Abstract
The goal of this study was to explore neural response to touch in children with and without autism spectrum disorder (ASD). Patterns of reduced (hypo-responsiveness) and enhanced (hyper-responsiveness) behavioral reaction to sensory input are prevalent in ASD, but their neural mechanisms are poorly understood. We measured event-related potentials (ERP) to a puff of air on the fingertip and collected parent report of tactile hypo- and hyper-responsiveness in children with ASD (n=21, mean(SD) age: 11.25(3.09), 2 female), and an age-matched typically developing (TD) comparison group (n=28, mean(SD) age:10.1(3.08, 2 female). A global measure of ERP response strength approximately 220–270 msec post-stimulus was associated with tactile hypo-responsiveness in ASD, while tactile hyper-responsiveness was associated with earlier neural response (approximately 120–220 msec post-stimulus) in both groups. These neural responses also related to autism severity. These results suggest that, in ASD, tactile hypo- and hyper-responsiveness may reflect different waypoints in the neural processing stream of sensory input. The timing of the relationship for hyper-responsiveness is consistent with somatosensory association cortical response, while that for hypo-responsiveness is more consistent with later processes that may involve allocation of attention or emotional valence to the stimulus.
Introduction
The advent of DSM-5 has brought renewed attention to differences in sensory responsiveness in autism spectrum disorder (ASD), but the presentation of these symptoms is complex and highly variable. For example, children with autism may show either tactile hypo- (Foss-Feig, Heacock, & Cascio, 2012) or hyper-responsiveness (Baranek and Berkson, 1994; Cascio et al., 2013) behavior, both of which may result from poor sensory filtering that impacts tactile perception (Puts, Wodka, Tommerdahl, Mostofsky, & Edden, 2014), but which are differentially associated with autism-associated genetic variants (Schauder, Muller, Veenstra-VanderWeele, & Cascio, 2015; Tavassoli, Auyeung, Murphy, Baron-Cohen, & Chakrabarti, 2012), suggesting divergent biological mechanisms. Widespread aberrant response to touch in young children with ASD has sparked interest in tactile-centered therapeutic approaches such as massage treatment (Silva et al., 2015), but these efforts would benefit greatly from a more comprehensive understanding of the neurobiology of aberrant response to touch in ASD.
Variability in the functioning of the nervous system at some stage (or stages) of tactile perception is clearly responsible for variability in tactile responsiveness, but we do not yet know at which stage(s) of processing this variability occurs. While basic sensory transmission between thalamus and primary somatosensory cortex may be affected, some recent studies implicate inhibitory mechanisms within primary somatosensory cortex (Puts et al., 2014; Tommerdahl, Tannan, Cascio, Baranek, & Whitsel, 2007), but other studies have not found evidence for altered inhibition in ASD (Coskun, Loveland, Pearson, Papanicolaou, & Sheth, 2013). It is also possible that differences in response are attributable to later-occurring, higher-order neural events such as sensory association, limbic response to sensory input, or orienting and allocation of attention. Rather than a single level, multiple levels of neurosensory processing, and feedback in addition to feedforward circuitry may be affected (Green et al., 2013; Khan et al., 2015). A method that has excellent temporal resolution is needed to better specify the level(s) at which processing of somatosensory stimuli occurs so that we can understand the source(s) of variability in tactile responsivity in individuals with ASD.
While the opposing behavioral profiles of sensory hypo- and hyper-responsiveness suggest divergent neural mechanisms (e.g., reduced or aberrantly enhanced neural processing, respectively), some have theorized that both patterns are behavioral strategies to deal with overwhelming sensory input (Gomot & Wicker, 2012; Markram & Markram, 2010). Determining the presence and nature of aberrant neural response associated with behavioral hyper- and hypo-responsiveness may help to clarify whether there are both convergent and divergent neural mechanisms that contribute to these behaviorally opposite patterns.
While multiple sensory systems are affected in ASD, focusing on a single sensory modality can be of value in isolating neural mechanisms. For the tactile system, magnetoencephalographic (Coskun et al., 2009; Marco et al., 2012), electroencephalographic (Miyazaki et al., 2007), and fMRI (Cascio et al., 2008) studies suggest that there are response differences on the level of primary somatosensory (SI) cortex in ASD, but specific associations with variability in tactile hypo- or hyper-responsiveness have not been reported. Our goal was to investigate the relationship between evoked neural response to touch and parent report of tactile hypo- and hyper-responsiveness using the exquisite temporal sensitivity of ERP to elucidate plausible waypoints in the temporal stream of tactile processing.
