fiber-specific markers, filensin and β-crystallin. Expression of Wnt-Fz signaling components was also reduced and a significant reduction in the active form of Dvl2 indicated inhibition of the pathway. Analysis of the TCF/Lef reporter mouse showed no evidence of canonical Wnt-Fz/β-catenin signaling during FGF-induced fiber differentiation.
Conclusions.
This study shows that Wnt-Fz signaling is a component of the FGF-initiated cascade that regulates fiber differentiation. The presence of groups of fibers with Fz and centrosome/primary cilium polarized to the leading edge of each cell is consistent with a role for noncanonical Wnt-Fz signaling in coordinating polarized behavior of differentiating fibers.
This study shows that Wnt-Fz signaling is activated during FGF-induced fiber differentiation. Fz and centrosome translocate to the apex of each elongating cell. This reiterates the polarized behavior of fibers in vivo and indicates a role for Wnt-Fz signaling in organizing the fiber cytoskeleton.
Introduction
It is now becoming recognized that cells within tissues commonly exhibit some degree of coordinated behavior within the plane so that they move/orient in a particular direction to generate polarized structures.1 The eye lens is one example of a tissue that develops a polarized structure through the highly coordinated behavior of its cells. Lens arises from ectoderm that overlies the optic vesicle (presumptive retina). Invagination of this thickened ectoderm forms the lens vesicle that differentiates into primary fibers posteriorly and an epithelial layer anteriorly. The divergent fates of these cells generate the distinctive polarity that is maintained as the lens grows throughout life. Epithelial cells divide, mostly in the germinative zone above the lens equator,2,3 and their progeny migrate below the equator where they elongate and differentiate into secondary fiber cells that progressively become added to the primary fiber mass. Like primary fibers, secondary fiber cells are also highly polarized with their apical ends associated with the overlying epithelium. As they elongate in the lens cortex, they develop convex curvature as they become progressively oriented toward the poles. As fibers form all around the lens equator, they eventually meet and form end-to-end associations with equivalent fibers from other segments of the lens. Precise alignment/orientation of fibers results in formation of distinct suture lines at the poles and in rodents (and at least initially in humans); these are characteristically Y-shaped.4 Because this highly ordered arrangement of fibers is critical for lens function, it is important to understand the mechanism(s) that generates such precise cellular architecture.
There is now compelling evidence that one, or several, members of the FGF growth factor family initiate and promote the fiber differentiation process.5–8 This information has been used to study the process of fiber differentiation in various in vivo and in vitro models. However, progress toward understanding lens morphogenesis depends, not only on knowing how to trigger fiber differentiation, but also how to recapitulate the processes that operate in vivo. To achieve this, we need to understand the factors downstream of FGF that, in addition to promoting the epithelial to fiber differentiation process, regulate the assembly of lens cells into the three-dimensional structure that transmits and focuses images onto the retina.
Recent work in our laboratory has focused on a role for members of the Wnt growth factor family in lens development. The Wnts are a large family of peptide growth factors that act as ligands for the Frizzled (Fz) family of transmembrane receptors. Historically, signaling by Wnts and Fzs have been classed as “canonical” or “noncanonical,” depending on the downstream pathways that are activated.9,10 The β-catenin (canonical) pathway is activated when Wnt ligand forms a complex with a Fz receptor and a low-density lipoprotein-related protein (Lrp) coreceptor. On formation of this complex, a domain of Dishevelled (Dvl) is activated and this leads to accumulation of stabilized β-catenin in the nucleus where it activates responsive promoters in collaboration with DNA-binding proteins of the TCF/Lef family.9 Although this pathway is known to mediate many Wnt effects in both vertebrate and invertebrate systems, it is becoming increasingly clear that noncanonical Wnt signaling also has important roles. In particular, the Wnt-Fz/planar cell polarity (Wnt-Fz/PCP) pathway has been a focus of much attention because of a growing awareness of its importance in coordinating directed cell migration and other oriented cell behaviors that are central to many developmental processes.1 Our recent studies in the lens indicate that as fibers undergo early stages of elongation, their alignment and orientation depends on the Wnt-Fz/PCP pathway. For example, in mice overexpressing secreted frizzled-related protein 2 (Sfrp2), a well-known regulator of Wnt-Fz signaling,11 fiber orientation is severely disrupted and this is associated with reduced expression/activation of downstream components of the PCP pathway.12–14 Given that FGF triggers fiber differentiation, we propose that a key part of this response involves Wnt-Fz signaling and that this regulates much of the coordinated cytoskeletal dynamics that underlie this process. In support of this, we now show that FGF-induced fiber differentiation in epithelial explants depends on activation of a Wnt-Fz signaling pathway.
Materials and Methods
Animals
All animal procedures were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the animal care guidelines published by the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals). All studies were approved by the Institutional Ethics Committee of the University of Sydney.
Preparation of Inverted Lens Epithelial Explants
Postnatal day 5 (P5) Wistar rats were sacrificed: eyes were removed and placed in prewarmed 37°C M199 medium with Earle's salts (Gibco; Invitrogen, Carlsbad, CA), supplemented with 50 IU/mL penicillin, 50 μg/mL streptomycin (Invitrogen), 0.2 mM l-glutamine, and 2.5 μg/mL Amphotericin B (ThermoScientific, Waltham, MA). With the use of a dissecting microscope and jeweler's forceps, the eyes were torn open at the optic nerve to release the lens. Lens epithelial explants were prepared by gently tearing the posterior lens capsule adjacent to the posterior suture and slowly removing the lens fiber mass. The anterior lens capsules were flattened and pinned in an inverted position to the base of the tissue culture dish such that epithelial cells were in direct contact with the base of the tissue culture dish with their lens capsule facing and exposed directly to the media. These are known as ‘inverted' explants (see Fig. 3B in Lovicu and McAvoy15) After explantation, culture medium was replaced with 1 mL of fresh, equilibrated M199 with the addition of either 50 or 200 ng/mL FGF-2 (R&D Systems, Minneapolis, MN). Control dishes for FGF treatments were supplemented with 0.05% or 0.2% BSA. Explants were maintained at 37°C in 5% CO2 for up to 8 days.
