Abstract
Background. Immunoglobulin G antibodies (Abs) to Plasmodium falciparum antigens have been associated with naturally acquired immunity to symptomatic malaria.
Methods. We probed protein microarrays covering 824 unique P. falciparum protein features with plasma from residents of a community in Kenya monitored for 12 weeks for (re)infection and symptomatic malaria after administration of antimalarial drugs. P. falciparum proteins recognized by Abs from 88 children (aged 1–14 years) and 86 adults (aged ≥18 years), measured at the beginning of the observation period, were ranked by Ab signal intensity.
Results. Abs from immune adults reacted with a total 163 of 824 P. falciparum proteins. Children gradually acquired Abs to the full repertoire of antigens recognized by adults. Abs to some antigens showed high seroconversion rates, reaching maximal levels early in childhood, whereas others did not reach adult levels until adolescence. No correlation between Ab signal intensity and time to (re)infection was observed. In contrast, Ab levels to 106 antigens were significantly higher in children who were protected from symptomatic malaria compared with those who were not. Abs to antigens predictive of protection included P. falciparum erythrocyte membrane protein 1, merozoite surface protein (MSP) 10, MSP2, liver-stage antigen 3, PF70, MSP7, and Plasmodium helical interspersed subtelomeric domain protein.
Conclusions. Protein microarrays may be useful in the search for malaria antigens associated with protective immunity.
Keywords: malaria, antibody, antigen, protein microarray, protective immunity
Despite reduction in Plasmodium falciparum transmission brought about by distribution of insecticidal bed nets, rapid diagnostic tests, and artemisinin combination therapy, malaria continues to be an important health problem throughout the tropics [1]. Infants and children experience the bulk of symptomatic P. falciparum infections, manifest primarily as uncomplicated malaria (parasitemia with fever, nonspecific systemic symptoms, and anemia with hemoglobin >5 g/dL). By contrast, adults with naturally acquired immunity, generated as a consequence of cumulative and repeated exposure to P. falciparum, maintain tight control of asexual parasite density with few symptomatic infections [2, 3]. Identifying the antigenic targets involved in the development of age-related acquired immunity is important to understanding why protection from P. falciparum infection and symptomatic malaria is slow to develop and informs prioritization of antigens that may be candidates for inclusion in a malaria vaccine.
Findings in residents of malaria-endemic areas and malaria-naive volunteers infected with P. falciparum sporozoites suggest that both humoral and cellular immunity are important to the development of protection [2]. With respect to defining the antigenic targets of antibodies (Abs), investigators in previous studies have primarily reported responses to recombinant proteins corresponding to native P. falciparum proteins with known or deduced function during various phases of the parasite's lifecycle. More recently, protein microarrays corresponding to ≥20% of the P. falciparum proteome deduced from the 3D7 genome sequence [4] and recombinant polypeptides produced in a cell-free wheat germ expression system [5] have been used to screen for immunogenicity of malaria proteins with known as well as unknown functions. With the goal of identifying a hierarchy of antigens relevant to the development of naturally acquired immunity, we probed microarrays corresponding to 824 P. falciparum protein features with plasma from children and adults living in a malaria-holoendemic community in western Kenya. Baseline Ab profiles were correlated with time to re(infection) and protection from symptomatic malaria in children followed during a 12-week period after cure of blood-stage infection with antimalarial drugs.
METHODS
Study Participants and Design
We obtained baseline (week 0) blood samples in July 2003 from 86 healthy asymptomatic adults (median age, 39.8 years; range, 18–78 years) and 88 healthy asymptomatic children (median age, 7.8 years; range, 1–14 years) who were lifetime residents of a single community in Nyanza Province, Kenya. This cohort has been described in detail in several publications [6–9]. Immediately after collection of blood samples, all participants were given a 6-dose regimen of artemether-lumefantrine to clear blood-stage infection without foreknowledge of whether the individual's blood smear was P. falciparum positive or negative. Finger prick blood samples were obtained weekly for the next 11 weeks to detect (re)infection by microscopic inspection of blood smears. Surveillance for symptomatic malaria (P. falciparum–positive blood smear accompanied by malaria symptoms and axillary temperature ≥37.5°C) was conducted weekly. In accordance with Kenyan national policy when our study was performed, asymptomatic P. falciparum infection was not treated with antimalarial drugs. Symptomatic malaria was treated with a 3-day course of artemether-lumefantrine.
Twenty-one children (median age, 7.4 years; range, 1–11 years) and 2 adults developed symptomatic malaria during the observation period. Seventy-two children (median age, 8.4 years; range, 1–14 years) remained asymptomatic (ie, were “protected”) regardless of whether or not P. falciparum (re)infection occurred. There was no statistically significant difference between the ages of protected children and those who experienced symptomatic malaria. Fifty-two adults and 82 children had a P. falciparum–positive blood smear during the 11-week follow-up.
Ethical Approval
Approval to conduct the study was obtained from the Institutional Review Board at University Hospitals Case Medical Center and the Kenya Medical Research Institute Ethical Review Committee. Adults signed written informed consent forms in the local language. Parents or guardians signed for minors <18 years old.
