Abstract
Background. Sensitive assays are needed for detection of residual human immunodeficiency virus (HIV) in patients with undetectable plasma viral loads to determine whether eradication strategies are effective. The gold standard quantitative viral outgrowth assay (QVOA) underestimates the magnitude of the viral reservoir. We sought to determine whether xenograft of leukocytes from HIV type 1 (HIV)–infected patients with undetectable plasma viral loads into immunocompromised mice would result in viral amplification.
Methods. Peripheral blood mononuclear cells or purified CD4+ T cells from HIV or simian immunodeficiency virus (SIV)–infected subjects with undetectable plasma viral loads were adoptively transferred into NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. The mice were monitored for viremia following depletion of human CD8+ T cells to minimize antiviral activity. In some cases, humanized mice were also treated with activating anti-CD3 antibody.
Results. With this murine viral outgrowth assay (MVOA), we successfully amplified replication-competent HIV or SIV from all subjects tested, including 5 HIV-positive patients receiving suppressive antiretroviral therapy (ART) and 6 elite controllers or suppressors who were maintaining undetectable viral loads without ART, including an elite suppressor from whom we were unable to recover virus by QVOA.
Conclusions. Our results suggest that the MVOA has the potential to serve as a powerful tool to identify residual HIV in patients with undetectable viral loads.
Keywords: HIV, SIV, quantitative viral outgrowth assay (QVOA), humanized mouse, cure
Human immunodeficiency virus type 1 (HIV) infection remains a pandemic without a cure. Recent reports of the failure of novel eradication strategies despite promising initial results of repeated undetectable viral loads in treated patients highlight the need for more-sensitive strategies to detect latent virus [1–8]. The gold standard quantitative viral outgrowth assay (QVOA) [9] fails to induce the majority of replication-competent proviruses, thereby underestimating the magnitude of the viral reservoir [10]. Conversely, although polymerase chain reaction (PCR)–based assays are exquisitely sensitive, they lack the ability to distinguish replication-competent from defective virus, therefore overestimating the size of the latent reservoir [11]. A novel method to screen large numbers of cells for replication-competent virus is urgently needed.
Transfer of infectious tissues between species can result in enhanced viral amplification and has historically been used as a strategy for the diagnosis of rabies virus infection [12]. Though natural infection only occurs in humans and chimpanzees, humanized mice can be infected with HIV [13] and have been used to study HIV persistence and latency [14, 15]. We hypothesized that xenograft of leukocytes from HIV-infected patients with undetectable plasma viral loads into severely immunocompromised mice would result in viral amplification within the aberrant murine host. Such amplification could potentially allow us to detect viral reservoirs within the peripheral blood of HIV-infected humans and simian immunodeficiency virus (SIV)–infected pigtailed macaques with plasma viral loads that are undetectable by standard quantitative reverse transcription (qRT)–PCR methods as a result of long-term antiretroviral therapy (ART) or elite control.
MATERIALS AND METHODS
HIV-Infected Patient Donors
Whole-blood specimens were obtained from 5 HIV-positive patients receiving suppressive ART regimens and 6 HIV-positive elite suppressors for this study. Patients receiving ART had been infected for an average of 10 years (range, 2–22 years) and had been receiving ART for an average of 3 years (range, 1–6 years). These patients had an average CD4+ T-cell count of 620 cells/µL (range, 409–1001 cells/µL) at the time of this study. Elite suppressors had been infected for an average of 15 years (range, 5–29 years). Elite suppressors had always had plasma viral loads of <50 copies/mL, had a median of 0.06 infectious units per million (IUPM; range, <0.04 to 4.57 IUPM) detected by QVOA, and had an average CD4+ T-cell count of 1026 cells/µL (range, 630–1902 cells/µL). Patients receiving ART had a median proviral HIV DNA load of 1775 copies/million CD4+ T cells and a median HIV messenger RNA level of 258 copies/million CD4+ T cells. Elite suppressors had a median proviral HIV DNA load of 1097 copies/million CD4+ T cells and a median HIV mRNA level of <10 copies/million CD4+ T cells (Pohlmeyer et al, unpublished data).
SIV Infection and ART Treatment of Macaques
Male pigtailed macaques aged 2–3 years were dual inoculated by the intravenous route with the neurovirulent clone 17E/Fr and immunosuppressive swarm ΔB670 of SIV as previously described [16]. Starting on day 12 after inoculation, macaques were treated with antiretroviral drugs at the following doses with the combination of drugs indicated in Table 1: 10 mg/kg tenofovir (Gilead) once daily by subcutaneous injection and 10 mg/kg integrase inhibitor L000870812 (Merck), 24 mg/kg ritonavir (AbbVie), and either 270 mg/kg atazanavir (Bristol-Myers Squibb) or 480 mg/kg darunavir (Janssen) twice daily by mouth in a food treat [17]. Macaques were sedated weekly until day 42 after inoculation and then semiweekly thereafter with 10 mg/kg ketamine, underwent a full physical examination by a veterinarian, and had blood specimens collected for determination of viral load. Macaques were sedated 205 days after inoculation, and blood specimens were collected into acid citrate dextrose (Sigma) for peripheral blood mononuclear cell (PBMC) purification.
Table 1.