Application of Wnt-Fz Pathway Inhibitors
To determine if Wnt/Fz signaling is key to regulating fiber differentiation, the Wnt-Fz signaling inhibitors secreted frizzled related protein 1 (Sfrp1; R&D Systems) and inhibitor of Wnt production-2/Wnt antagonist II (IWP-2: Merck, Germany) were employed. Human recombinant Sfrp1 has been shown to act as decoy receptor by preventing Wnt ligand from interacting with membrane bound Fz receptor.16,17 IWP-2 inhibits cellular Wnt processing and secretion via selective blockage of the membrane bound acyltransferase, porcupine,18 and has previously been shown to inhibit Dvl2 phosphorylation without induction of axin stability.19 Lens epithelial explants were treated with either 4 μg/mL Sfrp1 or 20 μM IWP-2 for 4 hours before the addition of 200 ng/mL FGF or 0.2% BSA; explants remained in these culture conditions for 4 days. Control dishes, lacking inhibitor, were supplemented with an equivalent volume of the vehicle, dimethylsulfoxide (DMSO).
Antibodies
Primary antibodies used in this study were as follows: mouse antibodies against E-cadherin (clone 36; BD Transduction Labs, Lexington, KY), β-catenin (clone 14, BD Transduction Labs), GAPDH (HyTest Ltd, Finland) and α-Acetylated Tubulin (T-6793; Sigma-Aldrich, St. Louis, MO); rabbit antibodies against β-catenin (H102; Santa Cruz Biotechnology, Santa Cruz, CA), pericentrin (ab4448; Abcam, Cambridge, MA), Dvl2 (3224; Cell Signaling, Danvers, MA), Dvl3 (3218; Cell Signaling), Fz-3 (provided by Jeremy Nathans), Filensin (provided by Roy Quinlan 20) and β-crystallin (prepared as previously described 2); and goat antibody against Fz-6 (M-19, Santa Cruz Biotechnology). For Western blot analysis the following horseradish peroxidase (HRP) conjugated secondary antibodies were employed: goat anti-mouse IgG (Upstate, Billerica, MA), goat anti-rabbit IgG (Millipore, Billerica, MA) and rabbit anti-goat IgG (Invitrogen). For immunocytochemistry negative controls of mouse, rabbit and goat whole molecule IgGs were used (Jackson ImmunoResearch Laboratories, Westgrove, PA); secondary antibodies employed were Alexa Fluor 488 or 594-conjugated donkey anti-rabbit, mouse or goat IgG (Invitrogen).
Western Blot Analysis
Lens explants for each Western blotting experiment were obtained from littermates and extracts prepared from pools of 6 to 9 explants. Following 4 days in experimental conditions, explants were rinsed in cold PBS and lens proteins extracted in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS) containing complete mini-protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany) and 10 mM sodium fluoride. Lysates were precleared by centrifuging at 13,000 rpm at 4°C for 15 minutes, and the protein content of the soluble fraction was determined using an assay kit (QuantiPro BCA Assay Kit; Sigma-Aldrich) according to manufacturer's instructions. Equal amounts of protein per sample were loaded onto 8% SDS-PAGE gels for electrophoresis and transferred onto an polyvinylidene fluoride membrane (Invitrolon; Invitrogen) with a electrophoretic transfer cell (Mini Trans-Blot; Bio-Rad, Hercules, CA). Western blotting was carried out as previously described.21 Proteins were detected using an enhanced chemiluminescence (ECL) substrate (SuperSignal West Dura Extended Duration ECL Substrate; ThermoScientific) and visualized using a G:Box with imaging software (GeneSnap v.6.08; Syngene, Cambridge, UK) for quantification of band intensities.
Immunocytochemistry
Inverted lens epithelial explants were fixed in 100% methanol for 45 seconds at room temperature followed by four successive washes with PBS. Following fixation, explants were flipped over and pinned to the base of the same tissue culture dish such that lens cells faced uppermost toward the bathing medium with their capsule closest to the base of the dish. Nonspecific cellular sites were blocked with the addition of normal donkey serum (1:10) in 0.1% BSA in PBS with incubation for 1 hour at room temperature. Primary antibodies (1:50-1:750 dilution) were diluted in 0.1% BSA in PBS with normal donkey serum (1.5:100) and applied overnight at 4°C. To remove unbound antibody, explants were washed in 0.1% BSA in PBS three times for 5, 10, and 15 minutes, respectively. Secondary antibodies conjugated to Alexa 488 and 594 dyes were used at a dilution of 1:1000 in 0.1%BSA in PBS and applied for 2 hours in the dark at room temperature; this was followed by three washes in 0.1% BSA. Circular glass coverslips were mounted on top of lens explants using an aqueous mounting medium (Aqua Poly/Mount Solution; Polysciences, Inc., Warrington, PA). Fluorescence was visualized and images were collected using a confocal microscope (Zeiss LSM-5 Pascal; Carl Zeiss Meditec, Jena, Germany) with LSM Image Browser 5 software (Carl Zeiss Meditec). Measurements of optical thickness were taken from the central region of lens explants immunostained for β-catenin. Using a ×40 objective a Z-stack function was performed with first and last parameters set before the first appearance of fluorescence signal and loss of all fluorescence respectively. Z-stacks of 0.8-μm intervals were acquired generating 20 to 35 optical slices. Using image browser software (Zeiss LSM Image Browser 5; Carl Zeiss Meditec) Z-stacks were displayed as orthogonal sections merged into an xz reconstruction of the thickness of the Z-stack against the xy image. By moving through the Z-stack and xz image (from apical to basal [capsule] xy view), optical thickness was measured as the distance between the first detection of β-catenin in cell boundaries to last detection.
