Abstract
Purpose.
Glucocorticoid (GC)-induced glaucoma is an undesirable side effect of traditional GCs. Ocular hypertension responsible for GC-induced glaucoma is due to alterations in conventional outflow homeostasis. The present study evaluates a novel selective GC receptor agonist (SEGRA), GW870086X, in two different in vitro models of the human conventional outflow pathway.
Methods.
Primary cultures of human trabecular meshwork (TM) cell monolayers were treated with dexamethasone (DEX), prednisolone (PRED), or GW870086X for 5 days and then assayed for cellular expression and secretion of fibronectin, myocilin, tissue plasminogen activator (tPA), and/or matrix metalloproteinase-2 (MMP2). In parallel, TM cell monolayers on permeable filters treated for 5 days with GCs were assayed for changes in hydraulic conductivity.
Results.
All three GCs increased fibronectin and myocilin secretion in a concentration-dependent manner (P < 0.05). In addition, DEX increased cellular fibronectin and both DEX and PRED significantly increased cellular myocilin (P < 0.0001), while GW870086X did neither. Interestingly, DEX and PRED significantly decreased tPA expression (P ≤ 0.01), while GW870086X had the opposite effect and increased tPA expression in a concentration-dependent manner (P = 0.01). For MMP2, only DEX treatment consistently decreased secretion (P < 0.01). In a functional assay, only PRED treatment significantly decreased hydraulic conductivity of TM cell monolayers (P < 0.05).
Conclusions.
All three GCs induced differential responses from TM cells. While the novel SEGRA GW870086X increases fibronectin and myocilin secretion similar to two traditional GCs, effects on the matrix degradation enzymes MMP2 and tPA differed significantly, suggesting that GW870086X favors matrix turnover. Consequently, effects on conventional outflow homeostasis may also be dissimilar.
A novel SEGRA, GW870086X affects secretion of proteins involved in extracellular matrix turnover from trabecular meshwork cells differently than two traditional glucocorticoids and may lack or have a less severe side effect profile typically associated with these classical glucocorticoids.
Introduction
Synthetic glucocorticoids (GC) are widely used as drugs that decrease inflammation and suppress the immune system throughout the body. Systemically, GCs treat conditions such as lupus and rheumatoid arthritis. Topically, GCs are used to treat skin conditions such as dermatitis and eczema or ocular conditions such as macular edema and uveitis. One of the potentially harmful side effects of GC use with either systemic or ocular application is ocular hypertension and the associated potential for developing iatrogenic secondary open-angle glaucoma.1
Glucocorticoids elevate IOP by decreasing conventional, pressure-dependent outflow.2–5 For example, topical administration of a potent GC for 4 to 6 weeks can result in a 40% increase in IOP. Severity of drug effects on IOP is dependent upon the potency of the GC, the dose, and duration of treatment in concert with individual susceptibility6—although different patients treated with GC have different responses, falling into three categories: low responders, intermediate responders, and high responders.7,8 Interestingly, incidence of developing ocular hypertension and POAG later in life corresponds to the severity of IOP response to GCs.9,10 Suggestive of a genetic link, there are also reports of increased GC responsiveness in relatives of those with POAG.7,8
The mechanisms by which GCs induce ocular hypertension appear to involve at least two cellular processes in the conventional pathway, increased barrier function at the inner wall of Schlemm's canal (SC), and alterations in cell contractility and extracellular matrix (ECM) turnover in the trabecular meshwork (TM).1 Morphological examination of the TM of patients having GC-induced glaucoma shows an increased abundance of extracellular materials throughout the TM, a decrease in intratrabecular spaces, and evidence of metabolically active TM cells—a phenotype similar to POAG.11,12 Such effects can be reproduced in the laboratory in animal models, organ cultured human eyes, and cell culture models.13–15 Molecular signatures of GC-treated cells in culture include upregulation of the glaucoma-causing gene product myocilin; ECM proteins such as fibronectin (FN), glycosaminoglycans, and laminins; plus downregulation of ECM degradative proteins such as tissue plasminogen activator (tPA) and the matrix metalloproteinases (MMPs).16–22 Treatment of SC cells with corticosteroids results in increased complexity of cell-cell junctions and decrease in hydraulic conductivity of cell monolayers.23
The effects of GCs on conventional outflow biology have typically involved use of “classical” GC receptor (GR) agonists, such as dexamethasone (DEX) or prednisolone (PRED) acetate. Binding of these classical GR agonists to the GC receptor followed by translocation to the nucleus leads to positive as well as negative regulation of the expression of a panel of genes, events commonly referred to as transactivation and transrepression, respectively (reviewed by Barnes24). Recent evidence suggests that transrepression, such as activator protein-1 (AP-1) and nuclear factor κB (NFκB), accounts for much of the anti-inflammatory activity of GC, whereas the unwanted side effects may be linked to transactivation. This has sparked a search for GCs that preferentially exhibit transrepressive activities with the goal to improve the therapeutic index.25,26 Examples of such novel, “dissociated” selective GC receptor agonists (SEGRA) are BOL-303242-X27,28 and GW870086X (Uings et al., manuscript submitted, 2013). Evidence that reduced transactivation may limit effects on conventional outflow was recently demonstrated when treatment of monkey TM monolayers in culture with BOL-303242-X resulted in diminished effects on myocilin induction of mRNA and protein compared with DEX or PRED. In both transactivation and transrepression transcriptional reporter assays in human cells, GW870086X was found to be a highly potent GC agonist (pIC50 = 10.1 for inhibition of NFκB reporter activity, e.g.) and similar in potency to DEX in a range of gene transcriptional assays (Uings et al., manuscript submitted, 2013). While GW870086X shares full transrepressive activity with DEX, the maximum extent of transactivation is dramatically diminished in comparison with classical GCs, with GW870086X exhibiting only partial agonism ranging from zero to approximately 80% maximal efficacy compared with DEX depending on the gene of interest (Uings et al., manuscript submitted, 2013). Despite the altered transcriptional profile, compared with DEX, GW870086X acts as a potent anti-inflammatory molecule in rodent models of both lung and delayed-type hypersensitivity induced inflammation (Uings et al., manuscript submitted, 2013). However, its effect on conventional outflow cells is unknown.