Our ERP measure of tactile processing was derived using a multi-step approach. First, we determined a window of tactile processing that was common to both a group of children with ASD and a typically-developing (TD) reference group, based on the difference in global field power (GFP, (Skrandies, 1990)) between response to an air puff stimulus relative to a sham condition (in which there was no tactile stimulation but auditory and visual information was identical to the puff condition). GFP provides a snapshot of global neural activity at each post-stimulus time sample, and is considered to reflect across-trial consistency and/or simultaneity of cortical pyramidal neuron firing. The between-condition GFP difference will be abbreviated as GFPpuff-sham throughout the paper. Next, within this common window of tactile processing, we identified smaller ranges of distinct stable response called microstates in the TD reference group. Finally, within these small time windows, we examined how GFPpuff-sham interacted with clinical group to predict levels of tactile hypo- or hyper-responsiveness assessed by parent report.
Our goal was to begin to address the question of whether behavioral hyper- and hypo-responsivity might reflect a shared neural mechanism (i.e., both patterns reflecting defensiveness against “overwhelming” sensory input). If hyper- and hypo- tactile responsivity share the same neural mechanism, their association with our ERP-measure of tactile processing should be in the same direction. Contrary to the aforementioned “shared neural mechanism” hypothesis, we expected different relations between ERP measures of tactile processing with hyper-responsivity versus that with hypo-responsivity. Specifically, we hypothesized that (a) more tactile hypo-responsivity as identified by parent report would be associated with lower GFPpuff-sham, and (b) more tactile hyper-responsivity as identified by parent report would be associated with higher GFPpuff-sham.
Although not primary questions of interest, we also examined between-group differences in mean hypo- and hyper-responsivity and our ERP measure of tactile processing. Hypo-responsivity to touch has been shown to be more specific to ASD and more predictive of ASD core features than hyper-responsivity (Foss-Feig et al., 2012; Watson et al., 2011), which is common across many developmental disabilities (Baranek & Berkson, 1994). Therefore, we predicted that the ASD group would show both higher tactile hyper-responsivity and hypo-responsivity than the TD group, and thus it was not clear whether group differences in the ERP index of tactile processing would emerge or whether high and low GFP puff-sham values might cancel one another out. As an exploratory analysis, we examined whether ERP-measures of tactile processing were associated with core clinical features within the ASD group, using calibrated severity scores (Gotham, Pickles, & Lord, 2009) derived from the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000).
Materials and Methods
Participants
Twenty-one children with ASD and 28 children with TD between the ages of 5 and 17 years participated in the study. For participants in the ASD group, a diagnosis of ASD was confirmed with research-reliable administration of the (ADOS, n=21), the Autism Diagnostic Interview-Revised (ADI-R, (Lord, Rutter, & Le Couteur, 1994), n=19), and the judgment of a licensed clinical psychologist based on DSM-IV criteria. Participants in the TD group were excluded if they had a diagnosed psychiatric or learning disorder, a first-degree relative with an autism spectrum disorder, or elevated scores on the Social Communication Questionnaire (SCQ, (Rutter, Bailey, & Lord, 2003)) or Child Behavior Checklist (CBCL/6-18)(Achenbach & Rescorla, 2001). All participants were screened and excluded for genetic and neurological history. Participants’ cognitive ability was assessed using the Kaufman Brief Intelligence Test, Second Edition (KBIT-2, (Kaufman & Kaufman, 2004), n=6 ASD, n=8 TD) or the Wechsler Abbreviated Scales of Intelligence (WASI, (Wechsler, 1999), n=15 ASD, n=20 TD). The mean IQ of the ASD group was significantly lower than that of the TD group, driven by a significantly higher verbal IQ in the TD group (see Table 1).
Table 1.