TCF/Lef Reporter Gene Assay
P2 lenses were collected from TCF/Lef reporter mice,22 and inverted explants were set up and cultured for 4 days without (controls) or with 200 ng/mL FGF-2 as described above. X-gal staining was conducted according to manufacturer's instructions (GAL-S; Sigma-Aldrich). After 3 hrs incubation with X-gal reaction solution at 37°C, explants were rinsed and coverslipped. For whole lens X-gal staining, postnatal day 3 eyes were fixed with 2% paraformaldehyde for 30 min and then processed for colour reaction according to the method described previously.23 Paraffin sections were prepared from stained eyes for histological analysis.
Statistical Analysis
A 2 tailed t-test analysis (Excel software; Microsoft, Redmond, WA) and one-way ANOVA (with Tukey's posthoc analysis; IBM SPSS Statistics ver. 19 for Windows; SPSS Inc., Chicago, IL) were performed to determine statistical differences between experimental groups, set at P ≤ 0.05.
Results
FGF Upregulates Wnt-Fz Signaling Components
Studies conducted over many years in our laboratory and others have shown that FGF induces rat lens epithelial explants to undergo many of the morphological and molecular changes characteristic of fiber differentiation in vivo.5,24–26 As a first step to investigate involvement of Wnt-Fz signaling in fiber differentiation, we carried out Western blotting analyses on FGF-treated explants. Initially we cultured explants with a half-maximal (50 ng/mL) or maximal (200 ng/mL) fiber differentiating dose of FGF-2 to cover the possibility of major FGF dose-related differences in expression of Wnt-Fz signaling components.27 Consistent with previous studies,5 by 4 days both concentrations of FGF had induced cell elongation and other features characteristic of early fiber differentiation. Western blotting was conducted to investigate the status of key components of the Wnt-Fz pathway, Fz3, Fz6, Dvl2, and Dvl3 (Fig. 1A). For Fzs and Dvls, both concentrations of FGF induced about a 2- to 4-fold increase in their expression levels and the statistical analysis showed this to be a significant increase in all cases (Fig. 1B). Dvl2 was the only component that showed a greater response to the higher dose of FGF compared with the lower dose. Also for Dvl2, we routinely detected two bands, the upper being the phosphorylated (activated) form of Dvl.28,29 Both bands showed an increase in FGF-treated explants; however, the observation that the upper band increased significantly over controls (see Supplementary Material and Supplementary Table, http://www.iovs.org/content/54/3/1582/suppl/DC1), indicates enhanced activation of Wnt-Fz signaling. Both concentrations of FGF induced a switch from epithelial to fiber phenotype as shown by decreased epithelial-specific E-cadherin and increased intermediate filament marker, filensin, as the cells elongated into fibers. Taken together these results indicate that activation of a Wnt-Fz signaling pathway is associated with FGF-induced fiber differentiation.
Figure 1.
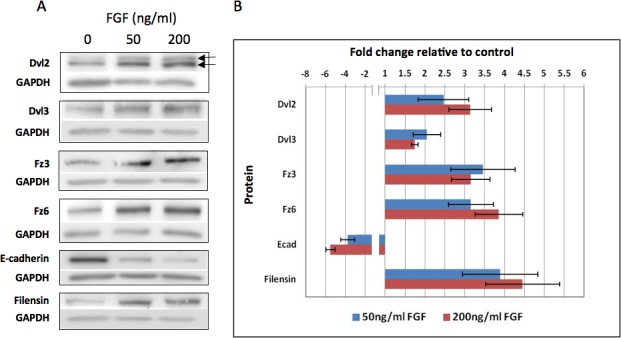
FGF upregulates Wnt-Fz signaling components as most cells switch from epithelial to fiber phenotype. (A) Western blots show that Dvl2, Dvl3, Fz3, and Fz6 are all upregulated in explants after 4 days exposure to either 50 or 200 ng/mL FGF-2. Dvl2 is present in two bands (arrows); the upper band being the phosphorylated form of Dvl2. Expression of the fiber-specific intermediate filament filensin is also upregulated in the FGF-treated explants. E-cadherin, the epithelial marker, is downregulated. (B) The histogram shows band density measurements at the two concentrations of FGF-2. In the case of Dvl2, total levels are presented (i.e., summation of upper and lower bands). In all cases, GAPDH loading controls were used to normalize the levels of protein detected and the results were presented as fold changes relative to the respective controls. Data presented shows the mean ± SEM (n = 4). Both Dvls and Fzs showed significant 2- to 4-fold increases in expression relative to controls at both concentrations of FGF. Dvl2 was the only signaling component that showed a significant (P = ≤ 0.05, 2-tailed t-test) concentration-related effect. For Dvl2, although both bands showed increased density, the observation that the upper phosphorylated (active Dvl2) band was significantly increased (P = ≤ 0.05) in the presence of FGF indicates that Wnt-Fz signaling is enhanced by FGF (see Supplementary Material and Supplementary Table, http://www.iovs.org/content/54/3/1582/suppl/DC1). For E-cadherin, the band density was substantially reduced by both concentrations of FGF. In the case of Dvl3, Fz3, Fz6, Filensin and E-cadherin both 50 ng/mL and 200 ng/mL showed significant difference compared to control (P = ≤ 0.05, 2-tailed t-test).