The purpose of the present study was to compare responses of GW870086X with DEX and PRED on several specific measures of ECM turnover status in the trabecular meshwork including myocilin, fibronectin, MMP2, and tPA. Moreover, to assess the overall condition of ECM turnover in the TM, we utilized an in vitro model of the juxtacanalicular region of the TM to assess effects of GW870086X on hydraulic conductivity of mature TM cells on permeable supports.
Materials and Methods
Cell Culture
Three strains of human trabecular meshwork (HTM) cells (HTM-83, -86, and -91) isolated from eyes of human donors aged 54 years, 3 months, and 44 years, and characterized as previously described29,30 were used in the present study. Human biological samples were sourced ethically from accredited US eye banks and their research use was in accordance with the terms of the informed consents of the donors and/or donor family.
HTM cells were cultured in T75 polystyrene plates (tissue culture treated; Becton, Dickinson and Company, Franklin Lakes, NJ) containing growth media (Dulbecco's modified Eagle's medium (DMEM), low-glucose DMEM; Invitrogen, Carlsbad, CA) supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.29 mg/mL glutamine (Invitrogen) in a controlled environment consisting of humidified air containing 5% CO2 at 37°C. For experiments, cells were seeded onto polystyrene 24 well plates (tissue culture treated; Becton, Dickinson and Company) or polycarbonate membrane filters (tissue culture treated, 12-mm diameter, 0.4-μm pore size; Corning, Inc., Corning, NY) at confluence. To facilitate differentiation in culture, the monolayers were allowed to mature 7 days supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT), then switched to 1% fetal bovine serum for an additional 7 days prior to experimentation.
Cell Treatments
After cell monolayers were allowed to mature at confluence for 2 weeks, treatments were started. Cells were exposed to DEX, PRED, or GW870086X at 0, 1, 3, 10, 30, 100 or 300 nM for 5 days. Compounds were supplied in powder form from GlaxoSmithKline and reconstituted in DMSO as 300-mM stock solutions that were diluted to give final drug concentrations in DMEM. Media (DMEM supplemented with 1% FBS) was changed after 48 hours and equal volumes of media from each group were collected after the final 72 hours of treatment, concentrated (×10), and added to an equal volume of ×2 sample buffer (Laemmli). Cells were then rinsed with PBS and scraped directly into 100-μL ×2 sample buffer/well. All samples were incubated at 100°C for 8 minutes and stored at −20°C until analysis.
Cell Perfusions
With human TM cells plated onto filters, we attempted to model the environment that exists in the juxtacanalicular region of the conventional outflow pathway where TM cells are in contact with cell neighbors and fluid flow passes by their apical surface. Thus, we predicted that changes in rate of ECM deposition by TM cells on filters would alter flow pathways (openings) between adjacent cells, and thus change hydraulic conductivity. Consistent with this idea and many reports in the literature (reviewed by Clark and Wordinger1), we used DEX and PRED treatment of TM cells on filters as a positive controls.31
For the present study, cells were plated onto filters at a density of 1 × 105, and cultured for 5 to 9 days. Cell monolayers were then exposed to media alone or media containing DEX, PRED, or GW870086X (30 nM or 100 nM) for 5 days. Hydraulic conductivity was measured as described previously31 with filters containing cells being placed in an Ussing-type chamber filled with HEPES-buffered DMEM (25 mM HEPES, pH 7.4, serum-free; Invitrogen, Carlsbad, CA). The chamber, tubing, and reservoir were gently filled with DMEM-HEPES, and the cells were allowed to acclimate for 15 minutes at 37°C with no pressure gradient. Experiments were begun by raising a reservoir of media to 13.6 cm above the midline of the filter containing cells (giving a 10-mm Hg pressure differential across the cells) and perfusing cells for 60 minutes at 37°C. Experiments were concluded by collecting effluent from the basal side of filters and storing on ice prior to concentrating ×10 initial volume using centrifugal concentrators (Millipore, Billerica, MA) at 7000g (Eppendorf, Hauppauge, NY). Filters were removed from chambers, rinsed in phosphate-buffered saline, and cells were scraped directly into 75-μL ×2 sample buffer (Laemmli). All samples were incubated at 100°C for 8 minutes and stored at −20°C until analysis.