Participant characteristics
| TD | ASD | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | N | Mean | Std. Deviation | ||
| Age | 28 | 10.1 | 3.08 | 21 | 11.25 | 3.09 | t(47)=1.25, p=.22 |
| Full scale IQ | 28 | 116.9 | 13.3 | 21 | 106.8 | 16.4 | t(47)= −2.37, p=.022 |
| Verbal IQ | 28 | 119.1 | 12.4 | 21 | 104.6 | 17.4 | t(47)= 3.41, p=.001 |
| Performance IQ | 28 | 110.5 | 14.7 | 21 | 106.8 | 17.7 | t(47)= −.79, p= .43 |
| ADOS severity | - | - | - | 21 | 7.47 | 2.0 | |
|
| |||||||
| ADI-R (A) | - | - | - | 19 | 17.16 | 4.92 | |
|
| |||||||
| ADI-R(Bv) | - | - | - | 19 | 15.37 | 4.8 | |
|
| |||||||
| ADI-R (C) | - | - | - | 19 | 5.95 | 3.15 | |
|
| |||||||
| ADI-R (D) | 19 | 2.84 | 1.12 | ||||
| % of n=28 | % of n=21 | ||||||
|
| |||||||
| Female | 7.1% | 9.5% | χ2(1)=.091, p=.76 | ||||
| Left-handed | 25% | 33% | χ2(1)=.408, p=.52 | ||||
ADI-R: A: Reciprocal social interaction score, Bv: Verbal communication score, C: Restricted, repetitive and stereotyped behaviors score, D: Evidence of abnormality before 36 months score
Tactile variable derivation
Parents completed two questionnaires assessing participants’ reactions to sensory stimuli in daily life. The Sensory Profile (Dunn, 1999) and the Sensory Experiences Questionnaire (SEQ, (Little et al., 2011)) capture sensory symptoms including hypo-responsiveness and hyper-responsiveness. We derived three variables representing tactile hypo-responsiveness and three variables representing hyper-responsiveness. Relevant items (addressing nature and degree of responsiveness to touch) from these two instruments were used separately as outcome variables and a third variable was created by combining normalized scores from the two instruments, after verifying that they were positively correlated above r=0.4. Table 2 summarizes the mean and standard deviations for each of these variables.
Table 2.
Sensory responsiveness scores
| TD | ASD | |||||
|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | N | Mean | Std. Deviation | |
| SP Hyper | 22 | 1.2727 | 0.40259 | 19 | 1.9895 | 0.7845 |
| SP Hypo | 22 | 1.3485 | 0.37765 | 19 | 2.1228 | 0.90411 |
| SEQ Hyper | 14 | 1.25 | 0.3669 | 15 | 2.2 | 0.71464 |
| SEQ Hypo | 14 | 1.25 | 0.42743 | 15 | 2.1 | 0.98561 |
| Combined Hyper | 14 | −0.6947097 | 0.47541162 | 15 | 0.6020818 | 0.94778643 |
| Combined Hypo | 14 | −0.6583482 | 0.4200319 | 15 | 0.5705685 | 1.01295753 |
SP: Sensory Profile, SEQ: Sensory Experiences Questionnaire
Experimental procedures
Tactile stimuli
A customized pneumatic stimulator was used to provide light tactile stimulation to the palmar surface of the dominant index finger. The stimulator setup and experimental parameters are detailed in Maitre et al.(Maitre, Barnett, & Key, 2012). Briefly, for each trial, a microcontroller opened one of two digital channels (one for the tactile stimulus, and one for the sham stimulus), delivering a 20 ms puff of air through 1.2 m of soft tubing to a nozzle secured 1.2 cm from the child’s immobilized fingertip at a constant pressure of 40 PSI (approximating brief light touch). For each 5-minute block, 60 tactile stimulation (“puff”) and 60 sham trials (identical to puff but the nozzle was directed away from the participant) were delivered in pseudorandom order, with a variable intertrial interval between 2000 and 2500 ms. To avoid habituation, pseudorandom stimulus sequences were constrained such that no more than two repetitions of the puff or sham stimulus would occur consecutively. Each participant completed 2 to 3 blocks, thus the duration of the experiment was 10–15 minutes. An experimenter monitored each child throughout the experiment to ensure compliance. Although all puffs were the same duration, participants were told that some would be longer than others, and asked to report how many “long” stimuli there were at the end of the experiment, in order to maintain attention. General positive feedback was given, and responses were not recorded or analyzed.
Electroencephalography data acquisition and preprocessing
A high-density geodesic array net of 128 Ag/AgCl electrodes embedded in soft sponges (EGI, Inc.; Eugene, OR) soaked in an electrolytic solution was used to record event-related potentials from the scalp. The electrodes were adjusted to ensure impedance levels below 40 kOhm. Continuous EEG data were sampled at 1000 Hz with 0.1–400 Hz filters, using Net Station (v. 4.2, EGI, Inc.). During data collection, electrodes were referenced to Cz (re-referenced offline to an average reference).