FGF Induces Translocation of Fz to Leading Edge of Elongating Fibers
Our recent studies have shown that when epithelial cells shift from the germinative zone and begin to elongate in the transitional zone, they exhibit PCP as shown by translocation of their centrosome/primary cilium to the leading edge of each elongating fiber cell (i.e., the side of the cell at its apex that faces the anterior pole). This is also accompanied by similar polarization of Fz6 and some other PCP proteins.13,14 To determine if a similar translocation of centrosome/primary cilium and Fz6 was a feature of FGF-induced fiber differentiation, we localized pericentrin and Fz6 in our explants (Fig. 2). Note that pericentrin is a major component of the centrosome that in many cell types, and certainly in lens cells, is associated with the primary cilium at the apical margin of the cell.13,14 In controls, Fz6 localizes to small, variable-sized domains at the margins of the typically cobblestone-packed epithelial cells (Figs. 2A, 2A1). In elongating fibers, strong Fz6 reactivity becomes prominent at one end of the cell. We identified this as the apical tip (or leading edge) of the elongating fibers because this is where the centrosomal marker, pericentrin, is also localized (Figs. 2B, 2B1). In controls, a spot of pericentrin immunoreactivity is located next to the nucleus and exhibits no spatial relationship with the Fz6 domain (Figs. 2A, 2A1); however, during fiber differentiation pericentrin becomes localized next to Fz6 at the apical cell margin (Figs. 2B, 2B1). This shows that as a result of their translocation, a close spatial relationship develops between Fz6 and the centrosome/primary cilium during FGF-induced fiber differentiation in explants and this mimics the relationship between these components that is evident at the apical tip of each fiber cell in vivo.13,14
Figure 2.
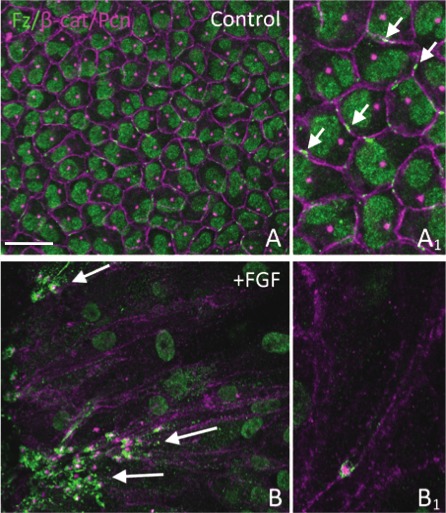
FGF promotes translocation and polarization of Fz6 and centrosome in rat explants. Rat lens explants were cultured with or without 200 ng/mL FGF for 8 days and immunostained for Fz6 (green) and β-catenin/pericentrin (purple). (A) In controls, Fz6 localizes to discrete membrane domains in the cobblestone-packed epithelial cells (enlarged in A1, arrows). Note that this Fz6 antibody gave non-specific signal in cell nuclei. Pericentrin immunoreactivity shows that the centrosome is located next to the nucleus and is not associated with Fz6. (B) During FGF-induced fiber elongation, Fz6 and the centrosome localize to the leading edge of elongating fibers and after 8 days groups of aligned fibers are commonly seen to be similarly polarized (arrows). At higher magnification, strong Fz6 reactivity is evident at the apical tip of each of these fibers and, as indicated by pericentrin reactivity, is intimately associated with the centrosome (B1). Scale bar: (A, B), 20 μm; (A1, B1), 10 μm.
Inhibition of Wnt-Fz Signaling Impacts on FGF-Induced Fiber Differentiation
To determine if the upregulation/activation of Wnt-Fz signaling components was involved in regulating the fiber differentiation process, we introduced modulators of Wnt-Fz signaling into the explant cultures. Previous studies in our laboratory have shown that overexpression of Sfrp2 in lenses of transgenic mice disrupts the orientation/alignment of their fibers.12 Availability of the closely related Sfrp1 protein allowed us to introduce this modulator of Wnt-Fz signaling into explant cultures and assess its effects on fiber differentiation. FGF treatment induced substantial elongation of cells throughout the explants as shown by acetylated-tubulin immunoreactivity (Fig. 3). This localizes the stabilized microtubules that are a feature of fiber differentiation in vivo. Pericentrin immunoreactivity localizes the centrosome and this identifies the apical tip (leading edge) of each elongated cell in FGF-treated explants (Fig. 3F, inset). Frequently, as in this image, groups of elongated cells as defined by acetylated-tubulin reactivity are seen to be similarly oriented.
Figure 3.
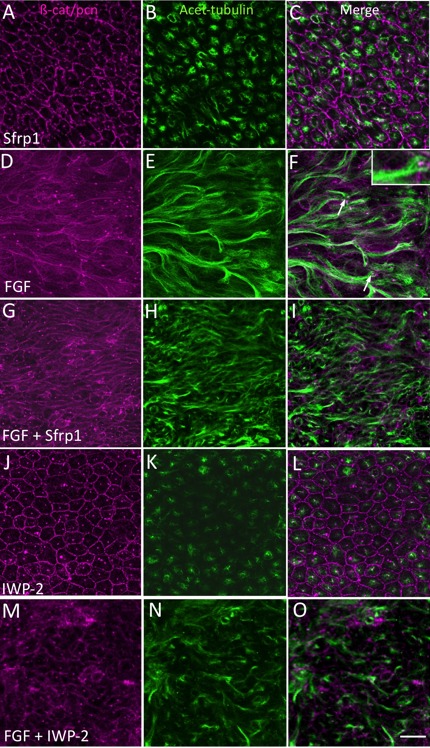
Sfrp1 or IWP-2 inhibits FGF-induced fiber elongation. Explants were cultured in 4 μg/mL Sfrp1 (A–C), 200 ng/mL FGF-2 (D–F), in the presence of both FGF and Sfrp1 (G–I), or in the presence of both FGF and IWP-2 (20 μM, J–L) for 4 days. β-catenin and pericentrin immunoreactivity (A, D, G, J) localizes cell margins and centrosomes, respectively. Acetylated-tubulin reactivity (B, E, H, K) localizes stabilized microtubules and helps visualize extent of cell elongation. In Sfrp1 alone, the cells maintain the cobblestone-like arrangement characteristic of lens epithelial cells in vivo (A–C). In FGF alone, cells show extensive elongation (D–F). Pericentrin reactivity localizes the centrosome and identifies the apical tip of each cell (F, inset) and groups of elongated cells are often seen to point in a similar direction (F, arrows). When Sfrp1 (G–I) or IWP-2 (M–O) is included with FGF, elongation is not as extensive as in explants with FGF alone. Scale bar: 20 μm.
