Abstract
Self-reported adherence to pre-exposure prophylaxis (PrEP) has limitations, raising interest in pharmacologic monitoring. Drug concentrations in hair and dried blood spots (DBS) are used to assess long-term-exposure; hair shipment/storage occurs at room temperature. The iPrEx Open Label Extension collected DBS routinely, with opt-in hair collection; concentrations were measured with liquid chromatography/tandem mass spectrometry. In 806 hair-DBS pairs, tenofovir (TFV) hair levels and TFV diphosphate (DP) in DBS were strongly correlated (Spearman coefficient r = 0.734; P < .001), as were hair TFV/DBS emtricitabine (FTC) triphosphate (TP) (r = 0.781; P < .001); hair FTC/DBS TFV-DP (r = 0.74; P < .001); hair FTC/DBS FTC-TP (r = 0.587; P < .001). Drug detectability was generally concordant by matrix. Hair TFV/FTC concentrations correlate strongly with DBS levels, which are predictive of PrEP outcomes.
Keywords: HIV prevention, pre-exposure prophylaxis, PrEP, pharmacologic monitoring, adherence, tenofovir/emtricitabine, hair concentrations, dried blood spot (DBS) concentrations, iPrEx Open Label Extension (OLE)
The utility of pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) prevention has been demonstrated in multiple trials. The efficacy of PrEP is highly dependent on adherence to the tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC)–based regimen. However, adherence assessed via self-report or pill counts inconsistently predicts prevention outcomes [1, 2]. Pharmacologic exposure measures, wherein drug levels are monitored in a biomatrix such as plasma, peripheral blood mononuclear cells (PBMCs), or dried blood spots (DBS), are of significant interest to the field [3]. Indeed, substantial discordance between self-reported measures of adherence and drug detection in PrEP trials, such as the global iPrEx study [4], FEM-PrEP [1], Partners PrEP [5, 6], and the Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial [2], have highlighted the critical need to incorporate objective pharmacologic exposure measures into PrEP demonstration projects and real-world settings.
Plasma or PBMC concentrations of tenofovir (TFV)/FTC or their phosphorylated forms have been most frequently incorporated into PrEP trials as pharmacologic measures [1, 2, 7]. Measurements of plasma antiretroviral concentrations represent more recent dosing (eg, 1–14 preceding days, depending on assay sensitivity) [6] and may be susceptible to “white-coat effects,” wherein adherence improves transiently before visits [8]. Exposure measures for drugs with longer half-lives, such as TFV and its phosphorylated forms, would ideally provide information over prolonged periods. For example, TFV diphosphate (DP) levels in PBMCs [4, 9] relay information on exposure over longer periods (7–14 days), although processing, isolating and counting PBMCs are costly and technically challenging. DBS—made from whole blood—are easier to collect and process than PBMCs [10], and drug levels in DBS (which consist mainly of red blood cells), provide longer-term exposure information (half-life 17 days) [10]; DBS assays, however, require standardization against hematocrit abnormalities for interpretation. Importantly, TFV or TFV-DP concentrations in all 3 matrices (plasma, PBMCs, and DBS) have shown strong relationships with the protective efficacy of PrEP in either global iPrEx [4, 9] or the Open Label Extension follow-up study (iPrEx OLE) [11].
Given the need for low-cost, feasible, longer-term measurements of exposure for PrEP, analyzing concentrations of TFV/FTC in small hair samples has been of emerging interest. Hair collection is noninvasive, nonbiohazardous, and does not require phlebotomy or cold storage, and hair levels reflect uptake from the systemic circulation over weeks to months. Hair concentrations of TFV are strongly and linearly related to dose [12], and hair levels of both TFV and FTC correlate with plasma and PBMC concentrations [3]. Hair and DBS collection both provide feasibility advantages in resource-limited settings, and concentrations in these 2 matrices represent longer-term exposure, although the correlation between hair and DBS measures is unknown. We report for the first time the correlation between drug levels and concordance of drug detection in hair and DBS in a large cohort receiving PrEP.
METHODS
Participants and Sample Collection
The iPrEx Open Label Extension (OLE) enrolled 1603 HIV-negative men who have sex with men and transgender women previously enrolled in 3 PrEP trials [11], of whom 1225 initiated PrEP. Visits were performed at baseline and at weeks 4, 8, 12, 24, 36, 48, 60, and 72. Whole blood was routinely collected for DBS preparation, as described elsewhere [10], at 4 and 8 weeks after PrEP initiation and then every 12 weeks. Hair samples were collected from consenting participants who opted in for this substudy every 12 weeks after PrEP initiation by cutting approximately 100 strands of hair as close as possible to the scalp in the occipital region and labeling the distal end. As described elsewhere [3], each hair sample is stored and shipped in aluminum foil at room temperature. The study protocol was approved by Institutional Review Boards of all participating sites, and all participants provided written informed consent in their preferred language.
