Abstract
Human immunodeficiency virus (HIV) infectivity increases as receptor/coreceptor expression levels increase. We determined peripheral CD4, CCR5, and CXCR4 expression levels in HIV-uninfected women who used depot medroxyprogesterone acetate (DMPA; n = 32), the levonorgestrel-releasing intrauterine device (LNG-IUD; n = 27), oral contraceptive pills (n = 32), or no hormonal contraception (n = 33). The use of LNG-IUD increased the proportion of CD4+ and CD8+ T cells that expressed CCR5; increases in the magnitude of T-cell subset CCR5 expression were observed with DMPA and LNG-IUD use (P < .01 for all comparisons). LNG-IUD and, to a lesser extent, DMPA use were associated with increased peripheral T-cell CCR5 expression.
Keywords: HIV-1, hormonal contraception, CCR5, medroxyprogesterone acetate, levonorgestrel, oral contraceptive pills, peripheral blood mononuclear cells, CD4, CXCR4
Hormonal contraceptive use may increase the risk of human immunodeficiency virus type 1 (HIV) acquisition and transmission, although controversy exists. The use of oral contraceptive pills (OCPs) or depot medroxyprogesterone acetate (DMPA) has been associated with a greater risk for HIV infection, and hormonal contraceptive use increases the risk of infection with more than one transmitted/founder virus [1–4]. A possible mechanism to explain why hormonal contraception use might increase susceptibility to sexual transmission of HIV remains unclear.
Receptor and coreceptor concentrations are key determinants of virus entry efficiency, kinetics, and infectivity, factors that are known to improve virus fitness [5–7]. We hypothesized that hormonal contraceptive–induced increases in CD4 and/or CCR5 expression could create a more favorable fitness landscape for virus replication and provide a mechanism to explain increased HIV acquisition rates in DMPA-treated women. To assess the relationship between levels of CD4, CCR5, and CXCR4 expression by peripheral blood mononuclear cells (PBMCs) in women receiving hormonal contraceptives, we used participants' cryopreserved samples from HIV-uninfected, at-risk women enrolled in the Women's Interagency HIV Study (WIHS) [8].
METHODS
Participant Selection
Specimens were obtained from 4 groups of HIV-uninfected women enrolled in the WIHS [8]. Participant samples were collected from 2004 to 2011 and analyzed from study visits during which participants indicated they were using OCPs, DMPA, or the levonorgestrel-releasing intrauterine device (LNG-IUD). Women with regular menstrual cycles were studied as a comparator group. Groups were matched by age and race. One-way analysis of variance for age and the Fisher exact test for race were used to ensure balanced age and race distribution across the 4 groups. This study was approved by the Partners Human Research Committee.
Flow Cytometry
Cryopreserved PBMC subpopulation proportions and surface expression levels of CD4, CXCR4, and CCR5 were determined by multicolor flow cytometry with 2 separate monoclonal antibody panels. Monocytes, monocytoid dendritic cells (mDCs), and plasmacytoid dendritic cells (pDCs) were identified using a-CD3-Pacific Blue, a-CD11c-PE, a-CD123-PerCP-Cy5.5, and a-CCR5-PE-Cy7 antibodies (BD Pharmingen, San Diego, California) and a-CD14-APC and a-CXCR4-PeCy5 antibodies (BioLegend, San Diego). The frequency of total CD4+ and CD8+ T lymphocytes and the proportion of central memory (TCM), effector memory (TEM), naive (TN), and terminally differentiated (TTD) CD4+ T cells were determined using a-CD3-Pacific Blue, a-CD4-APC-H7, a-CD8-PE, a-CCR7-PeCy7, a-CCR5-APC, and a-CXCR4-PerCP-Cy5.5 (BD Pharmingen) and a-CD45RA-FITC (Beckman Coulter, Brea, California). A total of 2.0–5.0 × 106 events (median, 2.5 × 106 events) were acquired using a BD LSRII flow cytometer (BD Biosciences, San Jose, California). Data were analyzed by FlowJo software 9.1 (Tree Star, Ashland, Oregon).