Macaquized Mice Amplify Simian Immunodeficiency Virus (SIV) From Peripheral Blood Mononuclear Cells (PBMCs) From SIV-Infected Macaques Receiving Antiretroviral Therapy (ART) With or Without a Detectable Plasma Viral Load
| Macaque | Days Infected | Donor Plasma Viral Load, Copies/mL | CD4+ T-Cell Count, Cells/µL | ART Regimen | SIV-Positive Mice | Mice, Total No. | SIV-Positive Mice, % | PBMCs Xenografted, No. | Time to Viremia in Mouse, d, Median | Peak Viremia in Mouse, Copies/mL, Median |
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 205 | 6.36 × 102 | 1284 | TDF, INSTI, RTV, ATV | 2 | 2 | 100 | 4.0 × 107 | 10.5 | 1.61 × 104 |
| V2 | 205 | 4.59 × 105 | 480 | TDF, INSTI, RTV, ATV | 1 | 1 | 100 | 4.0 × 107 | 7 | 1.25 × 105 |
| V3 | 205 | 9.50 × 102 | 533 | TDF, INSTI, RTV, DRV | 1 | 1 | 100 | 4.0 × 107 | 7 | 4.23 × 103 |
| V4 | 205 | 6.01 × 105 | 782 | TDF, INSTI, RTV, ATV | 1 | 1 | 100 | 4.0 × 107 | 7 | 6.71 × 102 |
| U1 | 0 | 0.00 | NM | Uninfected | 0 | 1 | 0 | 4.0 × 107 | ND | ND |
| S1 | 205 | <1.00 | 1141 | TDF, INSTI, RTV, DRV | 3 | 3 | 100 | 4.0 × 107 | 7 | 1.30 × 104 |
Abbreviations: ATV, atazanavir; DRV, darunavir; INSTI, integrase inhibitor L-000870812; ND, not detectible; NM, not measured; RTV, ritonavir; TDF, tenofovir.
Xenograft of Human or Macaque PBMCs or Purified Resting CD4+ T Cells Into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) Mice
Human and macaque PBMCs and resting CD4+ T cells were purified as previously described [17, 18]. NSG mice (Jackson Laboratories) were briefly manually restrained and injected intraperitoneally with the indicated number of PBMCs or purified resting CD4+ T cells resuspended in <0.5 mL of sterile saline (Tables 1–3). To allow for monitoring of engraftment and viral load, blood specimens were collected from manually restrained mice by facial sinus bleed no more than once weekly. CD8+ T cells were empirically depleted from humanized mice by intraperitoneal injection with 200–400 µg of anti-human CD8 monoclonal antibody (mAb; EBiosciences) and, in some cases, T cells activated with 100 µg anti-human CD3 mAb (OKT3; EBiosciences). Mice were monitored daily and promptly euthanized if they appeared ill. All mice were euthanized with inhalant CO2 according to the 2013 AVMA Guidelines on Euthanasia at the last time point indicated in the figures, and blood specimens, peritoneal wash fluid, spleens, and other organs were collected. Throughout the study, mice were pair or group housed in shoebox cages on a ventilated rack (Allentown), provided with free access to nutritionally complete rodent chow (Harlan), provided with a nestlet for enrichment, and handled in a biosafety cabinet.
Table 3.
Humanized Mice Amplify Virus From Peripheral Blood Mononuclear Cells (PBMCs) or CD4+ T Cells From Human Immunodeficiency Virus (HIV) Type 1–Infected Elite Suppressors (ES) With Undetectable Plasma Viral Load
| Donor ES | Infection Duration, y | Donor Plasma Viral Load, Copies/mL | QVOA Finding, IUPM | Donor CD4+ T-Cell Count, Cells/µL | Viremic Mice, No. | Mice, Total No. | HIV-Positive Mice, % | Cells Xenografted, No. | Time to Viremia in Mouse, d, Mean | Peak Viremia in Mouse, Copies/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| ES22 | 5 | <50 | 0.04 (1 of 2.8 × 107) | 981 | 1 | 1 | 100 | 6.6 × 107a | 28 | 8.0 × 103 |
| ES5 | 24 | <50 | <0.04 (0 of 2.5 × 107) | 630 | 2 | 2 | 100 | 1.0 × 107, or 2.0 × 107 | 49 | 1.09 × 103 |
| ES3 | 23 | <50 | 0.05 (1 of 2.0 × 107) | 1248 | 1 | 2 | 50 | 2.2 × 107 | 4 | 5.0 × 103 |
| ES23 | 29 | <50 | 0.06 (2 of 3.6 × 107) | 708 | 2 | 2 | 100 | 2.0 × 107 | 28 | 1.16 × 103 |
| ES39 | 5 | <50 | 0.08 (2 of 2.5 × 107) | 686 | 2 | 2 | 100 | 2.6 × 107 | 35 | 1.05 × 104 |
| ES24 | 6 | <50 | 4.57 | 1902 | 1 | 1 | 100 | 1.8 × 107 | 34 | 3.79 × 105 |
Abbreviations: IUPM, infectious units per million cells; QVOA, quantitative viral outgrowth assay.
a Mouse was xenografted with PBMCs rather than CD4+ T cells.
Ethics Statement
All animal work was approved by the Johns Hopkins University Institutional Animal Care and Use Committee and determined to be in accordance with the guidelines outlined in the Animal Welfare Act and Regulations (US Department of Agriculture) and the Guide for the Care and Use of Laboratory Animals, Eighth Edition (National Institutes of Health).