Sfrp1 on its own had no effect on the morphology of epithelial cells or their characteristic cobblestone-like packing arrangement (Figs. 3A–C; compare with controls in Fig. 2A). When Sfrp1 was included with FGF the cells did not show the same degree of elongation as seen in explants cultured in FGF alone (Fig. 3; compare D–F with G–I). The inclusion of IWP-2 with FGF had a similar inhibitory effect. This Wnt signaling antagonist was selected to complement the Sfrp1 studies because it works by inhibiting Wnt secretion from cells.9,18 As with Sfrp1, the presence of IWP-2 alone had no effect on morphology of cells in explants (Figs. 3J–L); however, when IWP-2 was included with FGF, the cells were clearly shorter than cells in explants treated with FGF alone (Fig. 3; compare D–F with M–O). Analysis of β-crystallin immunoreactivity also showed that compared with FGF alone, the presence of either Sfrp1 or IWP-2 substantially reduced accumulation of this fiber-specific marker in explants (Fig. 4). Consistent with inhibition of elongation and β-crystallin accumulation, the explants treated with inhibitors were consistently thinner than explants treated with FGF alone (Table).
Figure 4.
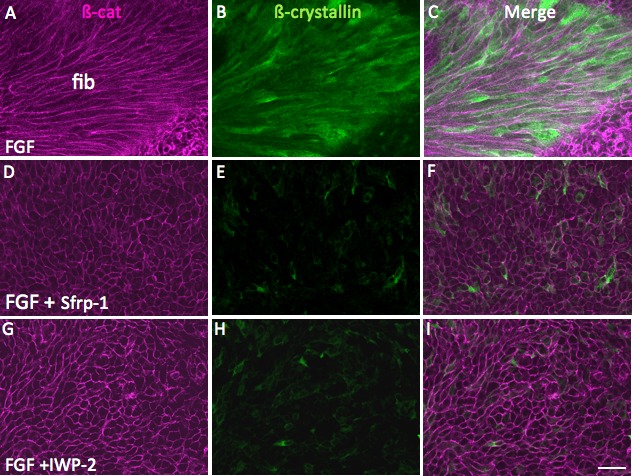
Sfrp1 or IWP-2 block FGF-induced β-crystallin accumulation. Explants were cultured in 200 ng/mL FGF-2 (A–C) or in the presence of both FGF and 4 μg/mL Sfrp1 (D–F) or FGF and 20 μM IWP-2 (G–I) for 4 days. β-catenin immunoreactivity (A, D, G) localizes to cell margins. In FGF-treated explants, β-crystallin accumulation is evident in the elongated fiber cells (B, C). When Sfrp1 (D) or IWP-2 (G) is included with FGF, elongation is abrogated and β-crystallin does not accumulate in the cells (E,F and H,I). Fib, fibers. Scale bar: 30 μm.
Table.
Measurement of the Optical Thickness of Explants (from β-catenin detection) Following Application of Wnt-Fz Signaling Inhibitors
|
Experimental Condition |
Optical Thickness of Explant, μm |
| Control | 14.88 ± 0.29 |
| FGF (200 ng/mL) | 26.16 ± 0.66 |
| Sfrp1 (4 μg/mL) | 16.46 ± 1.01 |
| Sfrp1 + FGF* | 18.94 ± 1.11 |
| IWP-2 (20 μM) | 13.07 ± 0.36 |
| IWP-2 + FGF* | 16.25 ± 1.01 |
Values in this table are derived from mean ± SEM (n = 4).
Significant difference between Sfrp/IWP-2 plus FGF compared with FGF alone group (P ≤ 0.05, ANOVA with Tukey's test).
Western blots were also conducted to assess the inhibitory effect of IWP-2 (Fig. 5A). In the presence of IWP-2, FGF-enhanced expression of Dvls was reduced. Importantly, the upper (phosphorylated) band of Dvl2 was reduced (Fig. 5A, upper arrow). Quantification reveals this to be a significant shift and provides evidence that IWP-2 has effectively inhibited Wnt-Fz signaling (Fig. 5B). Expression of Dvl3 and Fz6 was also significantly reduced. This reduced expression in the presence of the IWP-2 indicates that Wnt itself has a role in regulating its signaling pathway components. Such autoregulation within the Wnt pathway has been reported previously in both mammalian and Drosophila systems.30,31 Taken together, these results provide evidence that the blocking effect of IWP-2 on fiber differentiation is mediated through inhibition of the Wnt-Fz signaling pathway.
Figure 5.
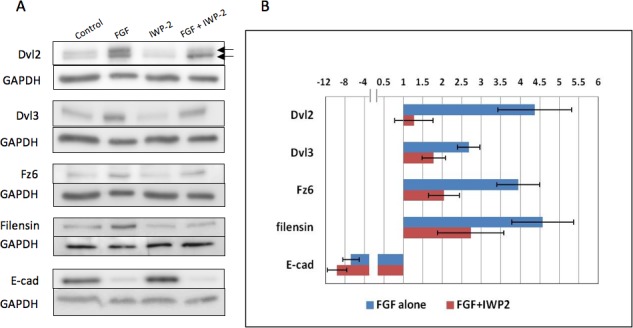
IWP-2 inhibits FGF-induced fiber differentiation. (A) Western blots for Dvl2, Dvl3, Fz6, Filensin, and E-cadherin in explants after 4 days exposure to either 200 ng/mL FGF-2, IWP-2 alone, FGF-2/IWP-2, or no FGF with DMSO vehicle only (controls). Dvl2, Dvl3, Fz6, and filensin are all upregulated after 4 days exposure to FGF. In contrast, E-cadherin expression is reduced. Explants cultured with IWP-2 show similar band densities for E-cadherin as in controls (no FGF with DMSO vehicle only). When IWP-2 is included with FGF, band densities for Dvl2, Dvl3, Fz6, and filensin are all reduced in comparison with explants cultured in FGF alone. E-cadherin is relatively unaffected by the FGF/IWP-2 combination. Dvl2 is present in two bands (arrows); the upper band being the phosphorylated form of Dvl2. In explants cultured with FGF/IWP-2, the upper band in particular is reduced in comparison to explants cultured in FGF alone. (B) The histogram shows band density measurements for Dvl2 (upper phosphorylated band only), Dvl3, Fz6, filensin, and E-cadherin. In all cases, GAPDH loading controls were used to normalize the levels of protein detected and the results were presented as fold changes relative to the respective controls. Data presented shows the mean ± SEM (n = 4). Similar to the results shown in Figure 1, FGF induced about a 2- to 4-fold increase in expression of Dvl2 (phosphorylated), Dvl3, Fz6, and filensin. Also, as before, E-cadherin was reduced in FGF-treated explants. In all cases, except for E-cadherin, the presence of IWP-2 significantly reduced protein expression when compared to groups treated with FGF alone (P = ≤ 0.05, ANOVA with Tukey's test). Notably, Dvl2 showed a significant decrease in the density of the upper phosphorylated (active Dvl2) band indicating reduced Dvl2 signaling activity.