For every experimental condition, a minimum of two different cell strains in at least five individual experiments were perfused for each treatment group to indicate that results were not an artifact of an individual donor or experiment. The hydraulic conductivity for each filter was normalized to its β-actin expression to account for differences in cell numbers between filters. Data for treatment groups were compared with controls where all controls for each experiment were averaged and used as baseline hydraulic conductivity (HC). We watched to make sure that hydraulic conductivity measurements were in the range of 0.5 to 10 μL/min/mm Hg/cm2 such that drug-induced changes would remain in the range of detection for the force displacement transducer.
Western Blot Analysis
For Western blotting, proteins were loaded into 10% polyacrylamide gel slabs and separated via SDS-PAGE. Fractionated proteins were then transferred electrophoretically to nitrocellulose membranes. Membranes containing transferred proteins were blocked in Tris-buffered saline with 0.2% Tween 20 (TBS-T) containing 5% nonfat dry milk. Primary antibodies (indicated below) were added to blocking buffer, and blots were incubated overnight at 4°C. Membranes were washed in TBS-T (4 times for 10 minutes) and incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies in TBS-T containing 5% milk. After incubation, membranes were washed with TBS-T as before. Protein-antibody complexes were visualized by a chemiluminescent HRP antibody detection reagent spray (HyGLO; Denville Scientific, Inc., Metuchen, NJ) on x-ray film (Phenix Research Company, Candler, NC). Where indicated, proteins were quantified by densitometry using a bioimaging system and image analysis software (GeneSnap/GeneTools; Syngene, Frederick, MD). Protein expression for all samples was normalized to β-actin expression to account for differences in cell densities at confluence.
Antibodies
Polyclonal rabbit anti-myocilin IgGs were produced against the amino half of myocilin by our laboratory and characterized previously.33 Membranes were incubated with blocking buffer containing anti-myocilin IgG at a final concentration of 300 ng/mL. All other antibodies were purchased commercially and used at the indicated dilutions: anti-tissue plasminogen activator (1:1000) and anti-fibronectin (1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), monoclonal anti-MMP2 (1:1000, Calbiochem, Gibbstown, NJ) and monoclonal anti-β-actin (1:5000, Sigma, St. Louis, MO). All horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Statistical Analyses
Only digitized bands captured onto x-ray film that corresponded to proteins of interest and fell within the linear range of the x-ray film were used for densitometric analyses. All Western blot densitometry and hydraulic conductivity data was analyzed on the log10 scale to improve normality and equalize variability.
Myocilin, tPA, and MMP2 expression in TM cells and media: log10 (concentration+1) versus log10 (response) slope estimates, standard errors, and P values were obtained from a hierarchical random-intercept analysis of covariance model (ANCOVA) with log10 (concentration+1) and cellular β-actin (cells data only) as covariates. Two levels of random variability were assumed in the intercept, corresponding to the donor-to-donor and experiment-within-donor variance components.
Fibronectin expression in TM cells, all filters (cells and effluent), and hydraulic conductivity: difference estimates with untreated control and standard errors were obtained from a hierarchical random-intercept ANCOVA with cellular β-actin as a covariate. Fibronectin secretion in media: difference estimates with untreated control and standard errors were obtained from a hierarchical random-intercept ANOVA model.
Simultaneous 95% confidence limits were calculated using Dunnett's method. Sidak's stepdown method was used to adjust the P values of the ANCOVA slope comparisons. The NLME library in the commercial data analysis software (S-PLUS v7 Enterprise Developer; Insightful Corporation, Seattle, WA) was used for all statistical modeling. The stepdown Sidak adjusted P values were obtained by Proc Multtest using statistical analysis software (SAS v9.1; SAS Institute, Cary, NC).
Results
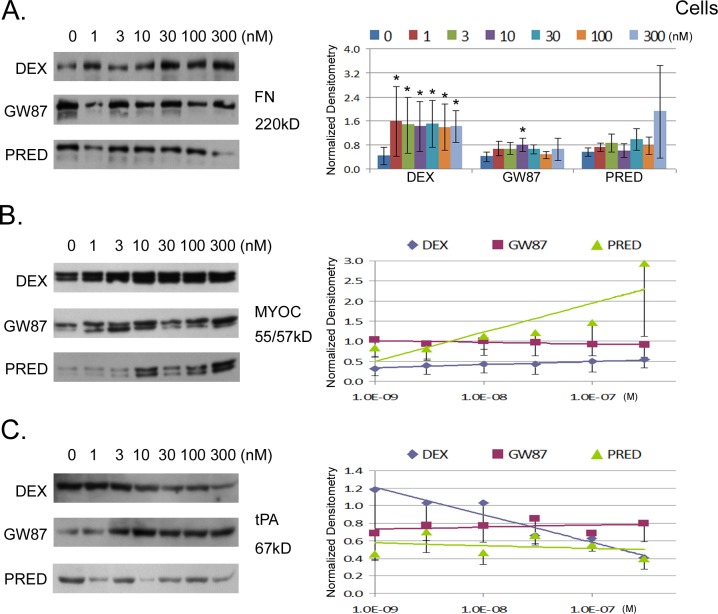
In all experiments, confluent human trabecular meshwork (TM) cell monolayers were differentiated in culture for 2 weeks. In preliminary experiments, we treated cells with 100 nM DEX and examined protein expression of fibronectin and myocilin over time. Cell lysates were prepared and analyzed for protein expression levels by Western blot analysis. We were able to detect increased expression of both proteins by 3 days, peaking at 5 days (data not shown). For all subsequent experiments, cells were treated with one of three corticosteroids, DEX, PRED acetate, or GW870086X for 5 days. Shown in Figure 1 are the concentration-related effects of treatments (0–300 nM) on the cellular protein expression levels of three different secreted proteins (myocilin, tPA, and fibronectin) known to change in response to classical GC. Due to the binary nature of fibronectin responses, data were analyzed at each GC concentration (see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/54/3/2100/suppl/DC1). Results in Figure 1 show that DEX treatment significantly increased cellular fibronectin approximately 4-fold at all concentrations tested, whereas GW870086X and PRED did not. While there was no dose/response relationship in terms of cellular accumulation for DEX, the variability of fibronectin response decreased with increasing dose.
