Abstract
Mammalian with-no-lysine [K] (WNK) kinases are a family of four serine-threonine protein kinases, WNK1-4. Mutations of WNK1 and WNK4 in humans cause pseudohypoaldosteronism type II (PHA2), an autosomal-dominant disease characterized by hypertension and hyperkalemia. Increased Na+ reabsorption through Na+-Cl− cotransporter NCC in the distal convoluted tubule plays an important role in the pathogenesis of hypertension in patients with PHA2. However, how WNK1 and 4 regulate NCC and how mutations of WNKs cause activation of NCC have been controversial. Here, we review current state of literature supporting a compelling model that WNK1 and WNK4 both contribute to stimulation of NCC. The precise combined effects of WNK1 and WNK4 on NCC remain unclear, but likely are positive rather than antagonistic. The recent discovery that WNK kinases may function as an intracellular chloride sensor adds a new dimension to the physiological role of WNK kinases. Intracellular chloride-dependent regulation of WNK’s may underlie the mechanism of regulation of NCC by extracellular K+. Definite answer yet will require future investigation by tubular perfusion in mice with altered WNK kinase expression.
Keywords: WNK1, WNK4, NCC, NKCC2, Kelch-like, Cullin-3
Introduction
Mammalian WNK (with-no-lysine [K]) kinases are a family of four serine-threonine protein kinases, WNK1-4, with an atypical placement of the catalytic lysine (46). Mutations of two members, WNK1 and WNK4, in humans cause pseudohypoaldosteronism type II (PHA2), an autosomal-dominant disease characterized by hypertension and hyperkalemia (44). Mutations in the WNK1 gene are large deletions of the first intron leading to increased expression. Mutations in the WNK4 gene are missense mutations in the coding sequence outside the protein kinase domain. WNK1-4 are products of different genes that contain a conserved kinase domain in the amino terminus (38, 44, 46). WNK1 is widely expressed in multiple spliced forms (46). The full-length WNK1 is produced from the entire 28 exons (referred as “long WNK1”; L-WNK1) and has a ubiquitous distribution (44, 46). A shorter WNK1 transcript encoding a polypeptide lacking the amino terminal 1-437 amino acids of the long WNK1 is highly expressed in the kidney (referred to as KS-WNK1 for kidney-specific; KS-WNK1) (9, 22). The KS-WNK1 is produced by replacing the first 4 exons with an alternative exon 4A, which encodes 30 amino acids unique to KS-WNK1. The remaining exons 5 through 28 are the same as the long transcript.
Based on results of studies over the past 12+ years, it is generally accepted that increased Na+ reabsorption through Na+-Cl− cotransporter NCC in the distal convoluted tubule (DCT) and Na+-K+-2Cl− cotransporter NKCC2 in thick ascending limb of Henle’s loop (TAL) plays important pathogenic mechanism for the phenotype of hypertension in patients with PHA2 (18). However, how WNK1 and 4 regulate NCC and NKCC2 and how mutations of WNKs cause activation of the cotransporters have been highly controversial. Here, we review current state of literature on these subjects and discuss a unifying mechanism(s) for WNK kinases regulation of NCC- and NKCC2-mediated Na+ transport and by which dysregulation in PHA2 causes hypertension.
WNK4 as a negative versus positive regulator of NCC and the mechanism of regulation by WNK1
Using Xenopus oocyte expression system, initial studies by several groups reported that WNK4 is a direct inhibitor of NCC independently of its kinase activity and that WNK1 activates NCC by antagonizing WNK4 inhibition of NCC (45, 47, 48). The decrease in NCC activity caused by WNK4 was due to decreased cell-surface protein abundance. These initial findings of inhibition of NCC by WNK4 were supported by several subsequent studies using mammalian cultured cells (4). Bacterial artificial chromosome (BAC) transgenic mice overexpressing WNK4 showed hypotension and decreased NCC abundance further supports the notion that WNK4 is an inhibitor of NCC in vivo (15).
In contrast, biochemical studies have shown that WNK1 and WNK4 phosphorylate and activate oxidative-stress response kinase-1 (OSR1) and related ste20-related proline/alanine-rich kinase (SPAK). Activated OSR1 and SPAK can phosphorylate NCC and other SLC12 family members of transporter, NKCC1 and NKCC2, and increase their function in cells by stimulating the intrinsic activity and/or surface abundance (1, 20, 27, 41, 42). The notion that WNK1 and 4 both stimulates NCC and NKCC1/2 via phosphorylation and activation of intermediate kinases OSR1 and/or SPAK in vivo were supported by studies showing that Wnk4-null mice have hypotension and decreased NCC abundance in the kidney and diminished urinary excretion of Na+ in response to hydrochlorothiazide (5, 33). Wnk4-null mice also have decreased levels of phosphorylated SPAK in the kidney. Moreover, Spak knockout mice exhibited decreased NCC abundance and activity and that deletion of Spak abrogated hypertension and Na+ retention in a mouse model of human PHA2 created by knockin of PHA-causing Wnk4 mutation (8, 49, 50). Overall, these studies provide compelling results to oppose the results suggesting that WNK4 inhibits NCC and to support the notion that both WNK1 and WNK4 stimulate NCC in vivo.