Protein Microarrays and Ab Profiles
Construction of the P. falciparum protein arrays and analysis of Ab binding have been described in detail [10–13]. Briefly, protein microarrays were constructed by polymerase chain reaction amplification of each complete or partial open reading frame followed by in vivo recombination cloning and in vitro transcription/translation to generate malaria polypeptides used for microarray chip printing. The Pf824 array used in the present study, (P. falciparum Reactive Antigen Microarrays, Cat. # 25-MA-0010, Antigen Discovery Inc., Irvine, California), comprising 824 unique features corresponding to 699 different P. falciparum genes, was a down-selected array based on results from previous larger array studies. Each microarray chip contained multiple negative in vitro transcription/translation control spots that lack plasmid template and serially diluted human immunoglobulin (Ig) G, anti-IgG, and Epstein-Barr nuclear antigen 1.
Plasma samples diluted to 1:200 in Protein Array Blocking Buffer (Whatman) were preincubated in Escherichia coli lysate, and microarrays were probed with the pretreated plasma by incubation overnight at 4°C. The slides were washed 5 times in Tris buffer (pH 7.6) and incubated in biotin-conjugated goat anti–human Ig (anti-IgG fragment crystallizable region [Fc] γ fragment specific; Jackson Immuno Research) diluted 1:200. After washing, bound Abs were detected by incubation with streptavidin-conjugated SureLight P-3 (Columbia Biosciences). The slides were washed in Tris buffer containing 0.05% Tween 20, followed by a final wash with water. Air-dried slides were analyzed using a Perkin-Elmer ScanArray Express HT microarray scanner. Intensities were quantified using QuantArray software (Packard BioChip Technologies).
Statistical Analysis
Differences in Ab signal intensities were compared across age groups (adults aged ≥18 years vs children aged 1–14 years and subsets of children aged 1–5, 6–10, or 11–14 years), using the Kruskal–Wallis nonparametric method. Analyses of the relationship between Ab responses and P. falciparum (re)infection and protection from symptomatic malaria were limited to children. The time to (re)infection based on a P. falciparum–positive blood smear was determined with a Kaplan–Meier log-rank test for survival analysis (SAS 9.2 software; SAS Institute), based on the 52 highest Ab responses to the protein features.
Cox regression models were used to evaluate the association between Ab signal intensity and risk for time to (re)infection. Covariates in the analysis included P. falciparum infection status at baseline and age (continuous). Because of the large number of microarray Ab responses measured in the same individuals, the “global test” developed by Goeman et al [14] was used to determine whether any of the malaria antigen–specific Ab responses were associated with the time to (re)infection. The null hypothesis is that none of the Ab responses is significantly associated with (re)infection. The test is designed to have high power to detect small associations between most of the covariates and the rate of (re)infection. Microarray data were normalized and calibrated as described elsewhere [10]. Analysis was performed using the R statistical environment (http://www.r-project.org) and SAS (http://www.sas.com/) statistical software.
The vsn method (www.bioconductor.org) was applied to the quantified array signal intensities. Antigens were considered “reactive” when the mean signal intensity of adults or the 3 age groups of children was greater than the mean plus 2 standard deviations of the “no-DNA” controls. Bayes-regularized t tests were then used to identify significant differential Ab reactivity [15]. The Benjamini–Hochberg (BH) method was used to correct for the false discovery rate. Differences were considered significant at BH-corrected P < .05. To compare different groups, we used R software to calculate Kruskal–Wallis/Dunn multiple-comparison tests with corrected P values.
We calculated the annual seroconversion rate (SCR) of immunogenic polypeptides with Systat 11 software (Systat Software) by fitting age-specific seroprevalence data to a reversible catalytic model described by Drakeley and coworkers [16]. The maximum-likelihood method assumes the following binomial error distribution: Pt = λ/λ + ρ(1-e(-(λ + ρ)*t)) where Pt is the proportion of seropositive individuals in each age group t; λ, SCR; and ρ, seroreversion rate. Stage expression information was obtained by querying mass spec evidence in PlasmoDB. http://plasmodb.org/plasmo/showQuestion.do?questionFullName=GeneQuestions.GenesByMassSpec. Evidence of expression at the Merozoite, Trophozoite, Gametocyte, and Sporozoite stages were obtained from Florens et al. 2002 [17]. Liver stage antigens were inferred from orthologues in P yoelli with mass spec evidence [18].