Data were filtered off-line with a 0.3–40 Hz bandpass filter and segmented based on stimulus onset to include a 200 msec pre-stimulus baseline and a 500 ms post-stimulus interval. The resulting segments were screened for motor and ocular artifacts using NetStation’s automated artifact rejection tool, using voltage above 140 μV as a threshold for eye blinks, 55 μV for eye movements, and 200 μV as an indicator of poor quality signal. Automated artifact rejection was supplemented by a manual review. If more than 15 channels had poor quality data, the entire trial was discarded. Trials with ocular artifacts were corrected using NetStation’s ocular artifact correction tool (Gratton, Coles, & Donchin, 1983). For trials without ocular artifacts, data from poor quality channels were reconstructed with spherical spline interpolation (Perrin, Pernier, Bertrand, & Echallier, 1989).
Evoked potentials were re-referenced to an average reference, and baseline corrected. The average number of retained trials across the blocks was 48 (+/−27) puff and 52 (+/− 27) sham for the ASD group, and 52 (+/−27) puff and 55 (+/−26) sham for the TD group. The groups did not differ in trial retention rate for puff (t(47)=−.459, p=.65) or sham (t(47)=−.396, p=.69) trials.
Normative ERP variable derivation
To constrain our investigation to the relevant epochs of the response for tactile processing, we identified periods of significant difference in GFPpuff-sham in the TD and the ASD group, and then focused our attention on only the periods that were significant in both groups, in order to address the possibility that the window of response to touch might differ between the groups. Using Cartool software v. 3.53 (brainmapping.uniuge.ch/cartool, Denis Brunet), global field power (GFP) was calculated for each condition for each participant. Paired t-tests were used to identify periods of significant differences in GFP between puff and sham conditions separately in the TD and ASD samples. The threshold for contiguous timeframes (1 timeframe=1 msec) was 10, which has been shown to adequately control for experiment-wise error (Guthrie & Buchwald, 1991). Within the time window that was shared by both groups, five ranges of time frames of stable topography (microstates) in response to touch were identified in the TD grand average using Cartool’s cluster analysis-based segmenting function. For each of these microstates, the between-stimulus difference in normalized GFP (GFPpuff-sham) averaged across the temporal range of the microstate was calculated for each participant in both groups and used in the statistical analyses.
Statistical analysis plan
All variables were tested for extreme non-normality, the presence of which was addressed with bootstrapping (1000 bootstrapped samples) to estimate the p value of statistical tests. To examine associations between group, GFPpuff-sham, and parent-reported sensory responsivity, multiple linear regression models were used with tactile hypo- or hyper-responsiveness as the outcome variable. Significant statistical interactions were followed up with Pearson’s r correlations to test hypothesized associations within groups. Although our sample had a wide age range, we tested for and did not find any significant association between age and any of the ERP or sensory variables (p values > .17), thus we did not covary for age in our analyses. Although the sample differed in verbal and full scale IQ, these variables did not correlate with any of the parent-report variables or GFPpuff-sham. Therefore, IQ scores were not used as covariates.