In the presence of IWP-2, the FGF-enhanced expression of filensin was also significantly inhibited (Fig. 5). This is consistent with the reduced elongation/differentiation of fibers as shown by diminished immunoreactivity for acetylated-tubulin and β-crystallin in these explants (see Figs. 3, 4). Also as reported earlier, the epithelial marker E-cadherin is substantially reduced as the majority of cells in the explant progress down the fiber differentiation pathway in response to FGF. The presence of IWP-2 does not appear to impact on the expression of E-cadherin in FGF-treated explants indicating that Wnt-Fz signaling is not required for expression of this epithelial marker (Fig. 5).
β-Catenin Signaling in TCF/Lef Reporter Mice
As noted earlier, the translocation of Fz and the centrosome/primary cilium to the leading edge of elongating fibers mimics the situation in vivo when cells shift from the germinative zone into the fiber differentiation compartment below the lens equator. Polarization of Fz and centrosome/primary cilium to the leading edge of the elongating fibers is a morphological feature of Wnt-Fz/PCP signaling that has been implicated in regulating the orientation/alignment of fibers in vivo.13 However, previous studies have reported evidence supporting a role for canonical Wnt-Fz/β-catenin signaling in regulating aspects of fiber differentiation.32 In contrast, studies from a number of laboratories including our own, using the transgenic reporter mouse lines TOP-gal, BAT-gal, and TCF/Lef that are routinely used for assessment of canonical Wnt-Fz signaling has shown a distinct absence of reporter activity in the lens fibers (Fig. 6).14,33–37 Thus, involvement of canonical Wnt-Fz/β-catenin signaling in FGF-induced fiber differentiation is uncertain. To investigate this further, we set up explants from TCF/Lef reporter mice and treated them with FGF to induce fiber differentiation. We assayed for reporter activity by staining for β-galactosidase (gives blue reaction product) after 4 days of culture. In these explants, no blue coloration was evident indicating absence of reporter activity (Figs. 6A–D). Particularly noteworthy was the observation that when FGF-treated explants had multilayered and elongated there was no evidence of blue coloration in any of these cells. Similar results were obtained with explants from P15 mice that were cultured with FGF for 1, 2, or 3 days (data not shown). We also noted that cells from remnants of ciliary body that were left attached to the peripheral lens capsule during explantation, stained blue (Figs. 6A, 6B). This provides a useful positive control because canonical Wnt-Fz/β-catenin signaling activity is prominent in the ciliary body (Fig. 6E), and has been shown to be important for determining formation of ciliary body and iris from periphery of optic cup.38,39 Thus, explants treated with FGF to induce fiber differentiation mimic the situation in vivo, as indicated by reporter mouse lines, that Wnt-Fz/β-catenin signaling does not feature in the fiber differentiation process. Note that in additional experiments where we exposed explants to Wnt3a in an attempt to induce Wnt/β-catenin signaling, the overwhelming response was negative (see Supplementary Material and Supplementary Figure, http://www.iovs.org/content/54/3/1582/suppl/DC1). Taken together, this indicates that lens epithelial cells are strongly resistant to activation of the canonical Wnt signaling pathway.
Figure 6.
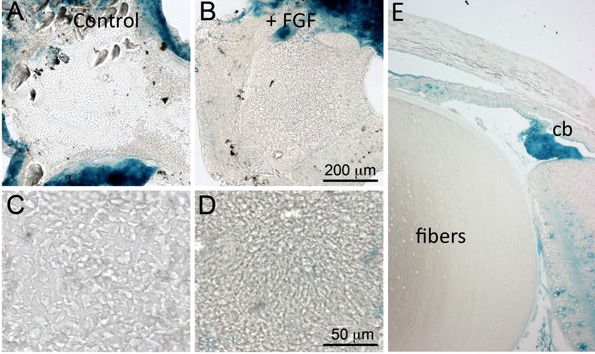
Canonical Wnt-Fz/β-catenin signaling is not a feature of FGF-induced fiber differentiation. Lens epithelial explants prepared from the TCF/Lef reporter mouse line were cultured for 4 days with no added factors (A, C) or 200 ng/mL FGF-2 (B, D). Low-power images stained for β-galactosidase to detect TCF/Lef reporter activity show that it is not detectable in lens cells of control or FGF-treated explants. Note that remnants of ciliary body associated with the peripheral capsule in the explants show strong blue coloration and act as a positive control for Wnt-Fz/β-catenin signaling (A, B). Higher power images show that control (C) explants retain the characteristic lens epithelial cobblestone packing arrangement whereas FGF-treated (D) show early fiber elongation changes. A sagittal section of a TCF/Lef mouse eye stained for β-galactosidase (E) shows prominent blue staining in the ciliary body (cb) but no blue staining in the lens fibers.