Flow Cytometry Detection of Macaque and Human Cells in Mice
To quantify the level of engraftment, flow cytometry was completed on blood specimens obtained from xenografted mice every 7 days following xenograft until necropsy; at necropsy, flow cytometry was performed blood specimens, spleens, and peritoneal wash fluid. For humanized mice, cells were stained with anti-mouse CD45 (clone 30F11; BV605), anti-human CD45 (clone HI30; PE-Cy7), CD3 (clone UCHT1; PacBlue), CD4 (clone RPA-T4; PE), CD8 (clone SK1; APC-H7), CD56 (clone B159; FITC), CD16 (clone 3G8; PerCP-Cy-5.5), CD25 (clone MA-251; APC), CD69 (clone FN50; APC), and HLA-DR (clone G46-6; APC; BD Biosciences) and were evaluated with a BD FACSCanto II. For macaquized mice, blood specimens were stained for anti-mouse CD45 (clone 30-F11; PE-Cy7), anti-NHP CD45 (clone D058–1283; BV605), CD3 (clone SP34-1; V500), CD4 (clone L200; PerCp-Cy5.5), CD8 (clone RPTA-T8; FITC), CD159 (Beckman Coulter clone Z199; PE), CD14 (clone M5E2; BV650), CD16 (BioLegend clone 3G8; AF700), CD25 (clone M-A251; PE), CD69 (clone FN50; APC), and HLA-DR (BioLegend clone L243; APC; all from BD Biosciences, unless otherwise noted) and evaluated using a BD LSRFortessa. Data were analyzed with FlowJo 7.6.5 (Supplementary Figure 1A).
Quantification of SIV and HIV in Plasma, Using qRT-PCR
RNA was isolated from plasma from human and macaque donors and xenografted mice, and viral RNA was quantified using qRT-PCR as previously described [19, 20]. Viral load was normalized to the original plasma input volume to determine copy equivalents per milliliter of plasma. Thus, the limit of detection varies between qRT-PCR runs.
Quantitation of Viral Outgrowth
The QVOA was performed on CD4+ T cells as previously described [9, 21–23]. CD4+ T cells were purified by negative selection, using Miltenyi beads, and were then stimulated with PHA (0.5 µg/mL) and a 5–10-fold excess of irradiated PBMCs from HIV-seronegative donors in medium containing 100 U/mL of interleukin 2. After 1–2 days of culture, the medium containing PHA was removed, and activated CD4+ T cell lymphoblasts from HIV-seronegative donors were added to amplify the virus. The cultures were split on day 7, and more CD4+ T-cell lymphoblasts were added. Virus was detected using a p24 enzyme-linked immunosorbent assay on days 14 and 21 of culture.
Quantitation of Proviral DNA and Cell-Associated RNA
For human samples, cell-associated DNA and mRNA levels were quantified by qPCR as previously described [19, 24]. For macaque samples, nucleic acids were isolated from 5 × 106 macaque PBMCs, using the AllPrep DNA/RNA isolation minikit (Qiagen). Level of intracellular SIV RNA and DNA were quantitated by digital-droplet PCR (BioRad), using the same SIV gag primers and probe and cycle conditions as described above for qRT-PCR.
Immunohistochemistry for the Detection of CD45+ Human and Macaque Cells in Tissues From Xenografted Mice
Mouse tissues were fixed in 10% neutral buffered formalin for a week and then embedded in paraffin. Antigen retrieval was performed by heating the slides in a microwave at 780 W for 8 minutes in 10 mM sodium citrate buffer at pH 6.0. The remainder of the protocol was performed using the Vector M.O.M. Immunodetection Kit (Vector Labs PK-2200) with anti-CD45 antibody (AbD Serotec MCA1921 Clone 2B11 + PD7/26/16) at a 1:100 dilution for 1 hour at room temperature. Slides were stained with DAB (Vector Labs SK-4100) and counterstained with hematoxylin (BioGenex HK100).
Data Management and Analysis
All data were organized using Microsoft Excel 2013, and statistical analyses were completed with GraphPad Prism. Nonparametric (Spearman) correlative analyses were used to compare the percentage of PBMCs or plasma viral RNA in the mouse with the number of cells or viral load, respectively, in the macaque or human donors.
RESULTS
Virus Can Be Detected in Plasma of NSG Mice Following Xenograft of PBMCs From SIV-Infected Macaques
We aimed to xenograft immunocompromised mice with PBMCs from HIV-infected patients with undetectable viral loads to determine whether we could detect virus with better sensitivity than the QVOA. To validate our xenograft technique, NSG mice were humanized with 8.8–25.2 million human PBMCs, control mice received no PBMCs, and whole-blood specimens were collected 7 days later for flow cytometry analysis. Intraperitoneal injection of >8.8 million PBMCs resulted in humanization of 8 of 9 mice, with CD45+ human cells detectable in the peripheral blood and colonizing the bone marrow, spleen, and peritoneal cavity (Supplementary Figure 1). Circulating CD45+ human cells consisted predominantly of activated CD4+ T cells (Supplementary Figure 1A), and the percentage of human cells circulating in the mouse's peripheral blood 7 days after humanization correlated with the number of xenografted PBMCs (Spearman R2 = 0.87; P = .0005).