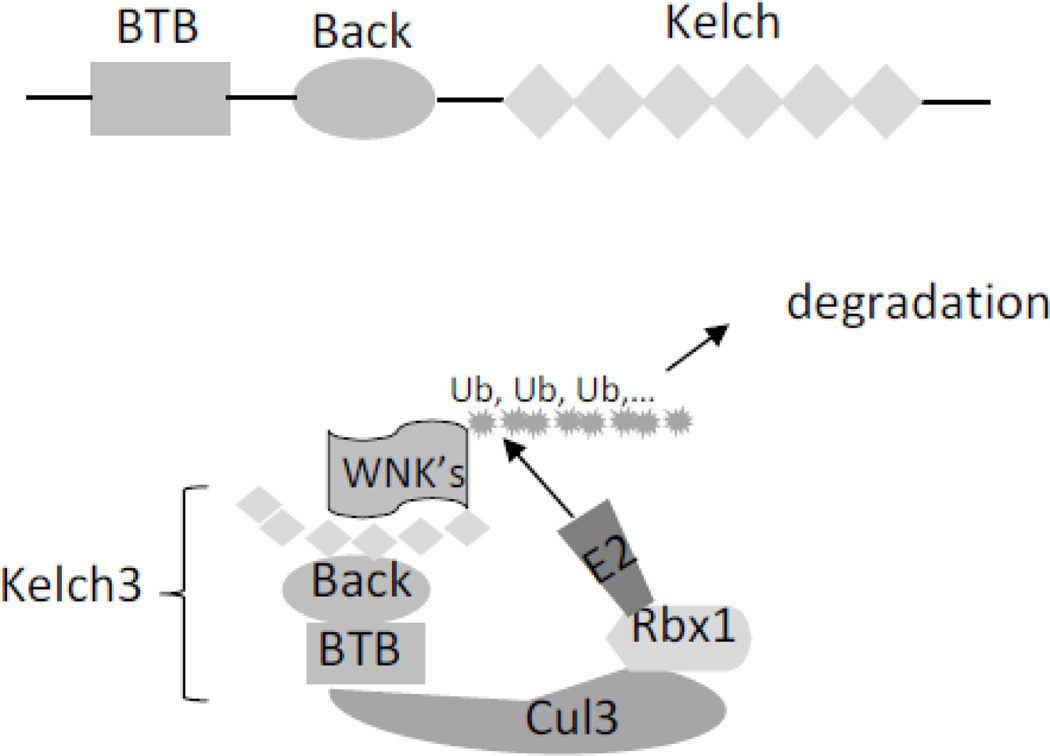
Kelch3 and cul3 regulate ubiquitination and degradation of WNKs providing support for a revised model that WNK1 and 4 both stimulate NCC
While mutations of WNK1 and WNK4 are recognized important causes of PHA2, only ~70% affected PHA2 individuals could be explained by mutations of these two genes (11). Two recent studies reported that mutations of KLHL3 and CUL3 also cause PHA2 (3, 16). KLHL3 and CUL3 encode kelch-like 3 (kelch3) and cullin3 (cul3) protein, respectively. Cul3 is one of the six cullins involved in the degradation of intracellular proteins by ubiquitination (10). Ubiquitin ligase including cul3-based cullin-RING ubiquitin ligases, also known as E3 ligases, is a key element in the ubiquitin or proteasome system that transfers ubiquitin moieties to substrates. Cul3 serves as a scaffold for the catalytic module of a RING finger protein (Rbx1) and a ubiquitin-conjugating enzyme (E2). Cul3 also serves as a scaffold for substrate adaptor proteins containing BTB domains that facilitate protein–protein interaction. The kelch-like (KLHL) proteins are a family of more than 40 substrate adaptor proteins that can function to connect cul3-RING ubiquitin ligase to its substrates (13). KLHL proteins contain one BTB domain, one BTB and C-terminal kelch (Back) domain, and five to six kelch domains. Each kelch domain forms one blade of a 6-bladed β-propeller structure, which is also involved in the protein–protein interaction. Kelch3 binds to cul3-RING ubiquitin ligase via BTB domain and to its substrate WNK kinases via the β-propeller structured-kelch domains (Figure 1).