RESULTS
Age-Related Changes in the Breadth and Magnitude of Malaria IgG Abs
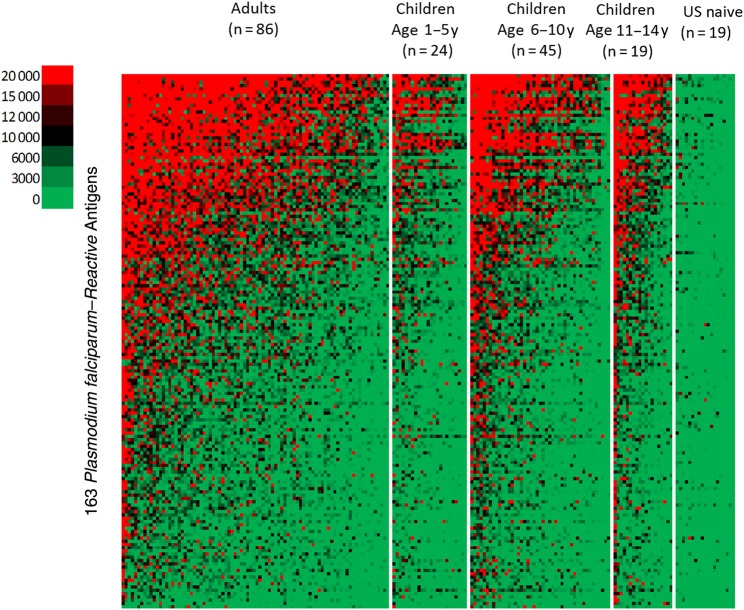
The pattern of Ab binding to 824 malaria protein features among adults and children within the 1–5-, 6–10-, and 11–14-year age groups is shown in the heat map (Figure 1). Ab binding in all age groups was limited to a subset of 163 of the 824 protein features on the microarray chip. In Figure 2A, the 163 antigens are sorted from left to right according to the mean Ab signal intensity for adults and compared with the average Ab reactivity for 1–5-year-old children. Supplementary Table 1 lists all 163 P. falciparum proteins in this order.
Figure 1.
Heat map showing the intensity and breadth of antibody binding to 163 reactive malaria protein features (rows) among Kenyan adults and children. Individual plasma samples are in columns grouped by age. Samples within each group are sorted by decreasing immunoreactivity. Red indicates positive reactivity; black, intermediate reactivity; green, no reactivity. Binding was limited to a subset of 163 proteins in all age groups. The level and breadth of antibody binding to the same proteins increased progressively with age.
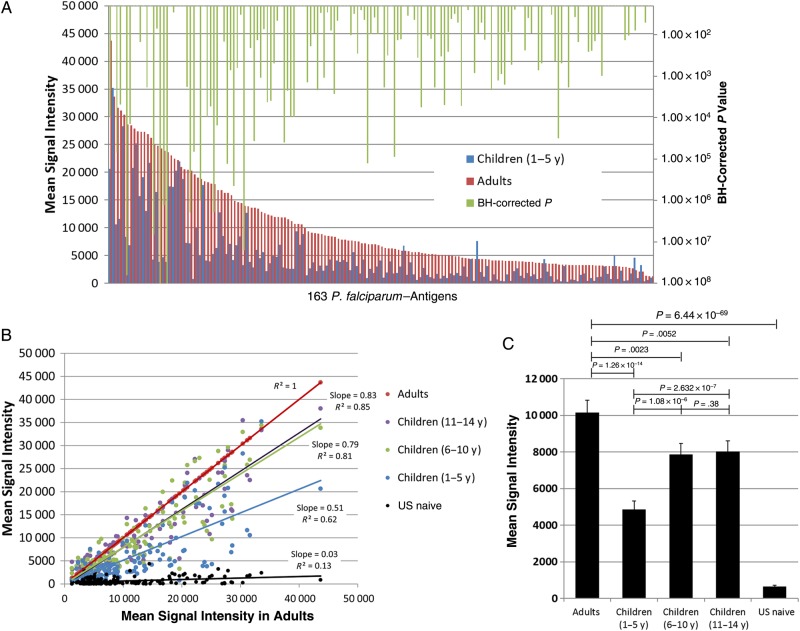
Figure 2.
Comparison of malaria antigen-specific antibody (Ab) responses of adults and children. A, Ab responses by adults (≥18 years old) and children 1–5 years old, sorted from left to right on the x-axis according to progressively decreasing signal intensity generated by adult Abs. Reactive antigens were defined by the mean signal intensity against the background (pixel intensity with no-DNA control signals subtracted) of Abs from adults greater than the mean plus 2 standard deviations of the no-DNA controls. Of 824 Plasmodium falciparum antigens on the array, 163 were reactive above the cutoff. Significant Benjamini-Hochberg (BH)–corrected P values (<.05) for adults versus 1–5-year-old children are shown in green for the comparison. B, Scatterplot of mean Ab signal intensities to 163 P. falciparum antigens from each age group of children (y-axis) against the mean intensities for adults (x-axis). C, Comparison of mean Ab signal intensities of Kenyan adults and children according to age group and malaria-naive adults from the United States. Kruskal–Wallis/Dunn multiple-comparison tests were performed with BH-corrected P values to compare the groups.
The overall Ab reactivity of children was less than that of adults, and Ab signal intensity to 110 of the 163 proteins was significantly lower among children than among adults after correction for false discovery (BH-corrected P < .05). Scatterplots of the mean Ab signal intensities to each antigen for adults (x-axis) against those for children of varying age (y-axis) show an increase in reactivity with age (Figure 2B). The Ab levels of children in the 6–10- and 11–14-year age groups were similar and approached those of adults. Malaria-naive individuals from the United States had scarce, low background reactivity. The global mean Ab signal intensities of all 163 reactive proteins combined are plotted in Figure 2C, showing a 2-fold difference between the 1–5-year-old children and adults. Differences between adults and the 3 age groups of children are highly significant.