Results
ERP index of tactile processing
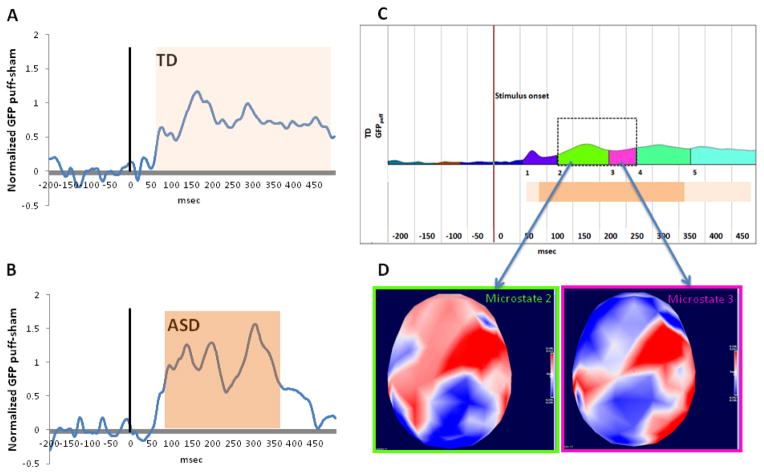
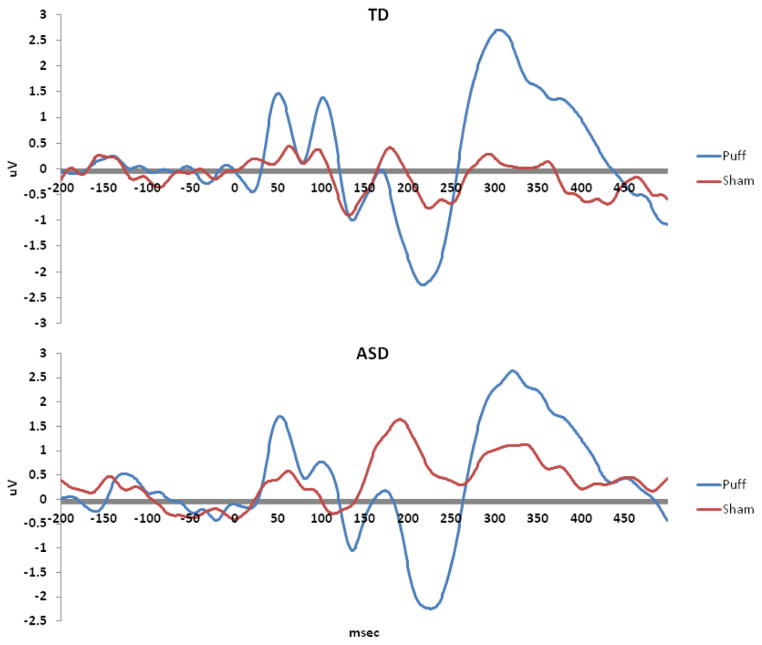
A long period of significant (p < .05) consecutive difference in GFPpuff-sham was identified in the TD group between 62 msec and 488 msec post-stimulus onset (i.e., a 426 ms window). The ASD group exhibited a smaller but overlapping window of significant consecutive GFPpuff-sham difference (between 85 and 365 msec post-stimulus onset, a 280 msec window). To ensure that the window analyzed was relevant for tactile processing in both groups, the smaller (ASD-derived) window was used to constrain subsequent analyses. The cluster analysis on the topographical maps for the tactile (puff) stimulus in the TD group revealed five distinct time periods (microstates) of stable response to touch: microstate 1 (55–120 ms post-stimulus), microstate 2 (121–218 msec post-stimulus), microstate 3 (219–268 msec post-stimulus), microstate 4 (269–372 msec post-stimulus), and microstate 5 (373–499 msec post-stimulus). Two of these normative microstates (microstates 2 and 3) were fully contained within the 280 msec post-stimulus window described above; thus subsequent analyses were limited to these two microstates. The periods of consecutive GFPpuff-sham difference for both groups and the template and topographic maps for the normative (TD) microstates are depicted in Figure 1. For ease of visualization and comparison with traditional analysis approaches, the raw data at a representative central electrode are depicted in Fig. 2.
Figure 1.
Normalized GFP different between puff and sham conditions in the TD (A) and ASD (B) groups. Vertical lines indicates onset of stimulus. Orange bars illustrate periods of significant GFP difference between puff and sham as measured by paired t tests within each group. (C): Five microstates in the TD (reference) group, defined as periods of stable topography to the puff stimulus, measured with Cartool’s cluster-based segmentation. Of these five, microstates 2 (121–218 msec post-stimulus) and 3 (219–268 msec post-stimulus) were wholly contained within the window of significant difference common to both ASD and TD (dark orange bar), and thus were the foci of subsequent analyses. (D): Topographic maps in the TD (reference) group, depicting GFP for the two microstates of interest.
Figure 2.
Raw ERP data (uV) for a representative central electrode contralateral to stimulation site (C3/C4) for (top) TD and (bottom) ASD groups.
Relation between GFPpuff-sham and parent-reported tactile hypo-responsiveness
When GFPpuff-sham and its interaction with group (i.e., the product term for group x GFPpuff-sham) for each microstate was regressed onto tactile hypo-responsiveness measured by the SEQ, there was a group* GFPpuff-sham interaction for microstate 3 (during the 219–268 msec window after stimulus onset) (rpart=−.403, p=.02). A significant negative correlation was noted between GFPpuff-sham for microstate 3 and hypo-responsiveness measured by SEQ for the ASD group (r=−.617, p=.01, 95% CI: [−.919 – −.144]), but not for TD (r=.271, p=.348, 95% CI: [−.062 – .625]).