Fig. 1.
Ubiquitination and degradation of WNK kinases by klech3-cul3 complex. Kelch3 protein contains one BTB domain, one BTB and C-terminal kelch (Back) domain, and six kelch domains. Kelch3 binds to cul3-RING ubiquitin ligase (consisting of a RING finger protein Rbx1 and E2 ubiquitin-conjugating enzyme) via BTB domain and to its substrate WNK kinases via kelch domains. Ubiquitination of WNK kinases leads to their degradation.
Findings that mutations of KLHL3 and CUL3 predicted to impair degradation of WNK kinase also cause PHA2 provide strong support for the notion that WNK1 and 4 stimulate NCC (3, 16, 30, 43). To further support this notion, knockin mice carrying PHA2-mimicking mutations in KLHL3 have increased abundance of WNK4 proteins and increased NCC activity (32). Interestingly, PHA2 patients with mutations in KLHL3 or CUL3 have relatively more severe phenotypes than those with WNK1 or WNK4, which can be explained by the notion that mutations in KLHL3 or CUL3 increase the abundance of both WNK1 and WNK4 (32). Overall, the preponderant data including many in vivo studies indicate that WNK1 and WNK4 separately each functions as activator of NCC. In contrast, data supporting that WNK4 inhibits NCC are almost entirely based on in vitro expression system with only one exception (15). Because WNK kinases activate NCC indirectly through SPAK and OSR1, forced expression of WNK1 or WNK4 in cell-based expression system conceivably may cause opposite results via dominant-negative effects. Additional explanation for inhibitory effect of WNK kinases under the condition of high intracellular Cl− concentration has also been proposed (see below). The only study reporting WNK4 inhibits NCC in vivo using BAC transgenic mice (15) has been disputed by an independent study by Uchida’s group (43), in which they reported that overexpression of WNK4 using the same WNK4- containing BAC clone lead to activation, not inhibition, of NCC.
Relationship between WNK1 and WNK4 regulation of NCC
Another controversy surrounding WNK kinase regulation of NCC is the relationship between WNK1 and WNK4. The initial study by Yang et al reported that WNK1 did not regulate NCC by itself but antagonizes WNK4 inhibition of NCC (47, 48). Recent study showed that there are multiple L-WNK1 isoforms with alternative splicing of exons 9, 11, 12 and 26 (39). L-WNK1 variant lacking only exon 11 (WNK1Δ11) is the most dominant form in the kidney. WNK1Δ11 activates NCC in the in vitro assay (6). The study by Yang et al used a L-WNK1 variant lacking both exons 11 and 12 (WNK1Δ11-12), which is relatively less abundant in the kidney tissue (6, 39). Compared to WNK1Δ11, WNK1Δ11-12 is much less potent in activating NCC in vitro (6). Moreover, the WNK1 cDNA construct used by Yang et al carried a point mutation that inactivates WNK1 stimulation of NCC (6), perhaps explaining the negative results reported by Yang et al (47, 48). Hadchouel et al recently showed that mice with increased WNK1 expression mimicking human PHA2 disease have increased NCC activity and concluded that WNK1 does activate NCC in vivo via SPAK (40). WNK1 and 4 interact and form heteromultimers in vitro (6, 35). To reconcile with those early reports that WNK4 is inhibitory to NCC and in keeping with the notion that WNK1 activates NCC, a more recent report by Hadchouel, Gamba, and coworkers proposed a revised model that WNK1 activates SPAK to activate NCC in DCT, and that WNK4 working upstream to antagonize WNK1 activation of NCC (6). The conclusion in the latter report that WNK4 antagonizes WNK1 was based on that Wnk4-KO did not reduce the activation of NCC caused by increased WNK1 expression in mice and that WNK4 forms heteromultimers with WNK1 to inhibit its function in vitro. This conclusion, however, did not take into account that several independent studies including results shown in their study have shown that NCC is markedly downregulated in Wnk4-KO mice (5, 6, 33). Wnk4-KO should not lead to downregulation of NCC if WNK4 works upstream of WNK1 to inhibit NCC.