To verify that we could detect lentivirus in plasma specimens from mice xenografted with leukocytes from infected individuals, we initially worked with an SIV-infected pigtailed macaque model of HIV infection [16]. We macaquized 5 of 5 NSG mice with 40 million PBMCs harvested from SIV-infected pigtailed macaques receiving ART, all with measurable plasma viral loads (median, 230 182 copies/mL; macaques V1–4 in Table 1). SIV RNA was detected in the plasma of all mice by 14 days after xenograft, with a median peak SIV load of 10 184 copies/mL (Table 1); the magnitude of the plasma viral load in the xenografted mouse did not correlate with the magnitude of the plasma viral load in the donor macaque (Spearman R2 = −0.02; P = .60). We did not detect any virus in a control mouse xenografted with 40 million PBMCs from an uninfected macaque (macaque U1 in Table 1) or in a control mouse that was not injected with macaque PBMCs (data not shown).
Replication-Competent HIV and SIV Can Be Detected by a Murine Viral Outgrowth Assay (MVOA) in NSG Mice Following Xenograft of PBMCs From Individuals Receiving Suppressive ART With a History of Undetectable Plasma Viral Loads
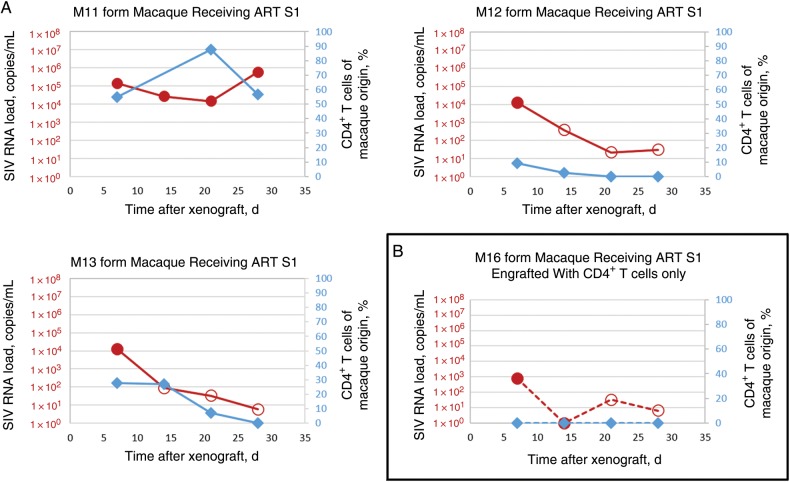
SIV-infected pigtailed macaques receiving ART are a valuable model for the study of latent reservoirs of HIV [17]. We sought to determine whether the MVOA could detect replication-competent virus in an SIV-infected pigtailed macaque receiving ART that had no plasma viral load detected by qRT-PCR for 78 days prior to PBMC donation, 312 copies per million PBMCs of proviral SIV gag DNA, and 18 copies of cell-associated SIV gag RNA per million PBMCs (macaque S1 in Table 1). We detected SIV 7 days after xenograft in plasma specimens from 3 of 3 mice, each injected with 40 million PBMCs from this donor (peak median MVOA finding, 13 032 copies/mL; Figure 1A). Resting CD4+ T cells are a resilient reservoir for latent virus [25], and xenografting 6.8 million resting CD4+ T cells from this macaque permitted detection of virus 7 days after xenograft (794 copies/mL; Figure 1B).
Figure 1.
Adoptive transfer of peripheral blood mononuclear cells (PBMCs) or CD4+ T cells from simian immunodeficiency virus (SIV)–infected macaques that were receiving antiretroviral therapy (ART) and had a history of undetectable viral loads into NSG mice results in amplification of SIV. Detection of virus with a murine viral outgrowth assay xenografted with PBMCs (A) or CD4+ T cells (B) from SIV-positive macaques over time following xenograft. Xenografted mouse plasma SIV loads are denoted by red circles, and circulating macaque CD4+ T cells in mouse are denoted by blue diamonds. Open circles represent the limit of detection for each sample in which no virus was detected.
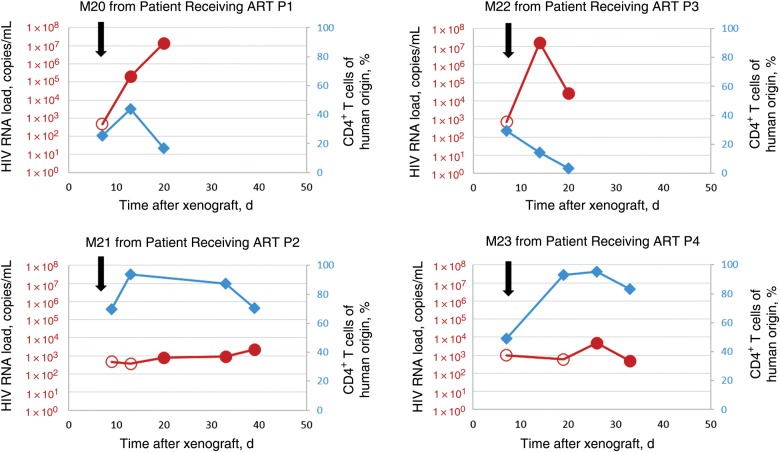
The MVOA was similarly able to detect replication-competent virus in 5 of 5 HIV-infected patients receiving long-term ART who had undetectable plasma viral loads for at least 1 year (average, 3 years; Table 2). One mouse per patient was engrafted with PBMCs, and CD8+ T cells were depleted with anti-human CD8 mAb 7 days after xenograft because of the concern that these cells may have antiviral activity. CD8+ T-cell depletion also mimics the conditions typically followed in the QVOA, [9, 21]. CD4+ T-cell activation was present in all engrafted mice (Supplementary Figure 1C). We detected HIV in the murine plasma an average of 20 days after xenograft (range, 13–26 days), with a median peak viral load of 4.6 × 103 copies/mL at an average of 25 days (Figure 2 and Supplementary Figure 2A).