Discussion
This study shows that FGF-induced fiber differentiation involves Wnt-Fz signaling. Evidence for this comes from the upregulation of Wnt-Fz signaling components, Fz3, Fz6, Dvl2, and Dvl3 during FGF-induced fiber differentiation. We also show that the phosphorylated (active) form of Dvl2 increases during this process. Inclusion of Wnt-Fz signaling inhibitors results in diminished elongation and reduced expression of fiber-specific markers, filensin and β-crystallin. The reduction in active Dvl2 in the presence of the Wnt inhibitor, IWP-2, also supports a causal link between Wnt-Fz signaling and fiber differentiation. Taken together this indicates a role for Wnt-Fz signaling in regulating the fiber differentiation process downstream of FGF induction.
Previous studies with lens explants have also indicated a role for Wnt-Fz signaling in fiber differentiation.32 Evidence was presented that Wnts promote the morphological aspects of fiber cell differentiation in a process that requires FGF signaling, but is independent of β-catenin. Nuclear localization of β-catenin in differentiating fibers at the lens equator and in FGF-treated explants was also reported. Other studies showed that embryonic lenses of genetically modified mice lacking β-catenin (and thus lacking canonical Wnt signals), had decreased levels of β-crystallin and impaired fiber differentiation.40 This formed the basis for the proposal that canonical Wnt-Fz/β-catenin signaling has a role in regulating some molecular events in fiber differentiation, notably accumulation of the fiber differentiation marker, β-crystallin. The Lyu and Joo32 study also reported that Wnt3a conditioned medium or vitreous could promote TOPFLASH (TCF reporter) activity in a transiently transfected mouse lens cell line. However, as already noted, the three widely used transgenic reporter mouse lines (TOP-gal, BAT-gal, and TCF/Lef), have shown a distinct absence of reporter activity in the lens fibers (Fig. 6).14,33–37 Moreover, our current studies with the TCF/Lef reporter mice that are commonly used to investigate canonical Wnt signaling in the eye, show that neither of the growth factor treatments used, FGF or Wnt3a, was able to promote signaling activity in explanted lens cells. Thus the current study provides no support for involvement of Wnt-Fz/β-catenin signaling in fiber differentiation. However it should be borne in mind that whilst reporter mice are generally reliable indicators of canonical Wnt-Fz signaling, there are also situations where false negatives could arise.41 In light of this, and given some of the data from previous studies, we cannot definitively exclude this pathway from the fiber differentiation process at this stage. In direct contrast to this uncertainty about canonical Wnt-Fz/β-catenin signaling, results from the current study add to the growing support for involvement of non-canonical Wnt-Fz signaling in fiber differentiation.12,13
Upregulation and activation of Wnt-Fz signaling during fiber differentiation is accompanied by the translocation of Fz protein from discrete membrane domains in the epithelial precursors (controls) to become prominently localized at the leading edge of elongating fibers. The centrosome/primary cilium, although initially showing no association with Fz, becomes similarly polarized to the leading edge of each elongating fiber (Fig. 2). This is reminiscent of the situation in vivo where, during the epithelial to fiber transition, Fz and centrosome/primary cilium become localized to the apical tip of each elongating fiber as it abuts against the epithelium (see Sugiyama et al.13). Their intimate association in this region may be important because the centrosome can function as a major docking station with multiple functions in cell signaling and its position next to Fz may be critical for polarized cell behavior, such as formation of protrusive processes during directed cell migration.42 Activated Dvls are likely to be key mediators in this context because they are known to be required for apical docking of basal bodies/centrosome,43 as well as functioning as a scaffold for cytoskeletal components and associated kinases, phosphatases, and adaptor proteins.44 Furthermore, given that studies in other systems show that Dvl is involved in the stabilization of microtubules,45 increased Dvl activity may be important for promoting local orientation and stabilization of microtubules during fiber differentiation. This is consistent with strong localization of acetylated-tubulin (indicating stabilized microtubules) associated with the centrosome at the leading edge (apical tips) of the elongating fibers (Fig. 3F).
Regulation of Dvl may also be one way that FGF influences Wnt-Fz signaling as studies in other developmental systems have shown that FGF is required for membrane localization of Dvl as well as polarization of cytoskeletal components during morphogenetic movements.46 While such a paradigm provides a plausible basis for their cooperation in the lens, other studies indicate additional possibilities. For example, it has been demonstrated that Wnt and FGF signals can be integrated to achieve robust expression of transcription factors that are important for coordinating convergent extension (depends on PCP signaling) at the neural plate border in Zebrafish.47 Given these diverse possibilities, further work will be needed to determine the mechanism(s) whereby FGF regulates Wnt-Fz signaling in the current context.
In summary, this in vitro study shows that FGF activates Wnt-Fz signaling during lens fiber differentiation. This is evident from FGF-induced upregulation of key Wnt-Fz signaling components, Dvl2/3 and Fz3/6 as well as by promoting activation of Dvl. During FGF-induced Wnt-Fz signaling, in groups of elongating cells, Fz and the centrosome/primary cilium translocate to the apical tip/leading edge of each cell. Our previous studies have shown that PCP operates in the lens. The current study now shows that FGF promotes processes that reiterate features of the polarized behavior of differentiating fibers in vivo and is consistent with FGF preferentially promoting the noncanonical Wnt-Fz/PCP pathway rather than the canonical Wnt-Fz/β-catenin pathway. The inhibitor studies also show that Wnt-Fz signaling is downstream of FGF-induced fiber differentiation and given its known cytoskeletal organizing role in other systems, these results are consistent with noncanonical Wnt-Fz/PCP signaling organizing the fiber cell cytoskeleton and coordinating polarized behavior of fibers during lens morphogenesis and growth.
Acknowledgments
Thanks to Daniel Dufort for his gift of the TCF/Lef reporter mice and to Elizabeth Shelley and Li Wen for assistance with some of the procedures.