A unifying model of WNK kinase regulation of NCC
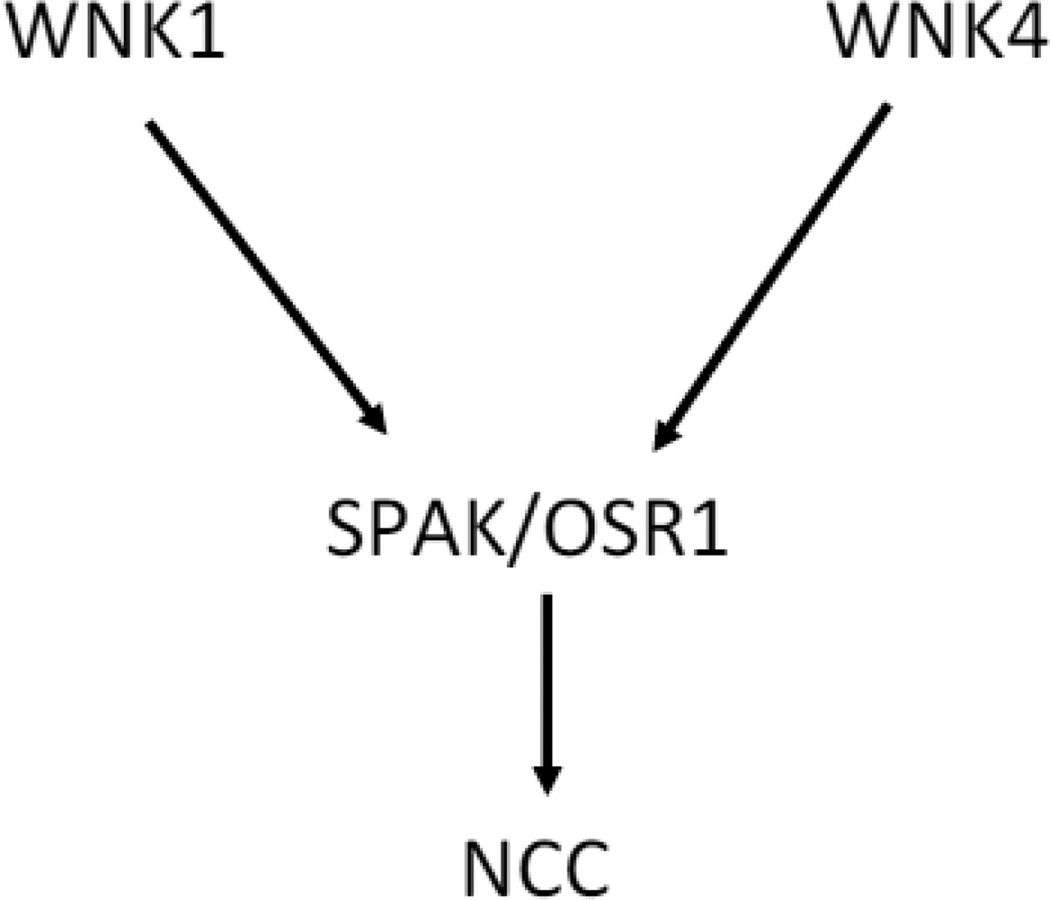
Overall, current available data strongly support a model that WNK1 and 4 both contribute to stimulation of NCC via OSR1/SPAK in vivo (Figure 2). This model is based on results of in vivo studies showing that loss-of-function of WNK4 or SPAK as in Wnk4-KO or Spak-KO, respectively, reduces NCC activity (5, 33, 49) and that gain-of-function of WNK4 as in mice overexpressing WNK4 or knockin mice carrying PHA2-mimicking mutations of Wnk4 or Klhl3 increases NCC activity (32, 43, 50). Moreover, mice overexpressing WNK1 generated by PHA2-mimicking deletion of the first intron of Wnk1 exhibit increased NCC activity (40). Regarding the results of the study by Hadchouel, Gamba, and coworkers (6), because both WNK1 and 4 activate NCC through the same OSR1/SPAK signaling cascade, increased function of WNK1 may activate OSR1/SPAK to an (maximal) extent such that loss-of-function of WNK4 does not affect the total activation of NCC. While WNK kinases may form heteromultimers in vitro (6, 35), it is not known whether it occurs in vivo. Thus, the precise combined effects of WNK1 and WNK4 in vivo remain unknown, but are likely positive (meaning mathematically additive, sub-additive or synergistic rather than antagonistic), and conceivably may depend on the ratio of WNK kinases versus SPAK/OSR1 in each setting. Among other WNK isoforms, WNK3 is also expressed in the kidney while WNK2 is not. In vitro, WNK3 is a potent activator of NCC (29). Mutations of WNK3 have not been reported in patients with PHA2. Wnk3-KO mice have no obvious phenotype, but may be due to compensatory upregulation of WNK1/4 (21). The role of WNK3 in regulating NCC in vivo remains unclear.