Hormone Concentrations
Plasma and serum concentrations of estradiol and progesterone were measured by competitive-binding immunoenzymatic assays (Access Immunoassay Systems, Beckman Coulter). Estradiol and progesterone measurements below the assay detection limit (<20 pg/mL and <0.08 ng/mL, respectively) were imputed for data analysis purposes at the midpoint, defined as 10 pg/mL estradiol and 0.04 ng/mL progesterone. Results from 2 pregnant participants were censored. Medroxyprogesterone acetate (MPA) was measured by a modified radioimmunoassay (RIA) method [9]. Levonorgestrel (LNG) was quantified by an RIA as described previously [10].
Statistical Analyses
Mean fluorescence intensities (MFIs), mean values, and 95% confidence intervals of PBMC subpopulation proportions that express CD4, CCR5, and CXCR4 were calculated. Intergroup differences in PBMC subpopulation HIV receptor and coreceptor expression levels and MFIs were determined using 1-way analyses of variance; Dunn multiple comparisons tests were used (GraphPad Prism 6, GraphPad, La Jolla, California). Linear regression analyses were performed to determine correlations between synthetic progestin levels and CCR5 expression (GraphPad Prism 6).
RESULTS
Participants' Demographics and Clinical Characteristics
Samples from 126 premenopausal HIV-uninfected women were analyzed (Table 1). We studied women who were using DMPA (n = 32), an LNG-containing IUD (n = 28), or OCP (n = 32). Women with regular menstrual cycles served as a comparator group (n = 34). Two participants were pregnant at the time samples were collected, one each from the group that received LNG-IUD and the group that received no hormonal contraception. Data generated from these subjects were censored and not included in subsequent analyses. Participants were predominantly nonwhite (50% were black, and 30% were Hispanic) with mean CD4+ T-cell counts typical of HIV-uninfected individuals. No significant differences in age, race, or CD4+ T-cell counts were noted across groups. Significantly fewer women receiving DMPA had received an undergraduate or higher academic degree (P = .03). No significant differences were observed in the proportion of smokers or the number of smoking-years across hormonal contraceptive groups.
Table 1.
Characteristics of At-Risk, Human Immunodeficiency Virus–Negative Women
| Characteristic | Study Group |
P Value | |||
|---|---|---|---|---|---|
| DMPA (n = 32) | LNG-IUD (n = 27) | OCP (n = 32) | No Hormonal Contraception (n = 33) | ||
| Age at visit, y | 32.5 (30.1–34.9) | 35.6 (32.6–38.6) | 33.7 (31.6–35.8) | 33.3 (31.8–34.8) | .26 |
| Race | .11 | ||||
| White, non-Hispanic | 1 (3.1) | 4 (14.8) | 7 (21.9) | 2 (6.1) | |
| Black, non-Hispanic | 20 (62.5) | 10 (37.0) | 11 (34.4) | 22 (66.7) | |
| Hispanic | 8 (25.0) | 10 (37.0) | 11 (34.4) | 8 (24.2) | |
| Other | 3 (9.4) | 3 (11.1) | 3 (9.4) | 1 (3.0) | |
| Education level | .02 | ||||
| Less than college | 27 (84.4) | 15 (55.6) | 16 (50.0) | 22 (66.7) | |
| College or greater | 5 (15.6) | 12 (44.4) | 16 (50.0) | 11 (33.3) | |
| CD4+ T-cell count at visit, cells/μL | 911 (794–1028) | 1090 (869–1312) | 953 (768–1137) | 1168 (1031–1305) | .05 |
| Current smoker at visit | 13 (40.6) | 10 (37.0) | 7 (21.9) | 11 (33.3) | .11 |
| Years of smoking | 7.8 (4.2–11.4) | 9.1 (4.8–13.3) | 5.5 (2.9–8.1) | 6.6 (3.7–9.6) | .63 |
Data are mean value (95% confidence interval) or no. (%) of participants.
Abbreviations: DMPA, depot medroxyprogesterone acetate; LNG-IUD, levonorgestrel intrauterine device; OCP, oral contraceptive pills.
