Abstract
The quantitative viral outgrowth assay (QVOA) provides a precise minimal estimate of the reservoir of resting CD4+ T-cell infection (resting cell infection [RCI]). However, the variability of RCI over time during antiretroviral therapy (ART), relevant to assess potential effects of latency-reversing agents or other interventions, has not been fully described. We performed QVOA on resting CD4+ T cells obtained via leukapheresis from 37 human immunodeficiency virus (HIV)–infected patients receiving stable suppressive ART for a period of 6 years. Patients who started ART during acute (n = 17) or chronic (n = 20) HIV infection were studied once HIV RNA levels were <50 copies/mL for ≥6 months. Using random effects analysis of 160 RCI measurements, we found that RCI declined significantly over time (P < .001), with an estimated mean half-life of 3.6 years (95% confidence interval, 2.3–8.1 years), remarkably consistent with findings of prior studies. There was no evidence of more rapid decay in acute versus chronic HIV infection (P = .99) for patients suppressed ≥6 months. RCI was reliably estimated with longitudinal measurements generally showing <2-fold variation from the previous measure. When QVOA is performed in this format, RCI decreases of >6-fold were rare. We suggest that a 6-fold decline is a relevant threshold to reliably identify effects of antilatency interventions on RCI.
Keywords: HIV, latency, IUPM, RCI, QVOA, SCA
(See the editorial commentary by Siliciano and Siliciano on pages 1345–7.)
Latent, persistent infection of resting memory CD4+ T cells by human immunodeficiency virus (HIV) type 1 is established early after initial viremia [1–3]. This rare but extremely stable pool of cells contains replication-competent, integrated provirus that may produce infectious virus on activation of the host cell. Resting cell infection (RCI) is the major obstacle to a cure, allowing the virus to persist even in patients receiving long-term antiretroviral therapy (ART) and in the presence of immune surveillance [4–6].
Initial estimates of the half-life of RCI, as low as 6 months, led to hopes that eradication could be achieved simply with prolonged ART [7–9]. Estimates for eradication with continuous therapy in patients who started ART early in infection were as low as 7.7 years [7]. However, after the initial rapid phase of RCI decay after ART initiation, a much slower phase of decay is estimated to have a half-life of approximately 44 months [6, 10]. Thus, eradication based on ART and natural decay alone would take up to 73.4 years in a patient with a reservoir size of just 106 cells [10].
Modeling studies have suggested that activation of latently infected cells can maintain persistent low-level viremia (LLV) and produce intermittent viral blips while replenishing the latent reservoir through homeostatic proliferation and/or de novo infection events [5]. This persistent LLV, detectable with an ultrasensitive single-copy assay (SCA) [11], has been observed more frequently in patients with a higher frequency of RCI in a small cohort of patients starting ART during either acute HIV infection (AHI) or chronic HIV infection (CHI) [3]. ART during the acute phase of infection restricts but does not accelerate decay of RCI [3]. Given the many factors at play in maintaining RCI and the absence of any accelerated decay rate in patients with AHI, it has become apparent that disruption of latency will be necessary in therapeutic approaches to clear latent HIV infection.
Current eradication strategies, such as treatment with latency-reversing agents, aim to eliminate RCI. Their effectiveness can be evaluated by measuring RCI over time in the presence of ART, after a therapeutic intervention. Although the quantitative viral outgrowth assay (QVOA) is currently the reference standard for measuring RCI, it has also been described as widely variable and inaccurate [12, 13]. QVOA provides an estimate of the frequency of resting CD4+ T cells carrying inducible, replication-competent viral genomes. Intact, noninduced proviruses have been characterized that are capable of replication but not detectable with QVOA, suggesting that QVOA may underestimate the true size of the latent reservoir [13]. In this work, Ho et al [13] suggested that serial induction of pools of infected cells may allow the quantitation of formerly noninduced viruses. To date, however, no polymerase chain reaction (PCR)–based assay provides a precise and internally consistent indication of the amount of replication-competent HIV in resting cells; such assays often detect defective proviruses that cannot produce infectious virus [12, 14, 15]. Therefore, measurements of any change in RCI using a PCR-based method of detection may fail to detect a depletion of latently infected cells capable of producing infectious virus, because this will be masked by a large pool of cells containing defective proviruses insensitive to any intervention [12, 13].