Table 2.
Humanized Mice Amplify Human Immunodeficiency Virus (HIV) From Peripheral Blood Mononuclear Cells (PBMCs) Obtained From HIV Type 1–Infected Patients Who Were Receiving Antiretroviral Therapy (ART) and Had an Undetectable Plasma Viral Load
| Donor Patient | Infection Duration, y | Donor Plasma Viral Load, Copies/mL | Time Since Last Detectable Plasma Viral Load in Donor, y | Donor CD4+ T-Cell Count, Cells/µL | ART Regimen | PBMCs Xenografted, No. | Time to Viremia in Mouse, d | Peak Viremia in Mouse, Copies/mL |
|---|---|---|---|---|---|---|---|---|
| P1 | 2 | <50 | 1 | 653 | EFV, 3TC, TDF | 4.0 × 107 | 13 | 1.4 × 107 |
| P2 | 8 | <50 | 3 | 628 | EFV, 3TC, TDF | 2.8 × 107 | 20 | 2.3 × 103 |
| P3 | 22 | <50 | 6 | 1001 | DRV, 3TC, TDF | 5.5 × 107 | 14 | 1.7 × 107 |
| P4 | 13 | <50 | 1 | 409 | EFV, DRV, 3TC | 2.5 × 107 | 26 | 4.6 × 103 |
| P5 | 7 | <50 | 6 | 409 | EFV, RAL, 3TC | 3.5 × 107 | 26 | 2.3 × 103 |
Abbreviations: 3TC, lamivudine; DRV, darunavir; EFV, efavirenz; RAL, raltegravir; TDF, tenofovir.
Figure 2.
Adoptive transfer of peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus (HIV)–infected patients who were receiving antiretroviral therapy (ART) and had a history of undetectable viral loads into NSG mice results in amplification of HIV type 1. Shown are viral loads and percentages of circulating human CD4+ T cells in representative mice with xenografted mouse plasma viral load (red circles) and circulating human CD4+ T cells in mouse blood (blue diamonds). Human CD8+ T cells were depleted by the murine viral outgrowth assay in all humanized mice as indicated (black arrows). Open circles represent the limit of detection for each sample in which no virus was detected.
Replication-Competent HIV Can Be Detected by MVOA in NSG Mice Following Xenografting of CD4+ T Cells From HIV–Positive Elite Suppressors With Plasma Viral Loads Below the Limit of Detection
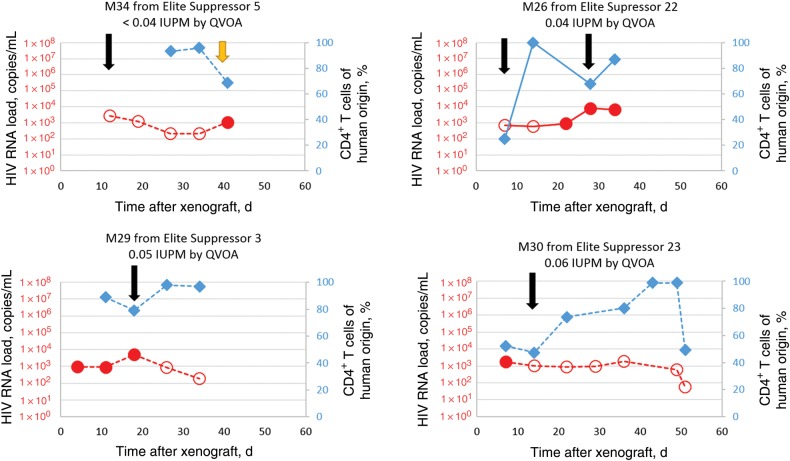
To further challenge the sensitivity of the MVOA, we engrafted mice with cells from elite suppressors. These patients control the replication of HIV to levels below the clinical limit of detection (<50 copies/mL) without ART [26–28] and have very low frequencies of latently infected CD4+ T cells [22]. We have previously shown that purified viral isolates from these patients are capable of replicating vigorously in humanized mice [23]. We xenografted 1–2 mice per patient with cells from 6 elite suppressors that had frequencies of infected cells ranging from <0.04 (undetectable) to 4.57 IUPM by QVOA (Table 3); 5 of these 6 patients had frequencies of ≤0.08 IUPM, which is thus much lower than the median frequency of 0.5–1 IUPM seen in patients receiving suppressive ART [25]. Mice injected with either PBMCs or purified CD4+ T cells were successfully engrafted. CD4+ T-cell activation was present in all engrafted mice (Supplementary Figure 1D). CD8+ T cells were depleted at the time points indicated with anti-human CD8 mAb (Figure 3 and Supplementary Figure 2B–D).We successfully amplified HIV from cells from all 6 elite suppressors following successful xenograft of 10–26 million purified CD4+ T cells or 66 million PBMCs by using this assay and from 9 of 10 mice xenografted with cells from these elite suppressors. In 3 of 9 viremic mice, amplification of HIV was first observed only after further activation of CD4+ T cells with the anti-CD3 mAb, OKT3 (Figure 3 and Supplementary Figure 2B and 2C); this is analogous to the activation with PHA routinely used in the QVOA [9] and also represents a strategy that amplified virus in a patient receiving a suppressive ART regimen [29]. We were able to amplify virus from elite suppressor 5 following engraftment of either 10 million or 20 million CD4+ T cells into mice (M34 in Figure 3 and M27 in Supplementary Figure 2B); in contrast, we were unable to recover virus when 25 million CD4+ T cells were tested in the QVOA. The magnitude of the plasma viral load in the xenografted mouse did not correlate with the IUPM as measured by QVOA in the donors (Spearman R2 = −0.10; P = .83). Elite suppressors’ CD8+ T cells are very effective at controlling viral replication in vitro [30–35] and in humanized mice [36], and the presence of residual CD8+ T cells may have contributed to the transient nature of the viremia in some mice (Supplementary Figure 3).