Footnotes
Supported by the National Institutes of Health (R01 EY0-3177), the National Health and Medical Research Council (NHMRC), the Sydney Foundation for Medical Research, and the Ophthalmic Research Institute of Australia.
Disclosure: L.J. Dawes, None; Y. Sugiyama, None; A.S. Tanedo, None; F.J. Lovicu, None; J.W. McAvoy, None
References
- 1. Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011; 21: 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McAvoy JW. Cell division, cell elongation and distribution of α-, β- and γ-crystallins in the rat lens. J Embryol Exp Morphol. 1978. a; 44: 149–165. [PubMed] [Google Scholar]
- 3. McAvoy JW. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morph. 1978. b; 45: 271–281. [PubMed] [Google Scholar]
- 4. Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. Int J Dev Biol. 2004; 48: 889–902. [DOI] [PubMed] [Google Scholar]
- 5. Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005; 280: 1–14. [DOI] [PubMed] [Google Scholar]
- 6. Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006; 17: 726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao H, Yang T, Madakashira BP, et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008; 318: 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu X, Hertzler K, Pan Y, Grobe K, Robinson ML, Zhang X. Genetic epistasis between heparan sulfate and FGF-Ras signaling controls lens development. Dev Biol. 2011; 355: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012; 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- 10. Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harb Perspect Biol. 2012; 4: pii: a007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mii Y, Taira M. Secreted Wnt “inhibitors” are not just inhibitors: regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev Growth Differ. 2011; 53: 911–923. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Stump RJ, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Dev Biol. 2008; 324: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugiyama Y, Stump RJ, Nguyen A, et al. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev Biol. 2010; 338: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011; 7: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lovicu FJ, McAvoy JW. Epithelial explants and their application to study developmental processes in the lens. Ed Tsonis PA. Animal Models in Eye Research. Academic Press; 2008; 134–147. [Google Scholar]
- 16. Balfico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999; 274: 16180–16187. [DOI] [PubMed] [Google Scholar]
- 17. LaMonica K, Bass M, Grabel L. The planar cell polarity pathway directs parietal endoderm migration. Dev Biol. 2009; 330: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009; 5: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacob LS, Wu X, Dodge ME, et al. Genome-wide RNAi screen reveals disease-associated genes that are common to hedgehog and Wnt signaling. Sci Signal. 2011; 4;ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandilands A, Wang X, Hutcheson AM, et al. Bfsp2 mutation found in mouse 129 strains causes the loss of CP49′ and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res. 2004; 78: 875–889. [DOI] [PubMed] [Google Scholar]
- 21. Stump RJ, Ang S, Chen Y, et al. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003; 259: 48–61. [DOI] [PubMed] [Google Scholar]
- 22. Mohamed OA, Clarke HJ, Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004; 231: 416–424. [DOI] [PubMed] [Google Scholar]
- 23. Robinson ML, Overbeek PA, Verran DJ, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995; 121: 505–514. [DOI] [PubMed] [Google Scholar]
- 24. Lovicu FJ, McAvoy JW. Structural analysis of lens epithelial explants induced to differentiate into fibers by fibroblast growth factor (FGF). Exp Eye Res. 1989; 49: 479–494. [DOI] [PubMed] [Google Scholar]
- 25. Golestaneh N, Fan J, Fariss RN, Lo WK, Zelenka PS, Chepelinsky AB. Lens major intrinsic protein (MIP)/aquaporin 0 expression in rat lens epithelia explants requires fibroblast growth factor-induced ERK and JNK signaling. J Biol Chem. 2004; 279: 31813–31822. [DOI] [PubMed] [Google Scholar]
- 26. Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol. 2009; 332: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989; 107: 221–228. [DOI] [PubMed] [Google Scholar]
- 28. González-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM. Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol Cell Biol. 2004; 24: 4757–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Endo Y, Wolf V, Muraiso K, et al. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005; 280: 777–786. [DOI] [PubMed] [Google Scholar]
- 30. Sivasankaran R, Calleja M, Morata G, Basler K. The Wingless target gene Dfz3 encodes a new member of the Drosophila Frizzled family. Mech Dev. 2000; 91: 427–431. [DOI] [PubMed] [Google Scholar]
- 31. Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002; 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyu J, Joo CK. Wnt signaling enhances FGF-2-triggered lens fiber cell differentiation. Development. 2004; 131: 1813–1824. [DOI] [PubMed] [Google Scholar]
- 33. Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kreslova J, Machon O, Ruzickova J, et al. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007; 45: 157–168. [DOI] [PubMed] [Google Scholar]
- 35. Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006; 47: 5088–5097. [DOI] [PubMed] [Google Scholar]
- 36. Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003; 227: 323–334. [DOI] [PubMed] [Google Scholar]
- 37. Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005; 285: 477–489. [DOI] [PubMed] [Google Scholar]
- 38. Liu H, Xu S, Wang Y, et al. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007; 308: 54–67. [DOI] [PubMed] [Google Scholar]
- 39. Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006; 133: 3167–3177. [DOI] [PubMed] [Google Scholar]
- 40. Cain S, Martinez G, Kokkinos MI, et al. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev. Biol. 2008; 321: 420–433. [DOI] [PubMed] [Google Scholar]
- 41. Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006; 25: 7505–7511. [DOI] [PubMed] [Google Scholar]
- 42. Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008; 85: 261–301. [DOI] [PubMed] [Google Scholar]
- 43. Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008; 40: 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006; 72: 153–166. [DOI] [PubMed] [Google Scholar]
- 45. Matsumoto S, Fumoto K, Okamoto T, Kaibuchi K, Kikuchi A. Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J. 2010; 29: 1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi W, Peyrot SM, Munro E, Levine M. FGF3 in the floor plate directs notochord convergent extension in the Ciona tadpole. Development. 2009; 136: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garnett AT, Square TA, Medeiros DMBMP. Wnt and FGF signals are integrated through evolutionarily conserved enhancers to achieve robust expression of Pax3 and Zic genes at the zebrafish neural plate border. Development. 2012; 139: 4220–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]