Kinetics of Antigen Specific Ab Acquisition
The results in Figure 2A show that by 5 years of age Abs to some antigens reached levels equal to that of adults. Acquisition of Abs to other antigens was slower, suggesting that SCRs for various antigens differ. Table 1 shows the SCR for Abs to the 23 most immunogenic antigens recognized by adults. Mean Ab signal intensity was normalized to the average intensity for adults. Abs to merozoite surface protein (MSP) 10, MSP2, 70KD EMP1-trafficking protein (Pf70), and P. falciparum erythrocyte membrane protein 1 (PfEMP1) intracellular acidic terminal segment (ATS) domain variants approached that of adults in the youngest children and remained elevated in adults. In contrast, Ab responses to liver-stage antigen (LSA) 3, MSP1, Plasmodium helical interspersed subtelomeric domain protein (PHIST), ring-exported protein 1, and P. falciparum reticulocyte homologue binding protein 2 (PfRh2), for example, were low in the youngest children and gradually increased during adolescence and adulthood. The SCR column in Table 1 shows rate constants ranging from 0.034 to 0.679 per year. (The columns labeled “NA” could not be assigned SCRs because Ab reactivity was already so high in the youngest children that an age-related rate of increase could not be calculated.) The SCRs for each of the 163 reactive antigens are shown in Supplementary Table 1. These results suggest a dynamic process in which the adult Ab profile to various P. falciparum antigens is acquired gradually and unevenly during childhood and adolescence.
Table 1.
Comparison of Antibody Signal Intensity Between Adults and Childrena
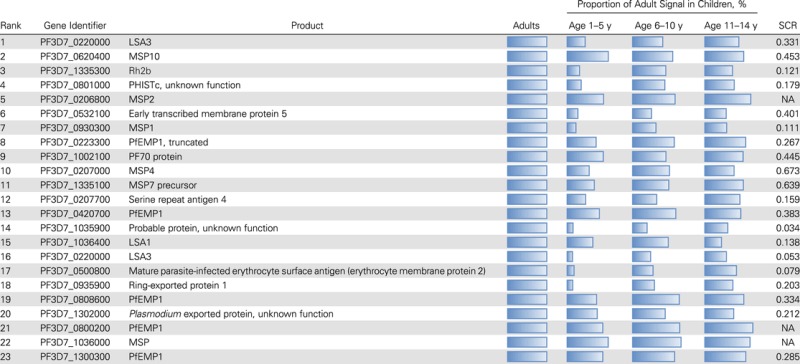 |
Abbreviations: LSA, liver-stage antigen; MSP, merozoite surface protein; NA, not assigned; PF70 protein, 70KD, EMP1-trafficking protein; Pfemp1, Plasmodium falciparum erythrocyte membrane protein 1; PHISTc, Plasmodium helical interspersed subtelomeric c family; Rh2b, reticulocyte binding protein 2 homologue b; SCR, seroconversion rate.
a The top 23 P. falciparum protein features to which antibodies from adults had the highest signal intensities are shown, along with the percentage of the adult signal in each age group of children. The SCR for each antigen is calculated as described in “Methods” section.
Immunodominant PfEMP1 Intracellular Domain
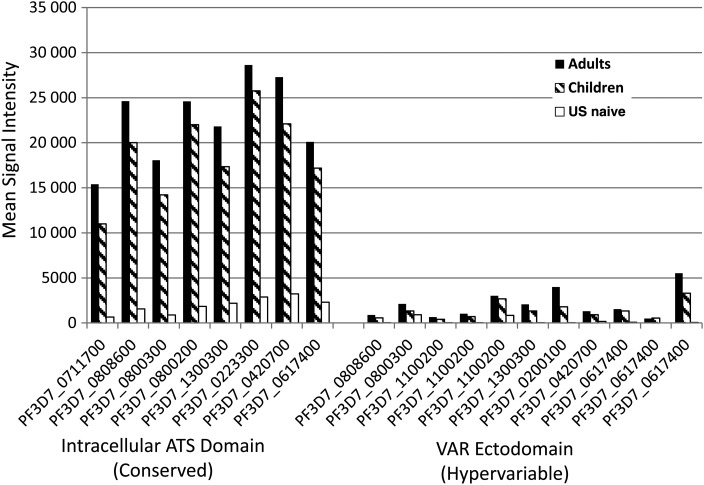
There are >60 distinct PfEMP1 loci spread across the 14 chromosomes of the P. falciparum genome, each locus encoding a different var gene [19]. Plasmodium falciparum–infected erythrocytes express only a single var gene at a time, and each of the corresponding PfEMP1proteins consist of 2 distinct domains. The C-terminal intracellular ATS protein domain is highly conserved and involved in intracellular signaling pathways. The N-terminal Variable (VAR) ectodomain is highly variable and involved in cytoadherence of infected erythrocytes to the vascular endothelium and other tissues. The results in Figure 3 compare Ab responses to the ATS and VAR domains printed on the microarray. Adults and children had similar Ab reactivity to the 2 domains, but Ab signal intensity in response to the ATS domain was significantly greater in both adults and children than for the VAR domain.
Figure 3.
Differential antibody (Ab) reactivity to intracellular versus extracellular domains of Plasmodium falciparum erythrocyte membrane protein 1, shown as mean Ab signal intensity generated by Abs from Kenyan and children and malaria-naive controls from the United States for the various intracellular acidic terminal segment (ATS) domains and VAR ectodomains printed on the microarray.