Laboratory Methods
The DBS from all relevant visits were stored at −20°C within 24 hours of collection and shipped on dry ice to our University of Colorado laboratory. At the time of analyses, a 3-mm DBS punch sample was extracted and analyzed for TFV-DP and FTC-triphosphate (TP) concentrations by validated liquid chromatography/tandem mass spectrometry (LC/MS-MS) methods, as described elsewhere [10]. The dynamic range of the assay was 2.5–2000 fmol per sample for TFV-DP and 0.1–200 pmol per sample for FTC-TP.
After storage and shipment at ambient temperature to our University of California, San Francisco laboratory, the proximal 1.5 cm of each hair samples (representing approximately 6 weeks of exposure) was cut finely with scissors and 5 mg was processed and analyzed using LC/MS-MS as described elsewhere [3, 12]. These assays have been validated from 0.002 to 0.400 ng/mg hair for TFV and from 0.02 to 4 ng/mg for FTC [12]. Of note, our assays for measuring TFV and FTC in hair samples have been peer reviewed and approved by the Division of AIDS Clinical-Pharmacology and Quality-Assurance (CPQA) program.
Statistical Analysis
Spearman correlation coefficients and scatterplots were examined to assess relationships between TFV and FTC levels in hair and TFV-DP and FTC-TP concentrations in red blood cells measured via DBS. The concordance of drug detection for each combination was also determined for paired samples.
RESULTS
Hair Collection in iPrEx OLE
Concentration results from 806 paired hair-DBS samples from 217 participants were available for comparison. Among 1603 HIV-negative individuals enrolled in iPrEx OLE, DBS were analyzed among all seroconverters and a random sample of seronegative participants, as described elsewhere [11]. All available hair and DBS samples from seroconverters and the random sample of non-seroconverters were used in the present analysis. Although 75% of participants in the overall study agreed to hair sampling, not enough hair samples were collected from the seroconversion visit in the 28 participants who acquired HIV during PrEP to perform a meaningful analysis of the protective effect of hair concentrations on HIV acquisition (12 declined or didn't have enough hair, 6 had hair collected but only at HIV RNA–negative time points; only 10 had hair collected at HIV RNA–positive time points).
Correlation Between DBS and Hair Drug Concentrations
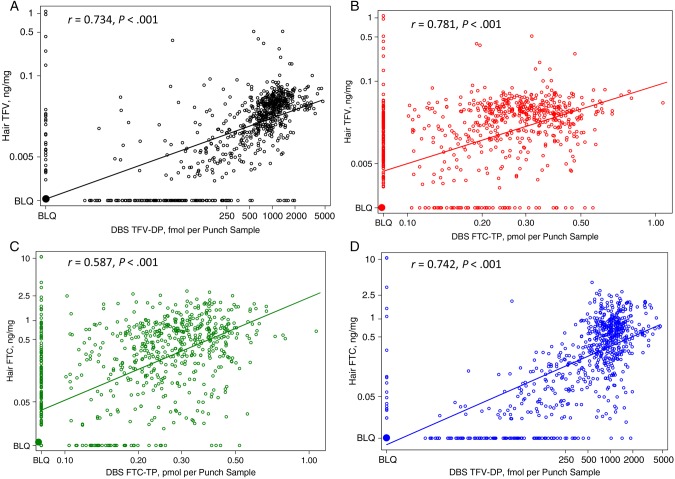
Scatterplots of the correlation between hair TFV or FTC levels and DBS TFV-DP or FTC-TP levels in the 806 paired samples are depicted in Figure 1. TFV concentrations in hair and TFV-DP levels in DBS were strongly correlated (r = 0.734; P < .001), as were hair TFV levels and DBS FTC-TP concentrations (r = 0.781; P < .001). FTC concentrations in hair were more strongly correlated with DBS TFV-DP levels (r = 0.742; P < .001) than with DBS FTC-TP levels (r = 0.587; P < .001).
Figure 1.
Scatterplots showing correlations between hair tenofovir (TFV) and dried blood spot (DBS) TFV-diphosphate (DP) levels (A); hair TFV and DBS emtricitabine (FTC)-triphosphate (TP) levels (B); hair FTC and DBS FTC-TP levels (C); and hair FTC and DBS TFV-DP levels (D). Abbreviation: BLQ, below limit of quantification.
Levels of TFV/ FTC Hair Concentrations Estimated to Confer Protective Efficacy
In iPrEx OLE [11], the protective levels of TFV-DP in DBS were quantified as 700 fmol per punch sample. From the correlation analysis, these DBS levels correspond to a median hair TFV concentration of 0.023 ng/mg (95% confidence interval [CI], .019–.025) and a hair FTC concentration of 0.37 ng/mg (95% CI, 28–.58).