PBMC HIV Receptor and Coreceptor Expression Levels
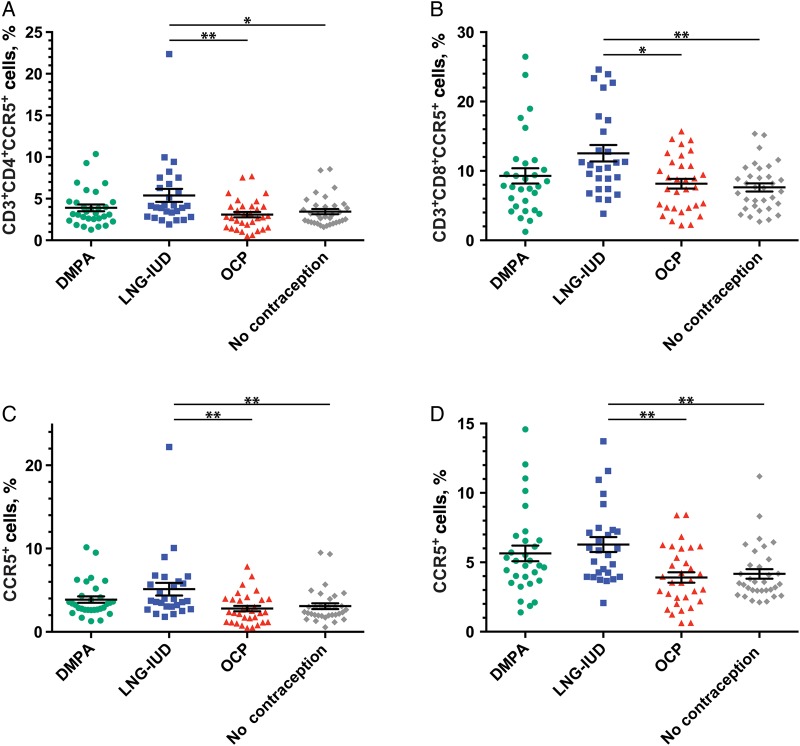
Within PBMC subpopulations, the proportions of CD4+ and CD8+ T cells that expressed CCR5 differed by hormonal contraception group (Figure 1). CD4+ T lymphocytes isolated from women who used the LNG-IUD expressed greater absolute proportions of CCR5 than women receiving OCPs (P < .01) and those receiving no contraception (P < .05). CD4+ T-cell CCR5 expression levels in women receiving DMPA did not differ significantly from those in the other contraceptive groups. Use of the LNG-IUD was associated with a 35% relative increase in the proportion of helper T cells that expressed CCR5 over that observed with the use of OCP and with a 29% increase, compared with the use of no contraception.
Figure 1.
Proportions of peripheral blood mononuclear cell (PBMC) and T-cell subpopulations that expressed CCR5 are shown as a function of contraceptive method. A, CD4+ helper T cells. B, CD8+ cytolytic T cells. C, CD4+ central memory T cells. D, CD4+ effector memory T cells. Data are mean values ± standard error of the mean.*P < .05 and **P < .01. Abbreviations: DMPA, depot medroxyprogesterone acetate; LNG-IUD, levonorgestrel intrauterine device; OCP, oral contraceptive pills.
Similarly, significantly greater absolute proportions of CCR5+CD8+ T cells were observed in participants in the LNG-IUD group than in women who received OCP (P < .05) or no contraception (P < .01). This corresponded to a 34% and 39% relative increase in CCR5 expression on CD8+ T cells from LNG-IUD participants, compared with participants receiving OCP or no contraception, respectively. The proportion of CCR5+CD8+ T cells in the DMPA-treated group did not differ significantly from that in other contraceptive groups. We did not observe significant differences in the proportion of monocytes or DCs that expressed CCR5 as a function of hormonal contraception group (Supplementary Figure 1). Consideration of DCs as separate mDC and pDC populations did not alter this observation (data not shown).
Statistically significant differences were observed when CD4+ TCM cell CCR5 expression in the LNG-IUD group was compared to that in the OCP group and the group that received no contraception (P < .01 for both comparisons). In CD4+ TEM, statistically significant differences were noted when the LNG-IUD group was compared to the OCP group and the group that received no contraception (P < .01 for both comparisons). The relative increases in CCR5 expression among LNG-IUD users, compared with OCP users and women who received no contraception, was 45% and 41%, respectively, among TCM cells and 38% and 35%, respectively, among TEM cells. No statistically significant differences in the proportions of TTD or TN cells that expressed CCR5 were seen across hormonal contraceptive groups (Supplementary Figure 1).