Fig. 2.
Model illustrating WNK1 and WNK4 both contribute to stimulation of NCC via OSR1/SPAK. This model is based on results of multiple studies performed in vivo showing that gain-of-function of WNK1 or WNK4 stimulates NCC and loss-of-function of WNK4 or SPAK inhibits NCC. The role of WNK3 in vivo remains to be defined. The precise relationship between WNK1 and WNK4 regulation of NCC is also unknown, but is likely positive (i.e., mathematically additive, sub-additive or synergistic) rather than antagonistic toward to each other (see text for details). Similarly, WNK1 and 4 likely also both contribute to stimulation of NKCC2 in TAL via OSR1/SPAK (not shown).
Regulation of catalytic activity of WNK kinases by intracellular Cl−
It has been long known that the activity NKCC1, a member of SLC12 family of transporters also including NKCC2 and NCC, is regulated by the intracellular Cl− (17). In shark rectal glands, increases in the intracellular Cl− concentration ([Cl−]i) inhibits while decreases in [Cl−]i stimulates NKCC1 (17). Using low chloride hypotonic stress in Xenopus oocyte expression system, Ponce-Coria et al first implicated that WNK3 is an intracellular Cl− sensor for regulation of NKCC2 by [Cl−]i (25). Piala et al recently reported that two key leucine residues (L369 and L371) in the DLG motif within the kinase domain of WNK1 form a chloride-binding site and that WNK1 autophosphorylation and activity are inhibited when chloride is bound to the site (23). The structure of kinase domain and these DLG motif leucine residues are conserved among all WNK kinases. This seminal discovery has spurred many studies to investigate the role of [Cl−]i in WNK kinase regulation of NCC. Bazua-Valenti et al reported that WNK1, WNK3 and WNK4 are all regulated by [Cl−]i with the affinity profile of WNK4 > WNK1 > WNK3 (2). These authors proposed that the function of WNK4 to inhibit or stimulate NCC may be dependent on [Cl−]i and that [Cl−]i-dependent regulation of activity of WNK4 may explain opposing effect of WNK4 on NCC in different reports. In high [Cl−]i when WNK4 is not active, WNK4 overexpression may cause inhibition of NCC via dominant-negative effects on other WNKs and/or SPAK/OSR1 present endogenously. Conversely, WNK4 overexpression will stimulate NCC under the condition of low [Cl−]i. As discussed above, multiple findings from in vivo mouse models that gain- and loss-of-function of WNK4 increases and decreases NCC activity, respectively, yet indicate that, in the basal condition in mice, WNK4 is stimulatory with respect to the regulation of NCC.
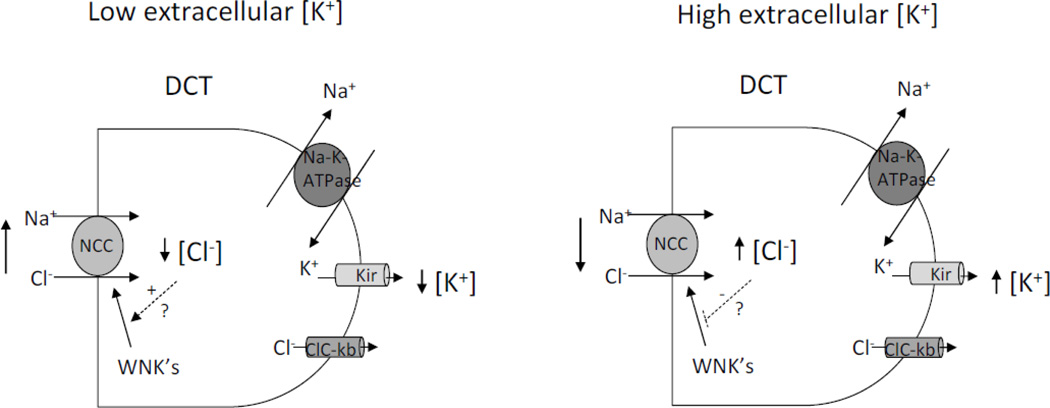
K+ secretion through K+ channels in the aldosterone-sensitive distal nephron involves exchange with Na+ reabsorption through the epithelial Na+ channel ENaC. The activity of NCC in DCT can affect Na+ delivery to ENaC in the downstream segments thus regulating K+ secretion. Studies have shown that dietary K+ loading downregulates NCC, which would increase Na+ delivery to ENaC thus enhancing K+ secretion (31, 37). The effect of K+ loading on NCC is not mediated by aldosterone because the effect is opposite to the expected effect of aldosterone on NCC. An increase in plasma aldosterone enhances the abundance of NCC (14). Moreover, downregulation of NCC by K+ loading persists in mice with defective synthesis of aldosterone and in mice with knockout of SGK1, a downstream mediator of aldosterone (31, 37). Of note, aldosterone stimulates Na+ reabsorption and K+ wasting while an increase in WNK kinase activity as in PHA2 causes Na+ reabsorption and yet K+ retention, an effect on K+ secretion in opposite to that in primary hyperaldosteronism. The contrasting effect of WNKs versus aldosterone on K+ secretion raises an interesting possibility that WNK kinase cascade may be the mechanism for regulation of NCC by dietary K+ intake. How could K+ regulate WNK kinases? Terker et al recently proposed a potentially interesting mechanism for K+ regulation of NCC via WNK kinases (34). They demonstrated that lowering extracellular [K+] is associated with a decrease in [Cl−]i, likely due to the effect on membrane potential and transmembrane Cl fluxes (34). Furthermore, the decrease in [Cl−]i is accompanied with increased phosphorylation on NCC via a WNK1-dependent manner. They concluded