Reliable measurement of RCI is necessary to assess potential effects of antilatency interventions. To our knowledge QVOA is the only reliable method by which replication-competent latent virus has been quantitated longitudinally. However, the variability of RCI measurements over time in patients receiving ART has not been fully described. Understanding RCI variability can help in determining a threshold to identify the effects of antilatency interventions and in conducting power analyses to design future cure studies.
METHODS
Study Participants
We studied a cohort of 37 subjects, of whom 17 were identified during AHI (plasma HIV RNA detected and HIV Western blot negative). All patients with AHI started ART within 45 days of the estimated date of infection, except 1 who started ART 6 months after infection (early infection). All patients had been aviremic (<50 copies/mL plasma RNA) for ≥6 months. All patients provided written informed consent, and studies were approved by the University of North Carolina Institutional Review Board.
QVOA and SCA
Patients underwent continuous-flow leukapheresis. Resting CD4+ T cells were isolated via negative selection, and RCI was determined with the QVOA, as described elsewhere [16]. Briefly, 30–65 million highly purified CD4+ resting cells were maximally stimulated in limiting dilutions with phytohemagglutinin (PHA) (Remel-PHA; Thermo Scientific), interleukin 2, and irradiated peripheral blood mononuclear cells (PBMCs) from a seronegative donor. Cultures were targeted twice with CCR5-high, CD8-depleted, PHA-stimulated PBMCs from an uninfected donor. To minimize introducing variation to the QVOA caused by using PBMCs from different donors, assays were performed with matched patient-donor pairs. Culture supernatants were tested for P24 expression by enzyme-linked immunosorbent assay on day 15, with confirmation on day 19. A maximum likelihood probability method was used to estimate the frequency of RCI for each patient, with results reported as infectious units per million resting CD4+ T cells (IUPM) [17, 18]. In a selected group of patients, LLV was measured using an ultrasensitive, quantitative real-time, reverse-transcriptase-PCR SCA that could quantify plasma HIV-1 RNA to a limit of detection of 1 copy/mL [11].
Statistical Analysis
Random effects regression on log10-transformed RCI measurements was used to evaluate RCI decay [10], compare initial levels and decay between AHI and CHI groups, and estimate sources of variability, analyzing RCI measurements obtained ≥12 months after ART initiation. For 4 time points (involving 3 subjects) with all negative cultures, RCI was estimated assuming that 1 culture at the highest input cells was positive, and one-half of this RCI was used for the statistical analyses; for 1 time point with all positive cultures, RCI was estimated assuming that 1 culture at the lowest input of cells was negative and analyzed at 2 times this estimated RCI. In patients for whom SCA was available, Pearson correlation was used in a univariate analysis to examine the relationship between mean SCA and IUPM values; SCA measurements below the assay limit were imputed as half of the reported detectable SCA value before calculation of the subject-specific mean SCA value.
RESULTS
RCI Decay in Virally Suppressed Patients
Overall, 37 participants (17 treated during AHI and 20 during CHI; aged 19–66 years) were studied, and provided a total of 160 individual QVOA measurements (median number, 4 per patient; median interval, 3 months; median RCI follow-up per patient, 1.2 years). The median ART duration at first RCI measurement was 21 months in the AHI and 78 months in the CHI cohort. The CD4+ T-cell count at first RCI was 744 cells for AHI and 584 cells for CHI. The mean RCI level at first measurement was 0.42 IUPM, well within the range of previously reported values [10].
RCI declined significantly over time (P < .001), with an estimated mean half-life of 3.6 years (95% confidence interval, 2.3–8.1 years). There was no evidence of more rapid decay for AHI compared with CHI (P = .99) (Figure 1).
Figure 1.