CD8+ T-cell CCR5 MFIs were significantly greater in the LNG-IUD group as compared to the no contraception group (P < .05). Significantly greater CD4+ T-cell CCR5 MFIs were noted with DMPA and LNG-IUD use, compared with no contraception use (P < .01 for both comparisons), and with DMPA and LNG-IUD, compared with OCP use (P < .001 for both comparisons). The relative magnitude of the statistically significant increases in CD4+ T-cell CCR5 MFI observed between contraceptive groups ranged from 7.1% to 10.6%. Statistically significant differences in CD4+ TCM cell and TEM cell CCR5 MFIs were observed when the DMPA and LNG-IUD groups were compared to the group that received no contraception (P < .01 for both comparisons) and when DMPA and LNG-IUD were compared to the OCP group (P < .001 for both comparisons). We did not observe significant differences in CCR5 MFI across treatment groups in CD4+ TTD or TN cells. The relative increases in the CCR5 MFI for the DMPA group over that for the OCP group and the group that received no contraception were 12% and 11%, respectively, for CD4+ TCM cells and 8% and 6%, respectively, for CD4+ TEM cells.
Monocyte and DC CCR5 MFIs did not significantly differ across groups (data not shown). We observed no statistically significant differences in the proportion of T cells, T-cell subsets, monocytes, or DCs that expressed CD4 or CXCR4 or in the median CD4/CXCR4 MFIs between contraceptive groups (data not shown). We further noted no difference in the CCR5 MFI between PBMC subpopulations that did or did not express CXCR4 (eg, CXCR4−CCR5+ vs CXCR4+CCR5+).
Sex Hormone and CCR5 Expression Levels
To investigate whether hormone levels correlated with CCR5 expression, we measured blood levels of estrogen, progesterone, MPA, and LNG (Supplementary Figure 2). We found no statistically significant correlation between MPA or LNG levels and the proportion of CD4+ T cells that expressed CCR5 (Supplementary Figure 3). We did not observe a correlation between CCR5 expression levels on CD4+ T cells and contraception treatment group with either progesterone or estradiol concentrations (data not shown). Similarly, no correlation was detected between T-cell subset CCR5 MFI, treatment group, and hormone concentrations.
DISCUSSION
HIV infection of target cells involves a competitive process between virus entry and inactivation. A shift in factors to favor entry, such as an increase in HIV receptor or coreceptor density, could subsequently increase the probability of transmission and infection following HIV exposure. We therefore used samples from 124 participants enrolled in the WIHS to investigate whether hormonal contraceptive use in at-risk, HIV-uninfected women was associated with greater surface expression of CD4, CCR5, or CXCR4.
No statistically significant differences in the proportions of T cells that expressed CCR5 were observed from DMPA recipients. Statistically significant increases in CCR5 expression were restricted to T cells from women who used the LNG-IUD. CD4+ and CD8+ T cells from LNG-IUD users expressed greater proportions of CCR5 than subjects receiving OCP or no contraception. Greater T-cell CCR5 expression was driven primarily by increases on central and effector memory T cells; terminally differentiated and naive T cells had unchanged surface CCR5 levels. The increased proportion of CD4+ T cells expressing CCR5 was accompanied by an increase in the amount of CCR5 expression, a statistically significant difference noted for CD4+ TCM cells and TEM cells from LNG-IUD and DMPA users, relative to women who received OCP or no contraception. CD4+ T cells are the primary HIV cellular targets; TCM cells and TEM cells are key components of the HIV reservoir in blood and tissue [11, 12].
Our observations suggest that progestin-only contraception can be associated with increased CCR5 expression in blood, although these increases did not correlate with hormone levels and suggests that the relationship we identified between CCR5 levels and progestin-based contraception use may be complex. Relative increases in the proportion of T cells from the LNG-IUD group that expressed CCR5 ranged from 30% to 50%, whereas MFI increases in the DMPA and LNG-IUD groups were on the order of 6%–12%. An explanation for why LNG release into the uterus would affect circulating CCR5 levels more so than systemic DMPA release is not immediately clear, although it is also possible that the IUD itself could induce a systemic change by an unknown mechanism. Studies document that use of the LNG-IUD results in detectable LNG blood concentrations, albeit at levels far lower than MPA levels achieved with intramuscular delivery of DMPA [13, 14]. The mechanism by which DMPA use increased CCR5 MFI but not the proportion of cells that expressed CCR5 requires additional investigation.