Figure 1.
Concentration-response relationship for three different glucocorticoid compounds and their effect on protein expression of human TM cell monolayers in culture. Cells were treated for 5 days in DMEM containing 1% FBS and then tested for expression of three candidate proteins. On the left are representative Western blots showing expression levels for each candidate protein: tissue fibronectin (FN, [A]) myocilin (MYOC, [B]), and tissue-type plasminogen activator (tPA, [C]). On the right are combined results (mean ± SEM) from densitometric analyses of Western blots taken from six experiments involving three different cell strains normalized to β-actin. Expression levels for FN were compared at every concentration and asterisks (*) indicate significant difference from untreated control at P < 0.05 (A). Due to concentration-related responses, slopes generated from changes in expression levels with treatment were compared for myocilin (B) and tPA (C): analyses of slopes are shown in Supplementary Table S2, (see Supplementary Material and Supplementary Table S2, http://www.iovs.org/content/54/3/2100/suppl/DC1).
In contrast, dose/response relationships could be analyzed for cellular myocilin and tPA. For these two, statistical comparisons were made between slopes derived from dose-related changes in cellular protein following treatment (see Supplementary Material and Supplementary Table S2, http://www.iovs.org/content/54/3/2100/suppl/DC1). The data show that both DEX and, to a much greater extent, PRED significantly increased cellular myocilin (P < 0.0001), while GW870086X did not (Fig. 1B). Moreover, DEX and PRED effects were significantly different from GW870086X (P < 0.05, Table 1). With respect to tPA protein, Figure 1C shows that both DEX and PRED decreased tPA levels with increasing dose, whereas GW870086X had the opposite effect, increasing tPA abundance with increasing dose. Differences between GW870086X and the other two classical GCs were significant at P ≤ 0.0002 (Table 1).
Table 1.
Statistical Comparison of Slopes Generated from Concentration-Related Responses of TM Cells to Glucocorticoid Treatment
|
Protein |
Source |
Comparison |
Estimate |
SE |
P* |
| Myocilin | Cells | Slope DEX vs. GW87 | 0.1129 | 0.0541 | 0.0393† |
| Myocilin | Cells | Slope PRED vs. GW87 | 0.1288 | 0.0537 | 0.0365† |
| tPA | Cells | Slope DEX vs. GW87 | −0.2047 | 0.0479 | <0.0002† |
| tPA | Cells | Slope PRED vs. GW87 | −0.1855 | 0.0477 | 0.0002† |
| Myocilin | Media | Slope DEX vs. GW87 | −0.0372 | 0.058 | 0.5225 |
| Myocilin | Media | Slope PRED vs. GW87 | 0.1314 | 0.058 | 0.0503† |
| MMP2 | Media | Slope DEX vs. GW87 | −0.0466 | 0.0491 | 0.5707 |
| MMP2 | Media | Slope PRED vs. GW87 | 0.0089 | 0.0491 | 0.8572 |
GW87 = GW870086X.
Sidak adjusted.
Significantly different.