Concordance of Detection of Hair and DBS Drug Levels
A comparison of detectability of the relevant antiretroviral or metabolite in the 2 matrices is shown in Table 1. Concentrations of drugs were mostly concordant in terms of detectability, with concordance of 82% for hair TFV and DBS TFV-DP levels, 90% for hair TFV and DBS FTC-TP levels, 81% for hair FTC and DBS FTC-TP levels, and 85% for hair FTC and DBS TFV-DP levels.
Table 1.
Concordance of Drug Detection of PrEP Drugs and Their Metabolites in Hair and DBS (806 Paired Samples)
| Drug Level | Detectable in Hair and DBS, % | Detectable in Hair but Not DBS, % | Detectable in DBS but Not Hair, % | Undetectable in Hair or DBS, % |
|---|---|---|---|---|
| TFV in hair, TFV-DP in DBS | 72 | 3 | 15 | 10 |
| TFV in hair, FTC-TP in DBS | 71 | 5 | 5 | 19 |
| FTC in hair, FTC-TP in DBS | 62 | 14 | 5 | 19 |
| FTC in hair, TFV-DP in DBS | 74 | 2 | 13 | 11 |
Abbreviations: DBS, dried blood spots; DP, diphosphate; FTC, emtricitabine; PrEP, pre-exposure prophylaxis; TFV, tenofovir; TP, triphosphate.
DISCUSSION
Given that pharmacologic measures of drug exposure have been crucial for trial interpretation in several PrEP studies [1, 2, 4], the incorporation of objective markers of drug adherence/exposure into PrEP settings is of growing interest. Easily stored samples that do not require a cold chain, such as hair, could be useful for drug monitoring in resource-constrained settings. Our study showed strong and significant correlations between concentrations of PrEP drugs in hair and DBS, as well as high levels of concordance (>80% for all combinations) for drug detectability in the 2 matrices. The current study is the first to analyze correlations between antiretroviral concentrations in hair and DBS.
Although plasma measures of TFV and/or FTC have been most commonly analyzed in PrEP trials [1, 2, 4–7], drug concentrations in plasma represent recent drug ingestion (eg, yes/no for taking drug or not). However, TFV and its moieties exhibit long half-lives in various matrices, enabling assessment of longer-term measures of cumulative drug exposure, such as those in DBS [10] and hair, that may be more relevant for PrEP monitoring. PBMC measurements of TFV-DP/ FTC-TP have proved useful [9], but PBMCs are generally difficult to collect, process, and transport. DBS are easy to prepare from whole-blood samples [10], although they must be shipped and stored at cold temperatures to allow measurement. Hair samples are easy to collect in real-world settings, and samples are stored and shipped at room temperature under nonbiohazardous conditions. However, it should be noted that current assays to quantify drug levels for PrEP, including those in both DBS and hair, use LC/MS-MS, which requires expensive equipment. Widespread applicability of biologic adherence measures in the context of PrEP rollout will rely on the availability of lower-cost methods to analyze drug levels, currently in development for hair assays [13].
Although whole blood was routinely collected at all time points in iPrEx OLE, hair collection was performed only on an opt-in basis; sampling required participants to sign an extra consent form and was only performed quarterly. The lack of hair sampling at relevant visits for participants who seroconverted during PrEP impeded our ability to directly analyze this measure in relationship to HIV seroconversion. However, from the correlation data [11], we can estimate that hair TFV concentrations of 0.023 ng/mg (95% CI, .019–.025 ng/mg) and hair FTC concentrations of 0.37 ng/mg (95% CI, .28–.58 ng/mg) are likely to confer protective benefit in the context of PrEP. The TFV hair concentration estimated to confer protection in this study is similar to that achieved by volunteers taking 4 doses of TFV per week in a separate study (median value, 0.023 ng/mg) [12].
Hair collection has been shown to be feasible and acceptable (>95%) among HIV-infected or at-risk individuals in Africa [3, 14]. A recent study from rural Kenya showed that community information campaigns and field staff training early in protocol development was likely to enhance hair collection rates [14]. A qualitative study from South Africa on the acceptability of hair collection underscored this point [15]. Of note, TFV concentrations in pubic hair samples were not reliably associated with dose in another study [12], which corroborates other findings that pubic hair, with its erratic growth rates and urine contamination, is not an informative biomatrix.