that a decrease in extracellular [K+] hyperpolarizes membrane potential, increases Cl− efflux, decreases [Cl−]i and activates WNK1, thus increasing NCC phosphorylation and activity. Overall, these studies performed in non-transporting cultured DCT or HEK cells support the notion that intracellular Cl− can regulate WNK kinase activity to alter NCC phosphorylation and activity. However, in DCT that actively reabsorbing NaCl, falls in [Cl−]i in response to decreases in basolateral [K+] would be expected to enhance NCC-mediated NaCl reabsorption due to favorable driving force (Figure 3). Conversely, an increase in extracellular [K+] would be expected to decrease Cl− efflux and increase [Cl−]i, leading to unfavorable driving force and decreased NCC-mediated NaCl reabsorption. To what extent the mechanism of [Cl−]i regulation of WNKs actually plays in the regulation of NCC by extracellular [K+] in DCT in vivo, particularly in the acute setting, remains to be determined experimentally.
Fig. 3.
Model illustrating how changes in basolateral [K+] may affect NCC-mediated transepithelial NaCl reabsorption in DCT. Shown is a simplified DCT cell model. NaCl enters apically through NCC cotransporter. Na+ exits cell through Na+-K+-ATPase. K+ is recycled across basolateral membrane through Kir inward rectifying K+ channel. Cl− exits cell through ClC-kb chloride channel. In DCT in vivo, a decrease in extracellular [K+] hyperpolarizes membrane potential, increases Cl− efflux, decreases intracellular [Cl−], and enhances NCC-mediated NaCl reabsorption due to increased driving force. A converse effect on intracellular [Cl−]i and NCC-mediated NaCl reabsorption is expected by an increase in extracellular [K+]. Note that a similar effect of changing extracellular [K+] on intracellular [Cl−] is expected if K+ and Cl− exit basolaterally through K+-Cl− symporter KCC (not shown). Changes in intracellular [Cl−] may also affect WNK kinase activity and thus NCC phosphorylation and activity if the magnitude of changes is sufficient and fits within the dynamic range of regulation of NCC by WNK kinase cascade. Future investigation using tubular perfusion is required to address this question.
Mechanism for regulation of NKCC2 by WNK kinases
In vitro biochemical and cell-based studies have provided evidence supporting the notion that activation of SPAK and/or OSR1 by WNK1 and 4 kinases also stimulate NKCC2-mediated Na+ reabsorption in the thick ascending limb (TAL) of Henle’s loop (1, 18, 20, 28, 42). Whether OSR1, SPAK or both is involved in vivo yet has been debated. Studies based on measurement of NKCC2 protein abundance in several SPAK loss-of-function mutant mouse models have produced apparent discrepant results (19, 26, 49). Rafiqi et al. reported that total and phosphorylated NKCC2 protein abundance were decreased in homozygous knock-in mice carrying a loss-of-function Spak allele, support the role of SPAK in the stimulation of NKCC2 (26). Another study yet showed that the abundance of phosphorylated NKCC2 was increased in SPAK-KO mice created by deletion of exons 9 and 10 of Spak gene (49). Authors in the latter study also found that OSR1 is more abundant than SPAK in TAL and that OSR1 is upregulated in TAL of SPAK-KO mice. They proposed that compensatory upregulation of OSR1 accounts for the increased NKCC2 abundance in their study. In a third study, McCormick et al. also found increased abundance of both total and phosphorylated NKCC2 in a separate mouse model of SPAK-KO mice created by gene-trap insertion (19). In the study by McCormick et al, they also identified a smaller kidney-specific SPAK (KS-SPAK) isoform that can inhibit the function of full-length SPAK (FL-SPAK) in vitro. KS-SPAK is more abundant than FL-SPAK in TAL. Since both exons 9 and 10 deletion and gene-trap insertion are expected to inactivate FL-SPAK as well as KS-SPAK, McCormick et al. suggested that increased abundance of NKCC2 observed in the gene-trap and in exon 9 and 10-deleted mice is due to loss of a combined net inhibitory effect of KS-SPAK plus FL-SPAK in TAL. This proposed mechanism yet cannot fully explain the apparent discrepancy in the above 3 studies because both FL-SPAK and KS-SPAK should also be inactivated in the knock-in mouse model employed in the study by Rafiqi et al (26).