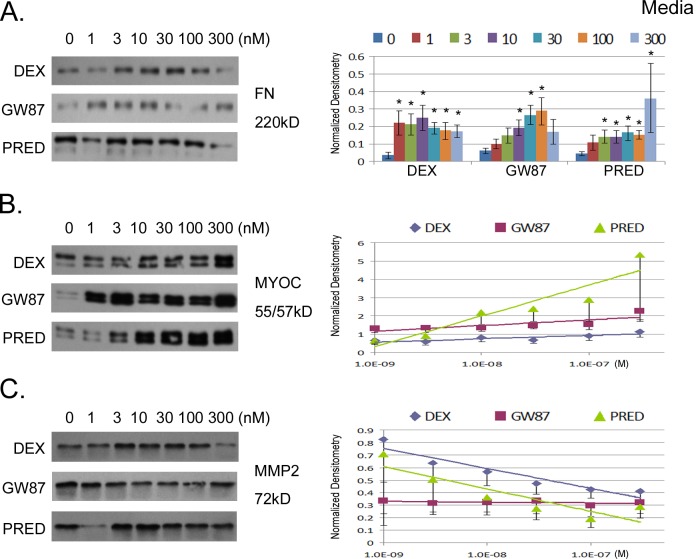
To determine effects of GCs on secreted levels of MMP2, myocilin, and fibronectin, conditioned media atop TM cell monolayers was collected for the final two days of treatment and concentrated 10-fold prior to analyses of protein content. Accumulated levels of secreted proteins were assessed by Western blot. Results in Figure 2 show that both DEX and PRED increased fibronectin at all concentrations, while only three concentrations of GW870086X (10–100 nM) significantly increased fibronectin secretion (P < 0.05; see Supplementary Material and Supplementary Table S3, http://www.iovs.org/content/54/3/2100/suppl/DC1). The profile of fibronectin secretion with increasing drug concentration was different between the three corticosteroids; DEX dramatically induced fibronectin equally at all concentrations, whereas PRED and GW870086X responses were dose-related. Figure 2 shows that extracellular myocilin levels were also significantly increased in a dose-related manner in response to all three corticosteroids (P < 0.0001). Interestingly, the slope of PRED-stimulated secretion of myocilin was greater than for GW870086X (Table 1). We were unable to consistently detect tPA in conditioned media from our three TM cell strains by Western blot (not shown), so we tested for another secreted protein (MMP2) whose expression has been shown previously to be negatively affected by GCs. Similar to effects on tPA, DEX significantly decreased MMP2 (P = 0.004), but PRED and GW870086X had no significant effects on MMP2 secretion (see Supplementary Material and Supplementary Table S4, http://www.iovs.org/content/54/3/2100/suppl/DC1).
Figure 2.
Concentration-response relationship for three different GC compounds and their effect on protein secretion from human TM cell monolayers in culture. Cells were treated for 5 days and then equal volumes of conditioned media (DMEM containing 1% FBS) were collected during the final 72 hours, concentrated (×10) and added to an equal volume of ×2 Laemmli buffer. On the left are representative Western blots showing secretion levels for each candidate protein: fibronectin (FN, [A]), myocilin (MYOC, [B]), and matrix metalloproteinase-2 (MMP2, [C]). On the right are combined results (mean ± SEM) from densitometric analyses of Western blots taken from six experiments involving three different cell strains normalized to β-actin. Expression levels for FN were compared at every concentration and asterisks (*) indicate significant difference from untreated control at P < 0.05. Due to concentration-related responses, slopes generated from changes in expression levels with treatment were compared for myocilin and MMP2: analyses of slopes are shown in Supplemental Table S4 (see Supplementary Material and Supplementary Table S4, http://www.iovs.org/content/54/3/2100/suppl/DC1).
Taken together, GW870086X differentially affected matrix generating and degrading processes in human TM cells compared with DEX and PRED. Both DEX and PRED shifted the balance toward matrix generation and decreased turnover. GW870086X was the only GC that increased a matrix-degrading component, tPA, and had a less pronounced effect on matrix deposition with an overall more favorable profile.
We next evaluated the effects of GW870086X, DEX, and PRED in a cellular perfusion system that models fluid flow through the juxtacanalicular region of the TM. Cells on filters were treated for 5 days at either 30 or 100 nM concentration of corticosteroid and then were placed into an Ussing-like chamber. Fluid flow across TM cells in the apical to basal direction was driven by a pressure gradient of 10 mm Hg. HC of cell monolayers was measured for 1 hour and combined results of multiple experiments (n = 5) testing two different TM cell strains isolated from two different eye donors are shown in Figure 3. While DEX lowered HC by ∼50% at both concentrations tested, data were not significantly different from control (P > 0.05; see Supplementary Material and Supplementary Table S5, http://www.iovs.org/content/54/3/2100/suppl/DC1). Results show that only PRED at both 30 nM and 100 nM significantly decreased HC compared with vehicle-treated controls (P < 0.05). GW870086X had no significant effect on HC in this model (see Supplementary Material and Supplementary Table S5, http://www.iovs.org/content/54/3/2100/suppl/DC1). Results were similar (i.e., significant findings did not change) whether data were normalized to β-actin content or not.
Figure 3.
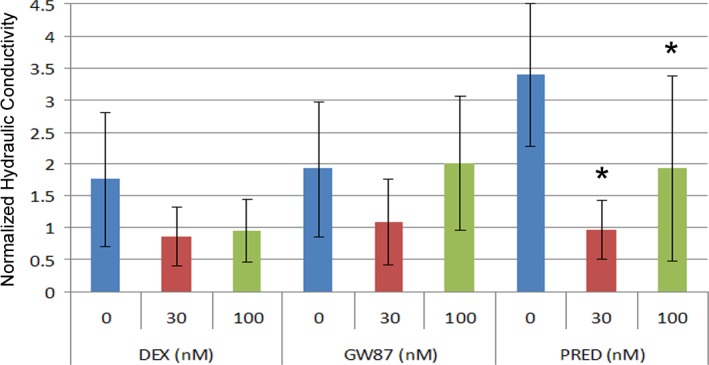
Effect of glucocorticoids on hydraulic conductivity of human TM cell monolayers. Cells on permeable filters were treated for 5 days with DEX, GW870086X (GW87), or PRED. Cells on filters were then assayed for HC in Ussing-like chamber in HEPES-buffered DMEM (serum-free). Shown are mean results (±SEM) of five experiments using two different TM cell strains normalized to β-actin. Asterisks (*) indicate significant difference from untreated control where P < 0.05.