Figure 3.
Adoptive transfer of peripheral blood mononuclear cells (PBMCs) or CD4+ T cells from human immunodeficiency virus (HIV)–infected elite suppressors with undetectable viral loads into NSG mice results in amplification of HIV type 1. Shown are viral loads and percentages of circulating human CD4+ T cells over time, following adoptive transfer, of CD4+ T cells (dashed lines) or PBMCs (solid lines) in representative mice with xenografted mouse plasma viral loads (red circles) and circulating human CD4+ T cells in mouse blood (blue diamonds). Human CD8+ T cells were depleted by the murine viral outgrowth assay as needed (black arrows). Three of 9 mice from which we were able to amplify virus from elite suppressors required anti-CD3 monoclonal antibody stimulation (yellow arrows) to amplify virus to detectable levels. Open circles represent the limit of detection for each sample in which no virus was detected. Abbreviations: IUPM, infectious units per million cells; QVOA, quantitative viral outgrowth assay.
DISCUSSION
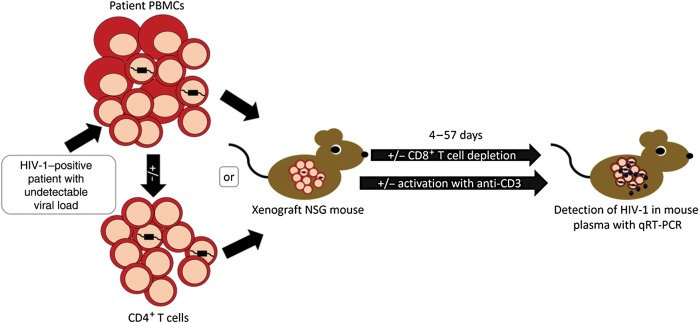
We demonstrated that the MVOA has the potential to serve as a powerful tool to identify reservoirs of HIV. By xenografting as few as 25 million PBMCs or 10 million CD4+ T cells, we amplified virus from HIV-positive patients and SIV-positive macaques with undetectable viral loads due to either long-term receipt of ART or natural control, including 1 elite suppressor from whom we were unable to recover virus with the QVOA. This assay, although not quantitative, may be more sensitive than the QVOA. Additionally, at a ratio of 1 mouse to every 10–50 million CD4+ T cells from patients, the MVOA can more efficiently be used to sample very large numbers of cells, compared with the 10:1 ratio of feeders to patient cells required in the QVOA (Figure 4) [9, 21]. The MVOA is promising not only as a simple and sensitive diagnostic assay for evaluating putative curative and preventive therapies in patients with otherwise undetectable plasma viral loads, but also because it may be adapted for future studies into the pathogenesis of the control of viral replication.
Figure 4.
The murine viral outgrowth assay (MVOA) amplifies replication-competent human immunodeficiency virus (HIV) from peripheral blood mononuclear cells (PBMCs) or CD4+ T cells from HIV-positive patients with undetectable plasma viral loads. Schematic representation of xenograft of NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice with patient cells and subsequent detection of HIV in the murine plasma. Abbreviation: qRT-PCR, quantitative reverse transcription–polymerase chain reaction.
The sensitivity of the MVOA is likely due to a number of factors, including the ability to screen large numbers of host cells (up to 60 million per mouse), xenogeneic human anti-mouse responses that lead to the activation of human CD4+ T cells, and the use of qRT-PCR to detect virus once amplified (similar to the QVOA modifications reported by Laird et al [21]). The efficacy of the MVOA may potentially be enhanced by exogenous activation of T cells. While the anti-CD3 mAb OKT3 alone was effective in flushing out virus in cells from 2 patients, this antibody eventually leads to T-cell depletion in human recipients [37, 38], and therefore a combination of anti-CD3 and anti-CD28 mAbs may be more effective in inducing prolonged T-cell activation.