In contrast, the group x GFPpuff-sham for microstate 3 predicting SP and composite measures for hypo-responsivity was nonsignificant (rpart=.117, p=.37, 95% CI: [−.129 – .650], rpart=−.121, p=.21, 95% CI: [−.479 – −.310], respectively), although significant main effects of GFPpuff-sham for microstate 3 were seen for both these measures (SP: rpart= −.130, p=.04, 95% CI: [−.187 – .002] composite: rpart=−.121, p=.04, 95% CI: [−.447 – −.044]). There were no significant effects of GFPpuff-sham or group x GFPpuff-sham interactions on hypo-responsiveness measured by SEQ for microstate 2 (p values > .062).
In summary, normative ERP measures of tactile processing during the 219 – 268 ms (microstate 3) range were associated with parent report of tactile hypo-responsivity such that lower response was associated with more hypo-responsiveness, and this relation was clearest in the ASD group. These results are summarized in Figure 3a.
Figure 3.

Relations between global field power (GFP) and tactile responsiveness. A: Hypo-responsiveness (measured by the SEQ) and its relation to GFP in microstate 2 (121–218 ms post-stimulus); B: Hyper-responsiveness (measured by the SP) and its relation to GFP in microstate 3 (219–268 ms post-stimulus).
Relation between GFPpuff-sham and parent-reported tactile hyper-responsiveness
There was a significant interaction between group and GFPpuff-sham in microstate 2 when regressed onto the SP hyper-responsiveness score (rpart=.350, p=.02, 95% CI: [.151 – .851]). Moderate correlations were observed between GFPpuff-sham and tactile hyper-responsiveness, in opposite directions for the two groups (ASD: (r= .405, p=.09, 95% CI: [.090 – .714]); TD: r= −.465, p=.03, 95%CI: [−.781 – .085]). These results should be interpreted cautiously, however, as the correlation in the ASD group did not reach statistical significance, and floor effects likely influenced the correlation in the TD group. There were no relations between GFPpuff-sham in microstate 3 or interactions with group and hyper-responsiveness measured by the SP (p values >.09). Additionally, there were no effects of GFPpuff-sham or GFPpuff-sham *group interactions for the other metrics of hyper-responsiveness (p values >.19) for microstate 2.
In summary, normative ERP measures of tactile processing during the 121–218 ms (microstate 2) range were associated with parent report of tactile hyper-responsivity in both groups, such that lower response was associated with less hyper-responsiveness in the ASD group, while the opposite pattern was seen in the TD group. These results are summarized in Figure 3b.
Secondary analysis assessing between-group differences
As predicted, the ASD group had significantly higher scores and greater variance than the TD group on all six parent-report measures of aberrant tactile responsiveness (SP hyper (t(25.97)=3.6, p=.001), SP hypo (t(23.38)=3.5, p=.002), SEQ hyper (t(21.2)=4.5, p<.001), SEQ hypo (t(19.36)=3.1, p=.001), and composite hyper (t(21.22)=4.67, p<.001) and hypo (t(19.22)=4.3, p<.001). Table 2 contains the descriptive statistics for the parent reports. There were no significant group differences in the GFPpuff-sham for either of the microstates (ANOVA/ANCOVA p values > .19).
Exploratory analysis
Because there was a significant association of GFPpuff-sham with some measure of tactile processing atypicality for both microstates 2 and 3 in the ASD sample, the associations of GFPpuff-sham for these microstates with severity of ASD symptoms were tested. GFPpuff-sham in both microstates was significantly negatively correlated with ADOS calibrated severity score (Gotham et al., 2009) (microstate 2: r= −.720, p=.001, 95% CI: [−.884 – −.428], microstate 3: r=−.478, p=.04, 95% CI: [.029 – .182]), reflecting an association between increased autism severity and lower GFP difference in the microstates associated with hyper- and hypo-responsivity.
Discussion
The goal of this study was to assess the relation of tactile hypo- and hyper-responsiveness to neural response to touch, as a first step toward resolving the question of whether these two opposite behavioral patterns share a common neural mechanism. Greater GFPpuff-sham in the 219–268 msec post-stimulus period was associated with less hypo-responsiveness, or more typical tactile processing. This association was specific to the ASD group, and suggests that tactile hypo-responsiveness may result from diminished neural response to touch in very late sensory association and/or early attentional processes, which are thought to be reflected in the 200–400 msec post-stimulus window (Kekoni, Hämäläinen, McCloud, Reinikainen, & Näätänen, 1996; Zopf, Giabbiconi, Gruber, & Müller, 2004). This is in contrast to previous findings of atypical primary/secondary somatosensory (SI/SII) response in ASD (Cascio et al., 2008; Marco et al., 2012; Miyazaki et al., 2007), which would be predicted within the first 200 msec post-stimulus (Hashimoto, Yoshikawa, & Sasaki, 1990; Mima, Nagamine, Nakamura, & Shibasaki, 1998). The current findings suggest that diminished responsiveness to touch in ASD may be more related to higher-order perceptual/attentional processes than to low level sensory processing.