Relationship of Ab Responses to Rate of Infection (or Reinfection) Among Children
Age-dependent acquisition of Abs to some P. falciparum proteins has been associated with protection from infection as measured by an increase in the time to (re)infection, for example, the 42-kDa C-terminal region of MSP1 in the study cohort described here [6]. In the current study, Kaplan–Meier survival time to (re)infection in children based on analysis of baseline Ab signal intensity to each of the 163 immunogenic protein features showed no significant associations. The P value of the global test statistic, represented as a weighted average of individual Ab responses, was .43, indicating that there is no evidence that any of the Ab responses were associated with time to (re)infection among children. Data in Supplementary Figure 1 provide a decomposition of the global test into the negative and positive contributions of Abs to individual P. falciparum protein features.
Ab Reactivity and Protection From Symptomatic Malaria Among Children
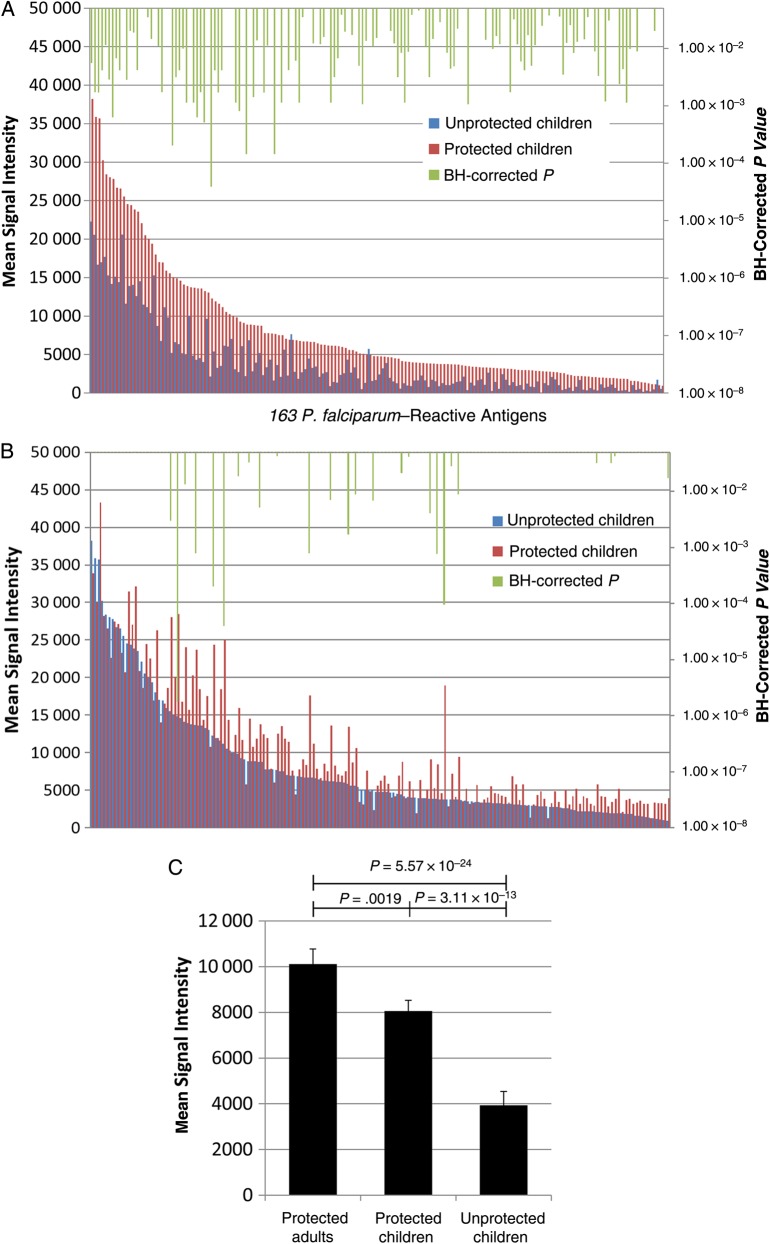
There were 88 children aged 1–14 years enrolled in this cohort. During the 12-week observation period, 21 children experienced symptomatic malaria (“unprotected’) and 67 children did not (“protected”). The median ages of the 2 groups, 7.35 and 8.43 years, were not significantly different (P = .13). Figure 4A shows mean Ab signal intensity to malaria protein features arranged from highest to lowest in protected children. Higher Ab levels were observed for protected compared with unprotected children for 106 of the immunoreactive proteins (BH-corrected P < .05).
Figure 4.
Antibody (Ab) responses associated with protection from symptomatic malaria in children. A, Comparison of Ab responses to 163 Plasmodium falciparum protein features in protected (n = 67) versus unprotected (n = 21) children. Significant Benjamini-Hochberg (BH)–corrected P values (<.05) are shown in green. B, Comparison of Ab responses to 163 P. falciparum protein features in protected children (n = 67) versus protected adults (n = 84). Significant BH-corrected P values (<.05) are shown in green. C, Comparison of mean Ab signal intensities to 163 P. falciparum antigens in protected adults, protected children, and unprotected children. Kruskal–Wallis/Dunn multiple-comparison tests were performed with BH-corrected P values to compare the groups.