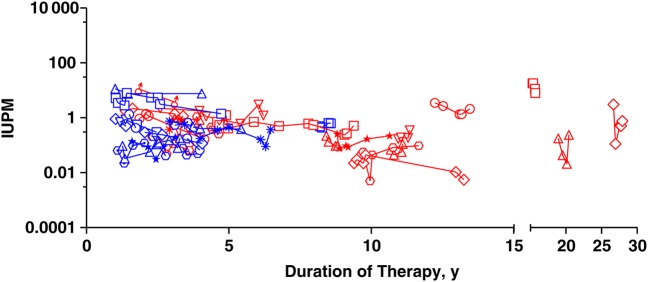
Decay of resting CD4+ T-cell infection in virally suppressed patients. Each data point represents an independent measurement. Each symbol/shape represents one patient. Blue symbols represent patients treated during acute human immunodeficiency virus (HIV) infection and red symbols those treated during chronic infection. Zidovudine (AZT) and didanosine (DDI) were the first line of therapy for 3 patients treated during chronic HIV infection. Abbreviation: IUPM, infectious units per million resting CD4+ T cells.
In a sensitivity analysis restricted to 21 subjects with sustained viral suppression and no interruptions in ART (9 with AHI and 12 with CHI, contributing a total of 91 RCI measurements), the estimated mean RCI half-life was 3.3 years (95% confidence interval, 2.0–8.0 years). There was no evidence (P = .68) that the half-life differed between this group and subjects with viral blips or LLV during the time period of RCI measurement (all <400 copies/mL; n = 13) or ART interruptions within 1 year of RCI measurement (n = 3).
Reproducibility of the QVOA
The ability to reliably measure RCI is critical for assessing potential effects of antilatency interventions, specifically the effects on the replication-competent reservoir. The variability between multiple measurements could provide important information to help determine a threshold for identifying these effects and conduct power analyses to design future studies. To that end, we assessed the variability over time in 160 individual RCI measurements.
Considering only within-subject variability, the estimated standard deviation for the change in log10 RCI was 0.38. Under assumed normality of RCI variability, this implies that a >2.5-fold RCI decrease within 3 months has a likelihood of 0.16 (16%) during stable ART with a RCI half-life of 3.6 years. A >6-fold RCI decrease has a likelihood of 0.023 (2.3%). Empirically evaluating the 123 pairs of consecutive RCI measurements, 81 (66%) had <2-fold increases or decreases, 21 (17%) had >2.5-fold decreases, and 3 (2.4%) had >6-fold decreases (Figure 2).
Figure 2.
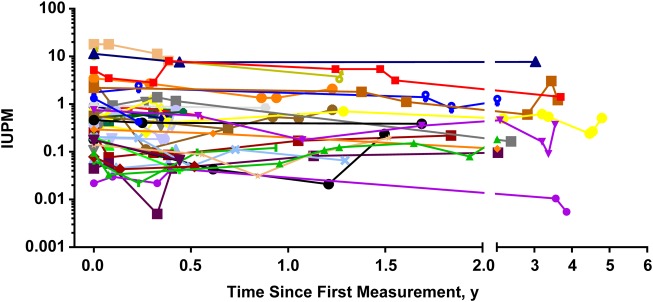
Reproducibility of the quantitative viral outgrowth assay. Multiple independent measurements of resting cell infection (RCI) in 37 suppressed patients over time show the limited variability of the assay. RCI was reliably estimated with longitudinal measurements generally showing <2-fold variation from the previous measurement. Abbreviation: IUPM, infectious units per million resting CD4+ T cells.
Correlation Between Low-Level Viremia and Reservoir Size
The initiation of ART leads to the rapid decline of viremia to below the limit of detection for conventional PCR assays. However, in approximately 80% of patients receiving ART, LLV detectable only with sensitive PCR assays is stable and unresponsive to intensification of therapy [19, 20]. The source of this viremia is currently not well characterized, but it has been postulated to be the release of virions from cells infected before ART administration. We examined the relationship between RCI and LLV, subjecting IUPM and SCA values from 26 patients with available LLV measurements to a univariate analysis using Pearson correlation. We observed a positive correlation (Figure 3; Pearson's r = 0.531) between the size of the resting CD4+ T-cell reservoir and LLV, consistent with previous reports [3].
Figure 3.