In contrast, the GFP difference in an earlier microstate (121–218 msec post-stimulus) interacted with group to predict tactile hyper-responsiveness. In this case, greater GFPpuff-sham was associated with more hyper-responsiveness in ASD, although the result fell short of statistical significance. The timing of this effect suggests that the neural basis of tactile hyper-responsiveness in ASD may be earlier (SII/posterior parietal) than that of hypo-responsiveness. The different directions of association between these two patterns and GFP difference, and the different timing of the association, suggest that tactile hypo- and hyper-responsiveness are unlikely to share a common neural mechanism, as might be predicted by the behavioral theory that both patterns arise from a desire to withdraw from stimuli that are perceived as overwhelming or too intense (Gomot & Wicker, 2012; Markram & Markram, 2010).
As has been previously found (Baranek & Berkson, 1994; Cascio, Lorenzi, & Baranek, 2013; Foss-Feig et al., 2012), the ASD group exhibited greater scores and higher variability for both tactile hyper- and hypo-responsivity than the TD group, as measured by parent report. The fact that both patterns are elevated in ASD highlights the need for research that emphasizes associations of aberrant processing with other variables, rather than group mean case-control comparisons in which variability in both directions may cancel out and obscure meaningful information in ASD. In this study, for example, overall group differences in ERP measures of neural response strength (GFP) were not observed, but there were clear relations between parent report of tactile hypo- and hyper-responsivity and the ERP measures of tactile processing. Given the opposite direction of the associations of the association between ERP-measured tactile processing and measures of hypo and hyper-responsivity and the greater hypo- and hyper-responsivity in ASD than in TD groups, it is not surprising that the groups did not differ on the ERP measure of tactile processing.
Finally, we noted a negative association between the neural response to touch in these two microstates and autism symptom severity scores derived from the ADOS. This supports the idea that both sensory hypo- and hyper-responsiveness are important contributors to the global autism behavioral phenotype. More specifically, this study provides further support of a link between atypical response to touch and autism symptoms (Cascio, Foss-Feig, Burnette, Heacock, & Cosby, 2012; Foss-Feig et al., 2012; Pryweller et al., 2014; Voos, Pelphrey, & Kaiser, 2013), suggesting that this is an area ripe for further exploration in ASD.
Our study had certain limitations, which warrant mention here. The first is that our behavioral measures of tactile hyper- and hypo-responsiveness were derived solely from parent report reflecting touch encountered in daily life, while we measured neural responses to controlled touch administered in a laboratory setting. Despite these differences, we did see meaningful interactions between group and neural response that predicted behavioral response. Limited variability in the parent report scores of hypo-responsiveness is an additional limitation of our behavioral data. We did not correct for the multiple tests performed, thus, our findings should be interpreted with caution until replicated. Finally, while GFP has distinct advantages as a reference-free, global measure of neural activity, it does not provide information about the spatial distribution of response, which may be relevant to understanding the relation between handedness and laterality of somatosensory response (Sörös et al., 1999). This has important implications given known differences in interhemispheric connections in ASD and other developmental disabilities (Alexander et al., 2007; Cascio et al., 2006; Hardan et al., 2009).
Despite these limitations, the results of the current study illustrate the nuance of both behavioral and neural responses to touch in ASD, and refute the idea that a single neurobiological model can explain all aberrant sensory responses. Although unexpected, the association between autism severity and diminished neural response to touch highlights the important role of basic sensory response in the complex social-communicative and repetitive behaviors that characterize autism. While this role is now recognized by the diagnostic criteria for ASD, future work should take advantage of the early development of sensation and perception relative to more complex behaviors and examine the relation between the two with prospective longitudinal studies.