The MVOA presented in this article is a sensitive assay of residual virus in the peripheral blood with a binary output, rather than a quantitative assay. However, the MVOA could potentially be adapted to produce a quantitative result, similar to the IUPM produced by QVOA [9]; multiple mice could be xenografted with serial dilutions of an HIV-positive patient's cells, thus allowing a similar calculation to be completed to determine an IUPM through the MVOA. Additionally, although the MVOA as described detected reservoirs in the peripheral blood, we anticipate that it may be able to similarly detect virus from cells isolated from tissue biopsy specimens from HIV-positive patients or animal models. The location of latent viral reservoirs within the tissues is an area of active investigation [17, 39], and therefore the MVOA may be used to elucidate the location of latent viral reservoirs in the tissues.
The MVOA has several limitations. The sensitivity of the assay is directly related to the volume of plasma obtained from the xenografted mouse at each time point. The level of engraftment is variable, and this affects the percentage of the adoptively transferred cells that are actually assayed. Finally, the time frame between engraftment and virus detection is also variable, and the transient nature of viremia in some mice may mean that blips may be missed in some cases. Our data suggests that residual CD8+ T-cell responses may play a role in controlling viremia (Supplementary Figure 3), but it is also possible that the observed blips of low-level viremia may be due to the release of defective virus, rather than to actual viral replication. Further studies will be needed to distinguish between these 2 scenarios.
Despite these limitations, the MVOA is unique in that it can determine whether viral rebound will occur when ART is discontinued in an in vivo system without any undue effect on the patient. Interestingly, the adoptive transfer of PBMCs from SIV-infected monkeys receiving ART into uninfected monkeys has been shown to be a sensitive method for detecting residual virus [40]. In our study, we evaluated the effect of discontinuation of common suppressive ART regimens on both HIV and SIV replication in PBMCs. We detected virus production by PBMCs isolated from these patients by MVOA within a time frame (<4 weeks) consistent with that reported following interruption of ART in patients who initiated treatment during the chronic phase of HIV infection [41]. Time to virus production in mice xenografted with cells from patients undergoing different antiretroviral regimens (ie, initiation of ART during primary infection and intensification of ART) could be compared to previous reports of the kinetics of viral recrudescence following cessation of these therapies [42, 43] to determine whether time to outgrowth in the MVOA is reflective of the efficacy of a therapy targeted to provide lasting control of viral replication in the patient.
The question of whether HIV has truly been eradicated from a patient is paramount following treatment with experimental curative regimens. Larger studies are needed to definitively determine whether the MVOA is more sensitive than the QVOA. However, the MVOA provides a novel simple and sensitive strategy for screening very large numbers of cells prior to the discontinuation of ART.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank S. Price and E. Forsyth, for technical assistance and advice; D. R. Graham, for suggesting the moniker “MVOA”; A. G. Swennes, for aid in properly referencing this work; the retrovirus laboratory team, for preparing and administering ART to the macaques; and the Johns Hopkins care staff, for careful attention to the health and well-being of the macaques and mice.
K. A. M. P., J. B. F., and J. N. B. designed the xenograft mouse studies; K. A. M. P., K. M. N., C. G. C., M. E. V., and C. E. L. completed in vivo mouse work; C. W. P., S. C., K. M. N., and M. L. optimized the technique for, processed, and analyzed murine viral loads; V. E. W.-S., K. M. N., C. G. C., and E. N. S. optimized the technique for, processed, and analyzed murine flow cytometry analyses; E. L. E. optimized and completed mouse immunohistochemistry analyses; J. N. B. designed human studies; J. N. B., C. W. P., V. E. W.-S., M. S., and S. C. processed human samples; J. L. M., J. E. C., M. C. Z., L. G., K. A. M. P., R. J. A., and S. E. Q. designed macaque studies; S. E. Q. and E. L. E. coordinated the macaque ART team; K. A. M. P. and R. J. A. completed in vivo macaque work; K. M. N. and B. B. optimized and processed macaque samples; S. E. Q. and M. L. processed and analyzed macaque viral loads; K. A. M. P. and J. N. B. wrote the manuscript; and C. W. P., V. E. W.-S., K. M. N., E. L. E., and S. E. Q. contributed text and figures.