Our study has limitations. Hormonal contraception use was self-reported and recorded at 6-month intervals. The menstrual cycle phase, follicular or luteal, was not recorded at the time of blood specimen collection and could introduce additional variability into our measurements. Immune activation and levels of interleukin 2, interferon α, and CCL5 (RANTES) are known to modulate CCR5 expression levels, but these factors were not measured in this study. It is possible that between-group differences in latent chronic viral infections could contribute to our observations. We could not address the effect of hormonal contraception use on female genital tract tissue CCR5 expression, where virus first encounters target cells. We speculate that the detection of increased HIV coreceptor expression in blood might be a harbinger of changes in the hormone-responsive tissues of the vagina and cervix.
These data suggest that hormonal contraception use can affect T-cell CCR5 expression in at-risk, HIV-uninfected women, although we did not identify a direct correlation between hormone and coreceptor levels. The effect size we identified in DMPA-treated women was modest, whereas LNG-IUD–associated effect sizes on the proportion of T-cell CCR5 expression were larger. While the differences we observed were statistically significant, it remains unclear whether CCR5 density changes of this magnitude can appreciably influence HIV acquisition risk. In vitro studies suggest that doubling the number of CCR5 molecules per target cell increases HIV entry and infectivity; increases of 10%–50% have not been adequately studied [5, 15]. Further comparative work in female reproductive tract tissues and blood will be necessary to determine whether contraception-associated increases in CCR5 expression may influence HIV acquisition risk.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Women's Interagency HIV Study (WIHS) study sites (principal investigators; financial support) are as follows: Bronx WIHS (Kathryn Anastos; U01-AI-035004), Brooklyn WIHS (Howard Minkoff and Deborah Gustafson; U01-AI-031834), Chicago WIHS (Mardge Cohen; U01-AI-034993), Metropolitan Washington WIHS (Mary Young; U01-AI-034994), Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien; U01-AI-034989), WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub; U01-AI-042590), and the Southern California WIHS (Alexandra Levine and Marek Nowicki [U01-HD-032632 [WIHS I–WIHS IV]).
Disclaimer. Data in this manuscript were collected by WIHS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH and the National Institute of Allergy and Infectious Diseases (NIAID) (through a subcontract of U01 AI034994 [to A. M. N. T.]). The WIHS is funded primarily by the NIAID, with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the NIH Office of Research on Women's Health. WIHS data collection is also supported by the NIH Clinical and Translation Science Award UL1-TR000004 to the University of California–San Francisco.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sagar M, Kirkegaard E, Long EM et al. Human immunodeficiency virus type 1 (HIV-1) diversity at time of infection is not restricted to certain risk groups or specific HIV-1 subtypes. J Virol 2004; 78:7279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison CS, Chen PL, Kwok C et al. Hormonal Contraception and the Risk of HIV Acquisition: An Individual Participant Data Meta-analysis. PLoS Med 12:e1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis 2015; 15:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffron R, Donnell D, Rees H et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis 2012; 12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt EJ, Durnin JP, Kabat D. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J Virol 2005; 79:4347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangel HR, Weber J, Chakraborty B et al. Role of the human immunodeficiency virus type 1 envelope gene in viral fitness. J Virol 2003; 77:9069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marozsan AJ, Moore DM, Lobritz MA et al. Differences in the fitness of two diverse wild-type human immunodeficiency virus type 1 isolates are related to the efficiency of cell binding and entry. J Virol 2005; 79:7121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacon MC, von Wyl V, Alden C et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiroi M, Stanczyk FZ, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR Jr. Radioimmunoassay of serum medroxyprogesterone acetate (Provera) in women following oral and intravaginal administration. Steroids 1975; 26:373–86. [DOI] [PubMed] [Google Scholar]
- 10.Stanczyk FZ, Hiroi M, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR Jr. Radioimmunoassay of serum d-norgestrel in women following oral and intravaginal administration. Contraception 1975; 12:279–98. [DOI] [PubMed] [Google Scholar]
- 11.Chomont N, El-Far M, Ancuta P et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yukl SA, Gianella S, Sinclair E et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis 2010; 202:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception 2009; 80:84–9. [DOI] [PubMed] [Google Scholar]
- 14.Seeber B, Ziehr SC, Gschliesser A et al. Quantitative levonorgestrel plasma level measurements in patients with regular and prolonged use of the levonorgestrel-releasing intrauterine system. Contraception 2012; 86:345–9. [DOI] [PubMed] [Google Scholar]
- 15.Johnston SH, Lobritz MA, Nguyen S et al. A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J Virol 2009; 83:11016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.