Of note, NKCC2 gene is alternatively spliced and there are as many as six different NKCC2 isoforms in mice (12, 24). Some splice variants are functionally inactive and may exert dominant-negative inhibition on the full-length variant (12, 24). All six splice variants have identical amino-terminal amino acids that can be recognized by anti-phospho-NKCC2 antibodies (18). Thus, abundance of phosphorylated NKCC2 should not be used as a sole indicator of NKCC2 activity. Recently, Cheng et al revisited the question by direct measurement of NKCC2-mediated Na+ reabsorption in isolated perfused TAL (7). They found that, compared to that in tubules isolated from wild-type mice, NKCC2-mediated Na+ reabsorption is markedly reduced in perfused TAL isolated from Spak-KO mice generated by deletion of exons 9 and 10 (expected to lack both FL- and KS-SPAK). These results indicate that the combined net effect of SPAK isoforms in TAL is stimulation of NKCC2-mediated Na+ reabsorption. OSR1 likely also contributes to stimulation of NKCC2, though the relative contribution by SPAK and OSR1 remain unknown. Finally, high dietary K+ intake also inhibits Na+ reabsorption in TAL, which also contributes to enhanced distal Na+ delivery for stimulation of K+ secretion (36). The mechanism for dietary K+ intake and extracellular [K+] to regulate NKCC2-mediated Na+ reabsorption in TAL is likely similar to that for NCC in DCT (as shown in Figure 3), involving alterations of [Cl−]i and driving force with or without additional role through [Cl−]i-dependent regulation of WNK kinase cascade.
Summary and future perspectives
Since the first report in 2003, the mechanism of regulation of NCC by WNK kinases has gone through numerous twists and turns, and multiple rounds of revision. Cumulative evidence to date based mostly on in vivo studies in mice has now provided a compelling model that WNK1 and WNK4 both contribute to stimulation of NCC. Because WNK kinases may multimerize, the relationship between regulation by WNK1 and 4, and potentially also by WNK3, in vivo remain unclear. Wnk1-KO mice is embryonic lethal. Mice lacking both WNK1 and 4 with conditional Wnk1-KO plus Wnk4-KO may be helpful to elucidate the interaction of WNK1 and 4 regulation of NCC in vivo. The mechanism for regulation of NKCC2 by WNK1 and 4 are likely similar to that for NCC, but await additional in vivo experiments. The recent discovery that WNK kinases may function as an intracellular chloride sensor adds a new dimension to the physiological role of WNK kinases. While it is intriguing to postulate that chloride-dependent regulation of WNK’s may underlie the mechanism of regulation of NCC and NKCC2 by extracellular K+, direct investigation by tubular perfusion in mice with altered WNK kinase expression will be paramount.
Acknowledgments
Work in authors’ lab is supported by grants from the National Institutes of Health of USA (DK59530 to CLH) and from National Science Council of Taiwan (MOST 103-2628-B-016-001-MY3 to CJC).