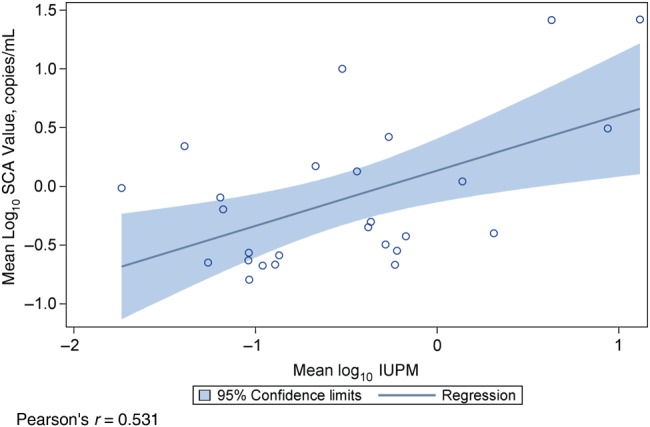
Correlation between low-level viremia (LLV) and the size of the resting CD4+ T-cell reservoir. In 26 patients for whom LLV measurements were available, Pearson's correlation was used in a univariate analysis to examine the relationship between mean single-copy assay (SCA) and infectious units per million resting CD4+ T cell (IUPM) values.
DISCUSSION
Memory resting CD4+ T cells remain the most well-defined reservoir of quiescent, archival, replication-competent HIV. Although not completely understood, infection of activated CD4+ T cells as they are transitioning to a resting memory state may be the primary mechanism by which latency is established; direct infection of resting cells has also been demonstrated [10, 21]. Several studies have suggested that HIV latency is regulated by the same mechanisms that govern host cell gene expression, including epigenetic transcriptional silencing, availability, and recruitment of host transcription factors and transcriptional interference (reviewed in [22]). There is clear evidence that cells that contain HIV DNA integrants can undergo homeostatic proliferation [23–25], and if these genomes are replication competent they will contribute to persistent infection. The use of the QVOA therefore remains the reference standard for quantifying quiescent HIV in memory resting CD4+ T cells, providing a true measure of replication-competent inducible provirus.
Analysis presented here shows that RCI decays with a half-life of 3.6 years or 43 months, a finding consistent with the 44-month half-life seen in studies by Siliciano et al [10]. From these measurements, it has been estimated that eradication based on ART and natural decay alone could take up to 73 years [6, 10]. Furthermore, we observed a correlation between RCI and LLV suggesting that, in at least a subset of patients, resting CD4+ T cells may be a source of this residual viremia. This is puzzling, given the stability of RCI over years, because it might be expected that the expression of virions from formerly latently infected resting T cells should lead to significant decay of this reservoir. Evidence has suggested that proviral genomes may undergo either homeostatic or dysfunctional proliferation [23–25], although the proportion of these genomes that are actually replication competent is unclear. Most of the genomes in transitional or effector memory subsets may in fact be defective [26]. A model that could link these disparate observations would hold that the rate of homeostatic proliferation in central memory cells that were latently infected with replication-competent genomes was perfectly matched by the rate at which this same cell population was stochastically activated to release virions. Therefore, clearance of residual infection will require targeting and disruption of the latent reservoir with an efficiency that significantly exceeds this proliferation rate of the replication-competent reservoir.
The ability of latency-reversing agents to induce expression of latent provirus is under intensive study (reviewed in [27]), and studies of the HDAC inhibitors vorinostat, panobinostat, and romidepsin have advanced into clinical trials [27]. Inducing expression of previously silenced promoters could provide an opportunity for immune recognition and clearance of infected cells, and therefore complimentary interventions, such as immune-augmentation therapies, are being studied for their ability to clear latent HIV infection.
However, to assess the effectiveness of such eradication strategies, the ability to reliably measure the frequency of RCI is essential. QVOA is the only method by which IUPM values have been longitudinally quantitated, and in the current study we assessed the variability of the assay, finding <2-fold variation in most cases. This is consistent with a model estimate of the likelihood of change based on within-subject variability. Change in IUPM values can be used to assess the effectiveness of antilatency interventions. Our results suggest that a >6-fold change would be rare during stable, suppressive ART and so should serve as a potential threshold for reduction in IUPM values that is not likely to represent natural variation. This finding is based on QVOA using large number of cells obtained from leukapheresis; with fewer cells examined, variability in RCI is expected to be larger. The magnitude of this threshold is both appropriate and significant because only large reductions in the frequency of RCI are expected to be clinical relevant. Results of a 2014 modeling study [28] suggest that reductions in the frequency of RCI on the order of several logs will be necessary to significantly delay time to viral rebound after ART interruption.