The top 23 antigens associated with protection from symptomatic malaria among children are listed in Table 2. These include blood-stage antigens and pre-erythrocytic antigens, eg, MSP1, 2, 4, 7 and 10, LSA1, PHIST and others. All 8 of the PfEMP1 intracellular ATS domains on the array are included in this list. Inspection of the mean Ab signal intensities to the 163 P. falciparum reactive antigens plotted in the same manner for protected children versus protected adults showed fewer significant differences (Figure 4B), particularly to the aforementioned proteins, indicating that the Ab profile of protected children approaches that of adults. With respect to the stages of the P. falciparum lifecycle at which the 163 immunogenic proteins are expressed, mass spectrometry data cited in PlasmoDB (plasmodb.org) [17, 18] indicated that expression by trophozoites was overrepresented relative to the 661 nonreactive proteins on the array (40.5% vs 25.0%) (Supplementary Table 2). Many P. falciparum proteins were expressed by >1 stage of the parasite lifecycle (Supplementary Table 3).
Table 2.
Corrected P Values for Mean Antibody Signal Intensities of Protected Versus Unprotected Children for the Top 23 Plasmodium falciparum Protein Features Recognized by Protected Children
| Rank | Gene ID | Product | P Valuea |
|---|---|---|---|
| 1 | PF3D7_0620400 | MSP10 | 5.57 × 10−3 |
| 2 | PF3D7_0206800 | MSP2 | 1.73 × 10−3 |
| 3 | PF3D7_0220000 | LSA3 | 1.70 × 10−3 |
| 4 | PF3D7_0223300 | PfEMP1, truncated | 4.14 × 10−3 |
| 5 | PF3D7_0420700 | PfEMP1 | 1.15 × 10−2 |
| 6 | PF3D7_0800200 | PfEMP1 | 2.87 × 10−3 |
| 7 | PF3D7_1002100 | Pf70 protein | 6.32 × 10−4 |
| 8 | PF3D7_1335100 | MSP7 precursor | 6.71 × 10−3 |
| 9 | PF3D7_0808600 | PfEMP1 | 4.14 × 10−3 |
| 10 | PF3D7_1036000 | MSP | 2.84 × 10−1 |
| 11 | PF3D7_0801000 | PHISTc, unknown function | 2.83 × 10−3 |
| 12 | PF3D7_0207000 | MSP4 | 2.01 × 10−2 |
| 13 | PF3D7_1335300 | Rh2b | 1.89 × 10−2 |
| 14 | PF3D7_1300300 | PfEMP1 | 4.20 × 10−3 |
| 15 | PF3D7_0617400 | PfEMP1 | 5.23 × 10−2 |
| 16 | PF3D7_1036400 | LSA1 | 6.70 × 10−2 |
| 17 | PF3D7_1302000 | Rh2b | 3.48 × 10−2 |
| 18 | PF3D7_0800300 | PfEMP1 | 1.46 × 10−2 |
| 19 | PF3D7_0207700 | Serine repeat antigen 4 | 6.35 × 10−1 |
| 20 | PF3D7_0711700 | PfEMP1 | 1.09 × 10−2 |
| 21 | PF3D7_0930300 | MSP1 | 1.72 × 10−3 |
| 22 | PF3D7_0207000 | MSP4 | 1.93 × 10−1 |
| 23 | PF3D7_0930300 | MSP1 | 2.54 × 10−1 |
Abbreviations: LSA, liver-stage antigen; MSP, merozoite surface protein; Pf70, 70KD EMP-1 trafficking protein; PfEMP1, P. falciparum erythrocyte membrane protein 1; PHISTc, Plasmodium helical interspersed subtelomeric c family; Rh2b, reticulocyte bindign protein 2 homologue b.
a P values were corrected using the Benjamini–Hochberg method.
Asymptomatic parasitemia in children has been associated with an increased risk of symptomatic malaria and elevated anti–P. falciparum Abs relative to children with P. falciparum–negative blood smears in some studies [20]. Consequently, infection status at baseline is a parameter that could influence our results. We therefore analyzed Ab responses in the subset of children with blood-stage P. falciparum infection at baseline. Results of this analysis (Supplementary Figure 2A and 2B) indicate the same conclusion – children with P. falciparum infection at baseline who were subsequently protected from symptomatic malaria had elevated Ab levels compared with those who were unprotected, and Ab signal intensity in the former group approaches that of adults.
Finally, we compared the global mean Ab reactivity against all 163 reactive antigens in the protected adults with reactivity levels in protected and unprotected children. Ab levels in protected children were significantly higher than those in unprotected children against this collection of 163 antigens (Figure 4C), approaching levels in adults. Supplementary Figure 3 displays Ab reactivity to each antigen according to age and protection from symptomatic malaria.
DISCUSSION
We probed 824 P. falciparum protein features corresponding to various domains of 699 protein-encoding genes to identify antigen-specific IgG Ab responses predictive of protection from symptomatic malaria and P. falciparum (re)infection in a cohort of adults and children living in a single holoendemic community in western Kenya. The overall magnitude and breadth of Ab responses among adults was greater than that in children. A total of 163 P. falciparum proteins, primarily but not only those expressed by blood-stage P. falciparum, were recognized across all age groups, confirming that Abs associated with naturally acquired immunity develop gradually after years of exposure to the parasite.