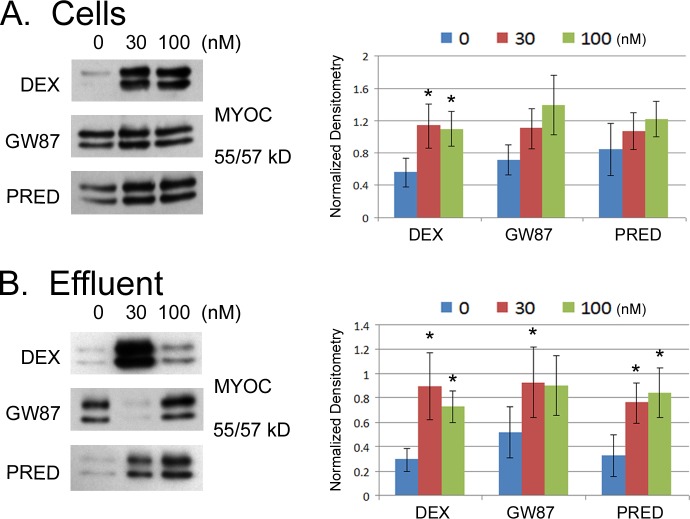
In perfusion experiments, we also monitored myocilin induction to verify activity of all three GCs (Fig. 4). Postperfusion cellular protein levels of myocilin appeared generally higher in all GC-treated cells compared with control; although levels were statistically different from control only for cells treated with DEX (P < 0.05, Table 6). We also collected effluent from perfused filters containing TM cells, concentrated the effluent, and monitored the accumulated secretion of myocilin. Similar to intracellular levels, extracellular levels of myocilin were higher in GC-treated cells compared with control. Here myocilin levels in effluent were significantly higher in all treatment groups (see Supplementary Material and Supplementary Table S6, http://www.iovs.org/content/54/3/2100/suppl/DC1), with DEX and PRED having equivalent but greater effects on MYOC secretion than GW870086X.
Figure 4.
Effects of glucocorticoids on myocilin expression/secretion in perfused human TM cells on permeable filters. Equal volumes of effluent from TM cells on filters perfused with serum-free HEPES-DMEM were collected, concentrated, and assayed for myocilin expression by Western blot (B). Immediately after perfusion, cell lysates from perfused cells were prepared and assayed for myocilin expression by Western blot (A). Results from representative Western blots are shown on left. Combined results from five different experiments using two different cell strains that were normalized to β-actin content are shown on right. Asterisks (*) indicate significant difference from untreated control where P < 0.05.
Discussion
The present study examined the effect of GC treatment on the expression and secretion profile of three different strains of primary cultures of human TM cells. We compared the responses of two classical GCs, DEX and PRED acetate, and a novel SEGRA, GW870086X, on four different TM proteins previously shown to change in response to GC treatment. In addition, we tested the effects of GCs on ECM turnover in a functional assay that examines fluid flow across TM cell monolayers. Unexpectedly, each of the three GCs affected hydraulic conductivity of TM monolayers and expression/secretion of the protein panel distinctively (Table 2). To our knowledge, this is the most comprehensive analysis of cultured TM cell responses to different GCs to date.
Table 2.
Summary of Human TM Cell Responses to Three Different Glucocorticoids
|
GC |
FN |
tPA |
MYOC |
MMP2 |
HC |
||
|
Cell |
CM |
Cell |
CM |
||||
| Dexamethasone | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | → |
| GW870086X | → | ↑ | ↑ | → | ↑ | → | → |
| Prednisolone | → | ↑ | ↓ | ↑ | ↑ | → | ↓ |
CM, conditioned media.
Similar to previous reports, we observed that DEX decreased the expression of two enzymes that mediate ECM degradation, tPA and MMP2.16,22 While PRED decreased tPA like DEX, GW870086X did the opposite and increased tPA expression. Unlike DEX, PRED and GW870086X were without effect on MMP2 secretion. We did not measure MMP inhibitors (e.g., TIMP-1, 2), which may also be affected differentially by these three GCs and impact MMP2. Previous studies have shown that depending upon the cell type, PRED decreases or has no effect on MMP2 secretion.33,34 Taken together, GW870086X is unique in that it does not appear to depress the expression/secretion of these two matrix degrading enzymes like two classical GCs. This characteristic of GW870086X holds promise in terms of the potential for limited detrimental effects on conventional outflow.
While all three compounds tested increased fibronectin secretion, DEX had the most pronounced impact on this ECM component by also increasing cellular levels. Responses by human TM cells in the present study were similar to levels reported previously where TM cells were treated with 100 nM DEX17 for 17 days and shown to approximately double fibronectin levels. Interestingly, stimulation of fibronectin secretion was observed to occur in the present study after only 5 days of treatment by all three compounds and in a binary fashion with a rank order of potency of DEX > PRED > GW870086X. Unlike with other endpoints tested, we do not understand the absence of a dose-response relationship with most GCs and FN. Like the previous study17 we saw variability in responses between cell strains. The increased response in our study may be related to the process of differentiation to which we subjected our TM cells.