Financial support. This work was supported by the National Institute of Health (NIH) National Institute of Allergy and Infectious Diseases (grants R56 AI080328 and R21 AI106491 to J. N. B.), the NIH National Institute of Mental Health (grant P01 MH070306 to J. E. C.), the Johns Hopkins University Center for AIDS Research (grant P30AI094189), the Collaboratory of AIDS Researchers for Eradication (to J. E. C.), the Spanish Health Institute (Sara Borrell grant to M. S.), the NIH National Center for Research Resources Office of Research Infrastructure Programs (ORIP; grant P40 OD013117, to R. J. A. through which macaques were obtained; grant K01 OD018244, to K. A. M. P.; and grant T32 OD011089, to J. B. F. and M. E. V.), Gilead (tenofovir donation for macaques), Bristol-Myers Squibb (atazanavir donation for macaques), Merck (integrase inhibitor L000870812 donation for macaques), AbbVie (ritonavir donation for macaques), and Janssen (darunavir donation for macaques).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Butler K, Gavin P, Coughlan S et al. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr Infect Dis J 2015; 34:e48–51. [DOI] [PubMed] [Google Scholar]
- 2.Henrich TJ, Hanhauser E, Marty FM et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Inter Med 2014; 161:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomet V, Trabattoni D, Zanchetta N et al. No cure of HIV infection in a child despite early treatment and apparent viral clearance. Lancet 2014; 384:1320. [DOI] [PubMed] [Google Scholar]
- 4.Fauci AS, Marston HD, Folkers GK. An HIV cure: feasibility, discovery, and implementation. JAMA 2013; 312:335–6. [DOI] [PubMed] [Google Scholar]
- 5.Persaud D, Luzuriaga K. Absence of HIV-1 after treatment cessation in an infant. N Engl J Med 2014; 370:678. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy M. HIV is detected in child thought to have been cured. BMJ 2014; 349:g4614. [DOI] [PubMed] [Google Scholar]
- 7.Luzuriaga K, Gay H, Ziemniak C et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Eng J Med 2015; 372:786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler KM, Gavin P, Coughlan S et al. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr Infect Dis J 2015; 34:51. [DOI] [PubMed] [Google Scholar]
- 9.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol 2004; 304:3–15. [DOI] [PubMed] [Google Scholar]
- 10.Ho Y-CC, Shan L, Hosmane NN et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson S, Graf EH, Dahl V et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Path 2013; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster WA, Casey GA, Charlton KM. The mouse inoculation test in rabies diagnosis: early diagnosis in mice during the incubation period. Can J Comp Med 1976; 40:322–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Denton PW, García JV. Humanized mouse models of HIV infection. AIDS Rev 2010; 13:135–48. [PMC free article] [PubMed] [Google Scholar]
- 14.Denton P, Olesen R, Choudhary S et al. Generation of HIV latency in humanized BLT mice. J Virol 2012; 86:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denton PW, Long JM, Wietgrefe SW et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Path 2013; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements J, Mankowski J, Gama L, Zink M. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol 2008; 14:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinoso J, Rabi S, Blankson J et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol 2009; 83:9247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckheit RW, Allen TG, Alme A et al. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat Com 2011; 3:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer S, Wiegand AP, Maldarelli F et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Micro 2003; 41:4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meulendyke K, Pletnikov M, Engle E, Tarwater P, Graham D, Zink M. Early minocycline treatment prevents a decrease in striatal dopamine in an SIV model of HIV-associated neurological disease. J Neuroimmune Pharmacol 2012; 7:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird GM, Eisele EE, Rabi SA et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Path 2012; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blankson JN, Bailey JR, Thayil S et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 2007; 81:2508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgado M, Swanson MD, Pohlmeyer CW et al. HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice. J Virol 2014; 88:3340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014; 20:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siliciano JD, Kajdas J, Finzi D et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 26.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007; 27:406–16. [DOI] [PubMed] [Google Scholar]
- 27.O'Connell KA, Bailey JR, Blankson JN. Elucidating the elite: mechanisms of control in HIV-1 infection. Trends Pharmacol Sci 2009; 30:631–7. [DOI] [PubMed] [Google Scholar]
- 28.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA 2010; 304:194–201. [DOI] [PubMed] [Google Scholar]
- 29.Prins JM, Jurriaans S, van Praag RM et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 1999; 13:2405–10. [DOI] [PubMed] [Google Scholar]
- 30.Migueles SA, Laborico AC, Shupert WL et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 2002; 3:1061–8. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Nason MC, West SM et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006; 107:4781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migueles SA, Osborne CM, Royce C et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 2008; 29:1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hersperger AR, Pereyra F, Nason M et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Path 2010; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sáez-Cirión A, Lacabaratz C, Lambotte O et al. HIV controllers exhibit potent CD8T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A 2007; 104:6776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckheit RW, Siliciano RF, Blankson JN. Primary CD8+ T cells from elite suppressors effectively eliminate non-productively HIV-1 infected resting and activated CD4+ T cells. Retrovirology 2012; 10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Quiros JC, Shupert WL, McNeil AC et al. Resistance to replication of human immunodeficiency virus challenge in SCID-Hu mice engrafted with peripheral blood mononuclear cells of nonprogressors is mediated by CD8(+) T cells and associated with a proliferative response to p24 antigen. J Virol 2000; 74:2023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosimi AB, Burton RC, Colvin RB et al. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation 1981; 32:535–9. [DOI] [PubMed] [Google Scholar]
- 38.Cosimi AB, Colvin RB, Burton RC et al. Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med 1981; 305:308–14. [DOI] [PubMed] [Google Scholar]
- 39.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS 2013; 8:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okoye A, Rohankhedkar M, Reyes M et al. Early treatment in acute SIV infection limits the size and distribution of the viral reservoir. Presented at: 21st Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 2014. [Google Scholar]
- 41.Wit FW, Blanckenberg DH, Brinkman K et al. Safety of long-term interruption of successful antiretroviral therapy: the ATHENA cohort study. AIDS 2005; 19:345–8. [PubMed] [Google Scholar]
- 42.Hamlyn E, Ewings FM, Porter K, Cooper DA. Plasma HIV viral rebound following protocol-indicated cessation of ART commenced in primary and chronic HIV infection. PLoS One 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutiérrez C, Hernández-Novoa B, Vallejo A et al. Dynamics of the HIV-1 latent reservoir after discontinuation of the intensification of antiretroviral treatment: results of two clinical trials. AIDS 2013; 27:2081–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.