Based on estimates of the SCR at which Abs reached their maximum levels [16], we observed that the complete adult Ab profile appears after 15 years of natural exposure to P. falciparum, and the rate at which Abs reach high levels characteristic of adults varies by >20-fold among the most immunogenic antigens. In principle, antigen specific SCRs could be used to inform identification of targets of naturally acquired immunity and components of vaccines intended to accelerate the development of Ab profiles in children that are similar to that of clinically immune adults. In this regard, antigens with high SCRs during childhood, such as MSP4, MSP7, and MSP10, would be low-priority targets, whereas those with low SCRs, such as LSA3, erythrocyte membrane protein 2 (mature parasite-infected erythrocyte antigen), and merozoite membrane protein M566 (PF3D7_1035900) [21, 22] would be high-priority targets. M566 has an internal domain structure with the sequence VDEEVAEELIEK repeated 18 times. Other well-characterized antigens, such as circumsporozoite protein, LSA1, and LSA3, also encode internal repeat domains that elicit Ab responses.
Based on differences in the magnitude of baseline Ab responses between children who were protected from symptomatic malaria and those who were not protected, we identified a repertoire of Abs to antigenic targets that correlates with protective immunity. Compared with children who experienced symptomatic malaria, protected children had significantly elevated Abs against dozens of P. falciparum antigens, consistent with other reports indicating that the breadth of Ab responses to malaria proteins rather than strong responses to a single protein or a small number, is critical [23, 24].
A study from Mali using a P. falciparum protein microarray different from that reported here also showed that 8–10-year-old children who did not experience symptomatic malaria during a period of seasonal transmission had significantly elevated Abs against 49 antigens, compared with age-matched children who did experience symptomatic malaria [10]. Abs to P. falciparum antigens that we observed to correlate with protection from symptomatic malaria include both those considered previously as vaccine candidates (eg, MSP1, MSP2, LSA1, and LSA3) [25–28] and less studied proteins involved in erythrocyte invasion by merozoites (MSP10 and MSP7) [24, 29] and trafficking of P. falciparum proteins from the parasite to the surface of the infected erythrocyte (PfEMP1, PF70, PHIST, and MSP7) [30–35].
Interestingly, we found no significant association of Ab responses to any of the 163 immunogenic P. falciparum proteins with the occurrence of or time to (re)infection, suggesting that Abs to antigens important to protection from asymptomatic versus symptomatic P. falciparum infection may differ. Alternatively, the immune mechanisms governing these related but phenotypically distinct processes may differ. An earlier report from the same cohort described here showed that combined Ab and T-cell interferon-γ responses to the single antigen studied, the 42-kDa C-terminal region of MSP1, had stronger predictive value for delay in time to (re)infection than Abs alone. In that study, there was no significant association of Ab or T-cell responses with protection from symptomatic malaria [6].
Our results with respect to PfEMP1 show that Abs to the conserved ATS domain were immunodominant, developed at an early age, and reached stable elevated levels in the youngest group of children that were nearly equivalent to that of levels in adults. In contrast, Abs to the hypervariable extracellular VAR domain were acquired more gradually and never reached the levels of the ATS domain. The immune system is presumably repeatedly exposed to the ATS domain during repeated cycles of erythrocyte lysis when merozoites are released after schizont rupture. The Abs to this domain would not be expected to play a functional role in inhibiting parasitemia or adherence of infected erythrocytes to endothelium or other tissues. However, the immunodominance of the ATS domain, even among children <5 years old, suggests that it is an indicator of cumulative parasite exposure.
Conversely, the extracellular VAR domain is associated with weak Ab responses across all age groups, suggesting that the hypervariable VAR sequence expressed as a single transcript by clonal parasites expressing a single VAR is an effective immune evasion mechanism. Because the immune system encounters only a single VAR domain or a small number of them during each blood-stage infection, the degree of exposure necessary to generate stable and high levels of Abs may be insufficient until late in childhood. In this context, it will be important to examine functional Ab responses to extracellular VAR domains, because recent observations have identified specific VAR protein sequences that bind to endothelial protein C receptor in a manner that is thought to modulate endothelial integrity and malaria pathogenesis [19].
Several features of the protein microarray format make it highly suitable for antigen discovery. In the context of prioritizing antigenic targets of protective immunity, the microarray enables screening of many proteins with known as well as unknown functions in malaria biology. In our view, screening the broad array of protein antigen targets with both known and unknown functions is advantageous, particularly in the case of liver-stage P. falciparum proteins for which there is very limited information regarding function. As with other high-throughput multiplex platforms, it is important to follow up candidates identified by microarrays with recombinant proteins or protein domains that are structurally similar to the native protein. In addition, serologic assays are ideally followed up with assays of Ab function that have been validated to correlate with protection from symptomatic malaria, for example, merozoite opsonization assays and quantification of P. falciparum growth inhibition using transgenic P. falciparum parasites in which specific invasion pathways can be evaluated [34, 36, 37]. Finally, a new generation of P. falciparum protein arrays may be tailored to the dominant alleles and protein sequences of polymorphic antigens thought to be relevant to protection from mild as well as severe malaria.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants in the community in Kenya for their cooperation and the director of the Kenya Medical Research Institute for approval to conduct this study. We also thank the team at Antigen Discovery for helpful discussions throughout the course of this work and related studies.