A previous study with a different SEGRA, BOL-303242-X, investigated effects on myocilin secretion from cultures of monkey TM cells.28 In that study, BOL-303242-X was less efficacious than DEX at stimulating myocilin secretion at equivalent concentrations. The present study compared GW870086X with DEX and PRED in parallel and monitored cellular expression and secretion of myocilin. PRED had the most profound effect on both parameters, particularly at the highest concentration tested. DEX also significantly upregulated myocilin in the cells and supernatant, but to a lesser extent than PRED. Overall, myocilin production appeared least impacted by GW870086X. The weaker stimulation was only evident as secreted product, but did not translate into any cellular accumulation, in marked contrast to DEX and PRED. Thus, the effect of GW870086X on myocilin was diminished compared with the classical GCs DEX and PRED—similar to the BOL-303242-X study. Differences between the two studies include human versus monkey cells, 5 vs. 4 days of drug treatment, and 72 vs. 48 hours of conditioned media collection.
To assess global effects of GCs on TM cell homeostasis, HC was monitored. In agreement with the idea that classical GCs decrease ECM turnover and/or strengthen cell-cell associations, HC of human TM monolayers decreased following GC treatment. While all three compounds decreased HC at least numerically, only PRED treatment was significantly different from control (P < 0.05), decreasing flow by ∼70% at 30-nM concentration. Similar to our previous study,31 DEX decreased fluid flow by ∼50% at both 30 and 100 nM. However, variability in control measurements prevented results from reaching significance (see Supplementary Material and Supplementary Table S5, http://www.iovs.org/content/54/3/2100/suppl/DC1). Consistent with its more favorable profile on the balance of matrix deposition and degradation, GW870086X did not significantly decrease HC. To ensure that the compounds were active in all experiments, we monitored cell-associated and secreted myocilin from TM cells on filters (Fig. 4). Secreted myocilin was significantly elevated in effluent from control following treatment with all three compounds.
The present study underscores the idea that all GCs are not created equal, especially with regard to upregulating untoward side effects at therapeutic doses, and that multiple endpoints in different models should be investigated to fully appreciate their range of effects. Based on its unique pharmacology, it was not surprising that the novel SEGRA, GW870086X, differed in its profile of effects on several parameters (compared with DEX and PRED) and was statistically significantly different in five of eight endpoints (compared in Table 1). In vitro, GR-related pharmacology of GW870086X differed from DEX primarily with respect to gene expression regulated by GR via transactivation (Uings et al., manuscript submitted, 2013). Depending on the gene studied, GW870086X behaved as a partial agonist with varying degrees of maximal efficacy relative to DEX (between 0 and ∼80%). In our study, GW870086X was not entirely devoid of transactivating activity either—on cellular tPA, secreted fibronectin, and myocilin, for example. However, it did so with lower efficacy or potency compared with DEX or PRED, which is consistent with a differentiated transcriptional profile (Uings et al., manuscript submitted, 2013). These studies highlight the need for a proper gene expression profiling of novel glucocorticoid agonists to fully understand their GR-related pharmacology. While a large number of genes are regulated by glucocorticoids (cf. transcriptome analysis [Uings et al., manuscript submitted, 2013]), some of them are transactivated or transrepressed directly at the promoter level via a glucocorticoid-response element while the up- or downregulation of other genes may be an indirect event. For example, while the distinct pharmacological profile of GW870086X can help explain differentiation from DEX and PRED on markers that are activated by the latter and unaffected by the former—for example cellular fibronectin and myocilin—a switch of response from a decrease (DEX, PRED) to an increase (GW870086X) for tPA is more difficult to explain and most likely due to indirect regulation of tPA protein expression. Additionally, alterations in cellular protein turnover may contribute to changes at the protein level as well. With that in mind, clinical studies are needed to determine whether the altered pharmacology indeed translates into clinical differentiation with respect to the AE profile of GCs while retaining desirable anti-inflammatory activity. Lastly, it was interesting that two well-characterized “traditional” GCs differed from each other in several compared endpoints. In the future, effects of GW870086X on intercellular junctions of Schlemm's canal, an essential part of the resistance-generation machinery in the conventional pathway, could be evaluated. Classical GCs have previously been shown to strengthen junctions between SC cells23 and in other endothelia. In addition, in vivo effects of GW870086X on outflow facility and IOP need to be determined. Based upon data in the present study, GW870086X affects TM cells differently than traditional GCs and may lack or have a less severe side effect profile typically associated with classical GCs.
Footnotes
Supported by a GlaxoSmithKline Research Contract (WDS).