In this regard, it has also been demonstrated that QVOA underestimates the frequency of RCI [13], because in a coculture assay a single exposure to even maximal mitogen stimulation will not result in the recovery of every replication-competent virus [13]. In this study of 8 patients, the frequency of CD4+ T cells encoding proviruses expressing apparently full-length HIV RNA was 4–50-fold greater than that of cells from which replication-competent HIV could be recovered, after a single stimulation, although recovery could be increased by serial stimulations. Therefore, the RCI as quantified by QVOA reflects a minimal measurement of the true latent reservoir, and the number of noninduced proviruses represents a maximal measurement of this reservoir. However, given current knowledge, a significant depletion of latent infection shown by QVOA would be expected to correlate with a depletion of the total replication-competent latent reservoir. Until improved assays are available, the documentation of a depletion of RCI by a >6-fold decline in QVOA measurements would represent a meaningful step toward the goal of HIV eradication.
Notes
Acknowledgments. We thank Brigitte Allard and Jenny J. Scepanski for technical support and Dr Yara Park and the staff of the University of North Carolina Blood Bank for clinical assistance. We are also very grateful for the contributions of the patients who have participated in these studies.
Financial support. This study was supported by the National Institutes of Health (grants DA030156 and AI096113 to D. M. M. and AI50410 to the University of North Carolina Center for AIDS Research) and the James B. Pendleton Charitable Trust (equipment grant).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 1998; 95:8869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1995; 1:1284–90. [DOI] [PubMed] [Google Scholar]
- 3.Archin NM, Vaidya NK, Kuruc JD et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A 2012; 109:9523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 2002; 53:557–93. [DOI] [PubMed] [Google Scholar]
- 5.Rong L, Perelson AS. Modeling latently infected cell activation: viral and latent reservoir persistence, and viral blips in HIV-infected patients on potent therapy. PLoS Comput Biol 2009; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finzi D, Blankson J, Siliciano JD et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999; 5:512–7. [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Justement JS, Moir S et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis 2007; 195:1762–4. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Ramratnam B, Tenner-Racz K et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med 1999; 340:1605–13. [DOI] [PubMed] [Google Scholar]
- 9.Ramratnam B, Mittler JE, Zhang L et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med 2000; 6:82–5. [DOI] [PubMed] [Google Scholar]
- 10.Siliciano JD, Kajdas J, Finzi D et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 11.Palmer S, Wiegand AP, Maldarelli F et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 2003; 41:4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson S, Graf EH, Dahl V et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013; 9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho YC, Shan L, Hosmane NN et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez G, Xu X, Chermann JC, Hirsch I. Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J Virol 1997; 71:2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, Siliciano RF. G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J Virol 2005; 79:1975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archin NM, Keedy KS, Espeseth A, Dang H, Hazuda DJ, Margolis DM. Expression of latent human immunodeficiency type 1 is induced by novel and selective histone deacetylase inhibitors. AIDS 2009; 23:1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers LE, McQuay LJ, Hollinger FB. Dilution assay statistics. J Clin Microbiol 1994; 32:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macken C. Design and analysis of serial limiting dilution assays with small sample sizes. J Immunol Methods 1999; 222:13–29. [DOI] [PubMed] [Google Scholar]
- 19.Maldarelli F, Palmer S, King MS et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinoso JB, Kim SY, Wiegand AM et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A 2009; 106:9403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agosto LM, Yu JJ, Dai J, Kaletsky R, Monie D, O'Doherty U. HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology 2007; 368:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton KM, Burch BD, Soriano-Sarabia N, Margolis DM. Prospects for treatment of latent HIV. Clin Pharmacol Ther 2013; 93:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomont N, El-Far M, Ancuta P et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldarelli F, Wu X, Su L et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner TA, McLaughlin S, Garg K et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014; 345:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano-Sarabia N, Bateson RE, Dahl NP et al. The quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J Virol 2014; 24:01900–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archin NM, Sung JM, Garrido C, Soriano-Sarabia N, Margolis DM. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat Rev Microbiol 2014; 12:750–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A 2014; 111:13475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