Financial support. This work was supported by the National Institutes of Health (grants AI098511 to A. E. D., AI043906 to A. M. M., AI089686 and AI095916 to P. L. F., and AI095192 to J. W. K.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Alonso PL, Tanner M. Public health challenges and prospects for malaria control and elimination. Nat Med 2013; 19:150–5. [DOI] [PubMed] [Google Scholar]
- 2.Crompton PD, Moebius J, Portugal S et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 2014; 32:157–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9:725–32. [DOI] [PubMed] [Google Scholar]
- 4.Doolan DL, Mu Y, Unal B et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 2008; 8:4680–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuboi T, Takeo S, Iriko H et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun 2008; 76:1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moormann AM, Sumba PO, Chelimo K et al. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis 2013; 208:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinkevych M, Petravic J, Chelimo K, Kazura JW, Moormann AM, Davenport MP. The dynamics of naturally acquired immunity to Plasmodium falciparum infection. PLoS Comput Biol 2012; 8:e1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinkevych M, Petravic J, Chelimo K et al. Density-dependent blood stage Plasmodium falciparum suppresses malaria super-infection in a malaria holoendemic population. Am J Trop Med Hyg 2013; 89:850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkevych M, Petravic J, Chelimo K et al. Decreased growth rate of P. falciparum blood stage parasitemia with age in a holoendemic population. J Infect Dis 2014; 209:1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crompton PD, Kayala MA, Traore B et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A 2010; 107:6958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felgner PL, Roestenberg M, Liang L et al. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci Rep 2013; 3:3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nnedu ON, O'Leary MP, Mutua D et al. Humoral immune responses to Plasmodium falciparum among HIV-1-infected Kenyan adults. Proteomics Clin Appl 2011; 5:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundaresh S, Doolan DL, Hirst S et al. Identification of humoral immune responses in protein microarrays using DNA microarray data analysis techniques. Bioinformatics 2006; 22:1760–6. [DOI] [PubMed] [Google Scholar]
- 14.Goeman JJ, Oosting J, Cleton-Jansen AM, Anninga JK, van Houwelingen HC. Testing association of a pathway with survival using gene expression data. Bioinformatics 2005; 21:1950–7. [DOI] [PubMed] [Google Scholar]
- 15.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t -test and statistical inferences of gene changes. Bioinformatics 2001; 17:509–19. [DOI] [PubMed] [Google Scholar]
- 16.Drakeley CJ, Corran PH, Coleman PG et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 2005; 102:5108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florens L, Washburn MP, Raine JD et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 2002; 419:520–6. [DOI] [PubMed] [Google Scholar]
- 18.Tarun AS, Peng X, Dumpit RF et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A 2008; 105:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JD, Rowe JA, Higgins MK, Lavstsen T. Malaria's deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol 2013; 15:1976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhouse B, Ho B, Hubbard A et al. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis 2011; 204:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gondeau C, Corradin G, Heitz F et al. The C-terminal domain of Plasmodium falciparum merozoite surface protein 3 self-assembles into alpha-helical coiled coil tetramer. Mol Biochem Parasitol 2009; 165:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce JH, Mills K, Triglia T, Cowman AF, Anders RF. Characterisation of two novel proteins from the asexual stage of Plasmodium falciparum, H101 and H103. Mol Biochem Parasitol 2005; 139:141–51. [DOI] [PubMed] [Google Scholar]
- 23.Osier FH, Mackinnon MJ, Crosnier C et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 2014; 6:247ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards JS, Arumugam TU, Reiling L et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 2013; 191:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest 2010; 120:4168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings JF, Spring MD, Schwenk RJ et al. Recombinant liver stage antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine 2010; 28:5135–44. [DOI] [PubMed] [Google Scholar]
- 27.Daubersies P, Thomas AW, Millet P et al. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat Med 2000; 6:1258–63. [DOI] [PubMed] [Google Scholar]
- 28.Genton B, Betuela I, Felger I et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in Papua New Guinea. J Infect Dis 2002; 185:820–7. [DOI] [PubMed] [Google Scholar]
- 29.Heiber A, Kruse F, Pick C et al. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog 2013; 9:e1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry AE, Trieu A, Fowkes FJ et al. The stability and complexity of antibody responses to the major surface antigen of Plasmodium falciparum are associated with age in a malaria endemic area. Mol Cell Proteomics 2011; 10:M111.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck JR, Muralidharan V, Oksman A, Goldberg DE. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 2014; 511:592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Koning-Ward TF, Gilson PR, Boddey JA et al. A newly discovered protein export machine in malaria parasites. Nature 2009; 459:945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsworth B, Matthews K, Nie CQ et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 2014; 511:587–91. [DOI] [PubMed] [Google Scholar]
- 34.Lopaticki S, Maier AG, Thompson J et al. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect Immun 2011; 79:1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol 2009; 7:341–54. [DOI] [PubMed] [Google Scholar]
- 36.Osier FH, Feng G, Boyle MJ et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014; 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright KE, Hjerrild KA, Bartlett J et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 2014; 515(7527):427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.