References
- 1.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006 Jul 18;103(29):10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G. The Effect of WNK4 on the Na+-Cl− Cotransporter Is Modulated by Intracellular Chloride. J Am Soc Nephrol. 2014 Dec 26; doi: 10.1681/ASN.2014050470. pii: ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyden LM, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012 Jan 22;482(7383):98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Inter. 2006;69:2162–2170. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- 5.Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109:7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chávez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal- Petiot E, Castañeda-Bueno M, Vázquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J, et al. WNK-SPAK-NCC Cascade Revisited: WNK1 Stimulates the Activity of the Na-Cl Cotransporter via SPAK, an Effect Antagonized by WNK4. Hypertension. 2014;64:1047–1053. doi: 10.1161/HYPERTENSIONAHA.114.04036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng CJ, Yoon J, Baum M, Huang CL. STE20/SPS1-related proline/alanine-rich kinase (SPAK) is critical for sodium reabsorption in isolated, perfused thick ascending limb. Am Physiol Renal Physiol. 2015 Mar 1;308(5):F437–F443. doi: 10.1152/ajprenal.00493.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu PY, Cheng CJ, Wu YC, Fang YW, Chau T, Uchida S, Sasaki S, Yang SS, Lin SH. SPAK deficiency corrects pseudohypoaldosteronism II caused by WNK4 mutation. PLoS One. 2013 Sep 11;8(9):e72969. doi: 10.1371/journal.pone.0072969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol. Cell Biol. 2003;23:9208–9221. doi: 10.1128/MCB.23.24.9208-9221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genschik P, Sumara I, Lechner E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications. EMBO J. 2013;32:2307–2320. doi: 10.1038/emboj.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadchouel J, Delaloy C, Faure S, Achard JM, Jeunemaitre X. Familial hyperkalemic hypertension. J Am Soc Nephrol. 2006;17:208–217. doi: 10.1681/ASN.2005030314. [DOI] [PubMed] [Google Scholar]
- 12.Hannemann A, Flatman PW. Phosphorylation and transport in the Na-K-2Cl cotransporters, NKCC1 and NKCC2A, compared in HEK-293 cells. PLos One. 2011;6:e17992. doi: 10.1371/journal.pone.0017992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji AX, Prive GG. Crystal structure of KLHL3 in complex with Cullin3. PLoS One. 2013;8:e60445. doi: 10.1371/journal.pone.0060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide- sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 16.Louis-Dit-Picard H, et al. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012 Mar 11;44(4):456–460. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 17.Lytle C, Forbush B., III Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am J Physiol. 1996;270:C437–C448. doi: 10.1152/ajpcell.1996.270.2.C437. [DOI] [PubMed] [Google Scholar]
- 18.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab. 2011 Sep 7;14(3):352–364. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 21.Oi K, Sohara E, Rai T, Misawa M, Chiga M, Alessi D, Sasaki 791 S, Uchida S. A minor role of WNK3 in regulating phosphorylation of renal NKCC2 and NCC cotransporters in vivo. Biology Open. 2012;1:120–127. doi: 10.1242/bio.2011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J. Am. Soc. Nephrol. 2003;14:2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- 23.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride Sensing by WNK1 Involves Inhibition of Autophosphorylation. Science signaling. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plata C, Mount DB, Rubio V, Hebert SC, Gamba G. Isoforms of the Na-K-2Cl cotransporter in murine TAL II. Functional characterization and activation by cAMP. Am J Physiol Renal Physiol. 1999;276:F359–F366. doi: 10.1152/ajprenal.1999.276.3.F359. [DOI] [PubMed] [Google Scholar]
- 25.Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, De Los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovi? S, Jovanovi? A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010 Feb;2(2):63–75. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 28.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and - independent pathways. J Cell Sci. 2011 Mar 1;124(Pt 5):789–800. doi: 10.1242/jcs.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinehart J, Kahle KT, De Los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA. 2013;110:7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 32.Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014 Oct 1;23(19):5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep. 2014 May 9;34(3) doi: 10.1042/BSR20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium Modulates Electrolyte Balance and Blood Pressure through Effects on Distal Cell Voltage and Chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 2012;441:325–337. doi: 10.1042/BJ20111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unwin R, Capasso G, Giebisch G. Potassium and sodium transport along the loop of Henle: effects of altered dietary potassium intake. Kidney Int. 1994;46:1092–1099. doi: 10.1038/ki.1994.371. [DOI] [PubMed] [Google Scholar]
- 37.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multicellular organisms. Oncogene. 2001;20:5562–5569. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- 39.Vidal-Petiot E, Cheval L, Faugeroux J, Malard T, Doucet A, Jeunemaitre X, Hadchouel J. A New Methodology for Quantification of Alternatively Spliced Exons Reveals a Highly Tissue-Specific Expression Pattern of WNK1 Isoforms. PLoS One. 2012;7:e37751. doi: 10.1371/journal.pone.0037751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidal-Petiot E, Elvira-Matelot E, Mutig K, Soukaseum C, Baudrie V, Wu S, Cheval L, Huc E, Cambillau M, Bachmann S, Doucet A, Jeunemaitre X, Hadchouel J. WNK1-related Familial Hyperkalemic Hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14366–14371. doi: 10.1073/pnas.1304230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-Mediated Ubiquitination of WNK4 Causes Human Hypertension. Cell reports. 2013;3:858–868. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 45.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: The Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol. Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 47.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest. 2005;115:1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010 Nov;21(11):1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007 May;5(5):331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]