Two phase II PrEP studies in Africa collected multiple biologic samples for exposure monitoring (plasma, PBMCs, and hair) and correlated drug levels in hair with drug or metabolite concentrations in plasma and PBMCs [3]. These studies did not collect DBS. In these phase II PrEP studies, the correlation between TFV levels in hair and plasma after 8 weeks was moderate (r = 0.41), as was the correlation between FTC levels in hair and plasma (r = 0.51). The coefficients for correlation between TFV or FTC levels in hair and their relevant phosphorylated concentrations in PBMCs at 8 weeks were similar (r = 0.43 and r = 0.50, respectively). In the current study, the correlation between TFV concentrations in hair and TFV-DP levels in DBS (both longer-term measures of exposure) was high (r = 0.734), and DBS measures of TFV-DP in the overall cohort were strongly associated with protective efficacy [11], implying that TFV hair levels may also have predicted efficacy.
The more moderate correlation observed between hair FTC hair and DBS FTC-TP levels may reflect the relatively short half-life of FTC-TP in DBS (approximately 30 hours). The discordance between drug detection in hair and DBS in 19% of pairs could be secondary to differential half-lives of TDF/FTC-based PrEP in different matrices; another study will examine the elimination kinetics of TFV in hair and DBS in the context of directly observed therapy (ClinicalTrials.gov, NCT02022657). Undetectable drug concentrations in hair (25% of samples for TFV) suggest no appreciable dosing over a significant duration before a visit.
The current study adds to the growing literature around the utility of hair monitoring in TDF/FTC-based PrEP. A large ongoing PrEP demonstration trial in 3 US cities has incorporated multiple pharmacologic measures of drug exposure, including hair assays, and will provide further data on the utility of hair measures in monitoring adherence. Future studies of pharmacologic monitoring in PrEP should probably examine the predictive utility of multiple measures of exposure simultaneously, because each measure reflects a different duration of exposure. Given the well-acknowledged limitations of self-report in monitoring adherence to PrEP, novel biologic measures of assessing exposure, including hair assays, should be further examined in real-world settings.
Notes
Acknowledgments. We thank the participants in the iPrEx OLE study and the dedicated study staff. We would also like to thank other members of the iPrEx study team leadership, including Javier Lama, Mauro Schechter, Esper G. Kallas, Valdilea Veloso, Kenneth Mayer, Susan Buchbinder, Orlando Montoya, Rivet Amico, Kim Koester, Kathy Mulligan, and Martin Casapia. We thank Leslie Z. Benet, PhD, Yong Huang, PhD, Shirley Yee, Karen Kuncz, and Alexander Louie in the Hair Analysis Laboratory at the University of California, San Francisco, for further work on the hair assays. We thank Ruth Greenblatt, Phyllis Tien, and Bradley Aouizerat, principal investigators of the San Francisco Women's Interagency HIV Study (WIHS) site, for the use of WIHS specimens as controls for the assays for this study.
Financial support. This work was supported by the Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, as a cooperative agreement (UO1 AI84735 to P. L. A., UO1 AI64002, 2R01 AI062333 to R. M. G., and UM1 AI068619). The NIAID also supported the hair assays for this work (R01 AI098472 [principal investigator M. G. with support to H. H.] and U01 AI034989 [San Francisco WIHS, principal investigator Ruth Greenblatt] for hair assay development work).
Potential conflicts of interest. Gilead Sciences donated emtricitabine/tenofovir disoproxil fumarate for participants in the study but provided no other financial support and did not contribute to data interpretation or manuscript development. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the iPrEx Study Team, Javier Lama, Mauro Schechter, Esper G. Kallas, Valdilea Veloso, Kenneth Mayer, Susan Buchbinder, Orlando Montoya, Rivet Amico, Kim Koester, Kathy Mulligan, Martin Casapia, Leslie Z. Benet, Yong Huang, Shirley Yee, Karen Kuncz, Alexander Louie, San Francisco, Ruth Greenblatt, Phyllis Tien, and Bradley Aouizerat
References
- 1.Van Damme L, Corneli A, Ahmed K et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrazzo JM, Ramjee G, Richardson BA et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxi SM, Liu A, Bacchetti P et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr 2015; 68:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnell D, Baeten JM, Bumpus NN et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 8.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance" limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials 2008; 9:238–46. [DOI] [PubMed] [Google Scholar]
- 9.Anderson PL, Glidden DV, Liu A et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-Mancilla JR, Zheng JH, Rower JE et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant RM, Anderson PL, McMahan V et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu AY, Yang Q, Huang Y et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014; 9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi M, Yang Q, Bacchetti P, Huang Y. Short communication: a low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses 2014; 30:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey MD, Salmen CR, Tessler RA et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 2014; 66:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coetzee B, Kagee A, Tomlinson M, Warnich L, Ikediobi O. Reactions, beliefs and concerns associated with providing hair specimens for medical research among a South African sample: a qualitative approach. Future Virol 2012; 7:1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]