Disclosure: W.D. Stamer, GSK (F), Alcon (F), Allergan (F), Cytokinetics (C), Aerie (C), Acucela (C), Novartis (R); E.A. Hoffman, None; E. Kurali, GSK (E); A.H. Krauss, GSK (E)
References
- 1. Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009; 88: 752–759. [DOI] [PubMed] [Google Scholar]
- 2. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963; 70: 482–491. [DOI] [PubMed] [Google Scholar]
- 3. Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963; 70: 500–507. [DOI] [PubMed] [Google Scholar]
- 4. Bernstein HN, Schwartz B. Effects of long-term systemic steroids on ocular pressure and tonographic values. Arch Ophthalmol. 1962; 68: 742–753. [DOI] [PubMed] [Google Scholar]
- 5. Goldmann H. Cortisone glaucoma. Arch Ophthalmol. 1962; 68: 621–626. [DOI] [PubMed] [Google Scholar]
- 6. Steroids Clark A. ocular hypertension and glaucoma. J Glaucoma. 1995; 4: 354–369. [PubMed] [Google Scholar]
- 7. Armaly MF. Statistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of response. Invest Ophthalmol. 1965; 4: 187–197. [PubMed] [Google Scholar]
- 8. Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol. 1965; 4: 198–205. [PubMed] [Google Scholar]
- 9. Kitazawa Y, Horie T. The prognosis of corticosteroid-responsive individuals. Arch Ophthalmol. 1981; 99: 819–823. [DOI] [PubMed] [Google Scholar]
- 10. Lewis JM, Priddy T, Judd J, et al. Intraocular pressure response to topical dexamethasone as a predictor for the development of primary open-angle glaucoma. Am J Ophthalmol. 1988; 106: 607–612. [DOI] [PubMed] [Google Scholar]
- 11. Rohen JW, Linner E, Witmer R. Electron microscopic studies on the trabecular meshwork in two cases of corticosteroid-glaucoma. Exp Eye Res. 1973; 17: 19–31. [DOI] [PubMed] [Google Scholar]
- 12. Johnson D, Gottanka J, Flügel C, Hoffmann F, Futa R, Lütjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch Ophthalmol. 1997; 115: 375–383. [DOI] [PubMed] [Google Scholar]
- 13. Gerometta R, Podos SM, Candia OA. Steroid-induced ocular hypertension in normal cattle. Arch Ophthalmol. 2004; 122: 1492–1497. [DOI] [PubMed] [Google Scholar]
- 14. Clark A, Wilson K, de Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest Ophthalmol Vis Sci. 1995; 36: 478–489. [PubMed] [Google Scholar]
- 15. Whitlock NA, McKnight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with dexamethasone. Invest Ophthalmol Vis Sci. 2010; 51: 6496–6503. [DOI] [PubMed] [Google Scholar]
- 16. Snyder R, Stamer WD, Kramer TR, Seftor RE. Corticosteroid treatment and trabecular meshwork proteases in cell and organ culture supernatants. Exp Eye Res. 1993; 57: 461–468. [DOI] [PubMed] [Google Scholar]
- 17. Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992; 33: 2242–2250. [PubMed] [Google Scholar]
- 18. Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993; 12: 783–793. [DOI] [PubMed] [Google Scholar]
- 19. Johnson DH, Bradley JM, Acott TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Invest Ophthalmol Vis Sci. 1990; 31: 2568–2571. [PubMed] [Google Scholar]
- 20. Dickerson JE Jr, Steely HT Jr, English-Wright SL, Clark AF. The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp Eye Res. 1998; 66: 731–738. [DOI] [PubMed] [Google Scholar]
- 21. Polansky JR, Fauss DJ, Chen P, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997; 211: 126–139. [DOI] [PubMed] [Google Scholar]
- 22. el-Shabrawi Y, Eckhardt M, Berghold A, et al. Synthesis pattern of matrix metalloproteinases (MMPs) and inhibitors (TIMPs) in human explant organ cultures after treatment with latanoprost and dexamethasone. Eye (Lond). 2000; 14 (pt 3A): 375–383. [DOI] [PubMed] [Google Scholar]
- 23. Underwood J, Murphy CG, Chen J, et al. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am J Physiol. 1999; 277: C330–C342. [DOI] [PubMed] [Google Scholar]
- 24. Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006; 27: 413–426. [DOI] [PubMed] [Google Scholar]
- 25. Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005; 26: 452–464. [DOI] [PubMed] [Google Scholar]
- 26. Schacke H, Rehwinkel H, Asadullah K, Cato AC. Insight into the molecular mechanisms of glucocorticoid receptor action promotes identification of novel ligands with an improved therapeutic index. Exp Dermatol. 2006; 15: 565–573. [DOI] [PubMed] [Google Scholar]
- 27. Zhang JZ, Cavet ME, VanderMeid KR, Salvador-Silva M, López FJ, Ward KW. BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells. Mol Vis. 2009; 15: 2606–2616. [PMC free article] [PubMed] [Google Scholar]
- 28. Pfeffer BA, DeWitt CA, Salvador-Silva M, Cavet ME, López FJ, Ward KW. Reduced myocilin expression in cultured monkey trabecular meshwork cells induced by a selective glucocorticoid receptor agonist: comparison with steroids. Invest Ophthalmol Vis Sci. 2010; 51: 437–446. [DOI] [PubMed] [Google Scholar]
- 29. Stamer W, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glautomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000; 20: 347–350. [PubMed] [Google Scholar]
- 30. Stamer W, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995; 14: 611–617. [DOI] [PubMed] [Google Scholar]
- 31. Wan Z, Woodward DF, Cornell CL. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007; 48: 4107–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture and characterization of endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1998; 39: 1804–1812. [PubMed] [Google Scholar]
- 33. Silva PL, Garcia CS, Maronas PA. Early short-term versus prolonged low-dose methylprednisolone therapy in acute lung injury. Eur Respir J. 2009; 33: 634–645. [DOI] [PubMed] [Google Scholar]
- 34. Smith VA, Hoh HB, Easty DL. Role of ocular matrix metalloproteinases in peripheral ulcerative keratitis. Br J Ophthalmol. 1999; 83: 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]