Acknowledgments
This work was supported by the National Institutes of Health (K01 MH090232 awarded to C.J.C., UL1 TR000445 from NCATS/NIH, and P30 HD015052). Effort for C.J.C. is also supported by R01 MH102272. The authors wish to thank Dorita Jones of the Vanderbilt Kennedy Center Psychophysiology Laboratory for performing data processing, Jennifer Foss-Feig for assistance with a pilot version of the study, and Nathalie Maitre, M.D., PhD for supplying the puffer stimulator and protocol. The Cartool software (brainmapping.unige.ch/cartool) has been programmed by Denis Brunet, from the Functional Brain Mapping Laboratory, Geneva, Switzerland, and is supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school age forms and profiles. Burlington, VT: 2001. [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. http://doi.org/10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Berkson G. Tactile defensiveness in children with developmental disabilities: responsiveness and habituation. Journal of Autism and Developmental Disorders. 1994;24(4):457–471. doi: 10.1007/BF02172128. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Burnette CP, Heacock JL, Cosby AA. The rubber hand illusion in children with autism spectrum disorders: delayed influence of combined tactile and visual input on proprioception. Autism. 2012;16:406–19. doi: 10.1177/1362361311430404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Lorenzi J, Baranek GT. Self-reported Pleasantness Ratings and Examiner-Coded Defensiveness in Response to Touch in Children with ASD: Effects of Stimulus Material and Bodily Location. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1961-1. http://doi.org/10.1007/s10803-013-1961-1. [DOI] [PMC free article] [PubMed]
- Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. 2008;38:127–37. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C, Styner M, Smith RG, Poe MD, Gerig G, Hazlett HC, Piven J. Reduced relationship to cortical white matter volume revealed by tractography-based segmentation of the corpus callosum in young children with developmental delay. The American Journal of Psychiatry. 2006;163(12):2157–2163. doi: 10.1176/ajp.2006.163.12.2157. http://doi.org/10.1176/appi.ajp.163.12.2157. [DOI] [PubMed] [Google Scholar]
- Coskun MA, Loveland KA, Pearson DA, Papanicolaou AC, Sheth BR. Interaction of finger representations in the cortex of individuals with autism: a functional window into cortical inhibition. Autism Research: Official Journal of the International Society for Autism Research. 2013;6(6):542–549. doi: 10.1002/aur.1314. http://doi.org/10.1002/aur.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, Castillo EM, Pearson DA, Loveland KA, Sheth BR. How somatic cortical maps differ in autistic and typical brains. Neuroreport. 2009;20(2):175–179. doi: 10.1097/WNR.0b013e32831f47d1. http://doi.org/10.1097/WNR.0b013e32831f47d1. [DOI] [PubMed] [Google Scholar]
- Dunn W. The Sensory Profile. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Foss-Feig JH, Heacock J, Cascio CJ. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Res Autism Spectr Disord. 2012;6:337–44. doi: 10.1016/j.rasd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Wicker B. A challenging, unpredictable world for people with autism spectrum disorder. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2012;83(2):240–247. doi: 10.1016/j.ijpsycho.2011.09.017. http://doi.org/10.1016/j.ijpsycho.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. http://doi.org/10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Bookheimer SY. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. http://doi.org/10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28(2):240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Pabalan M, Gupta N, Bansal R, Melhem NM, Fedorov S, Minshew NJ. Corpus callosum volume in children with autism. Psychiatry Research. 2009;174(1):57–61. doi: 10.1016/j.pscychresns.2009.03.005. http://doi.org/10.1016/j.pscychresns.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto I, Yoshikawa K, Sasaki M. Latencies of peripheral nerve and cerebral evoked responses to air-puff and electrical stimuli. Muscle & Nerve. 1990;13(12):1099–1104. doi: 10.1002/mus.880131203. http://doi.org/10.1002/mus.880131203. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. KBIT2: Kaufman Brief Intelligence Test-II. 2. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Kekoni J, Hämäläinen H, McCloud V, Reinikainen K, Näätänen R. Is the somatosensory N250 related to deviance discrimination or conscious target detection? Electroencephalography and Clinical Neurophysiology. 1996;100(2):115–125. doi: 10.1016/0013-4694(95)00231-6. [DOI] [PubMed] [Google Scholar]
- Khan S, Michmizos K, Tommerdahl M, Ganesan S, Kitzbichler MG, Zetino M, Kenet T. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain: A Journal of Neurology. 2015;138(Pt 5):1394–1409. doi: 10.1093/brain/awv043. http://doi.org/10.1093/brain/awv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little LM, Freuler AC, Houser MB, Guckian L, Carbine K, David FJ, Baranek GT. Psychometric validation of the Sensory Experiences Questionnaire. Am J Occup Ther. 2011;65:207–10. doi: 10.5014/ajot.2011.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP, Key APF. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. Journal of Child Neurology. 2012;27(10):1276–1283. doi: 10.1177/0883073811435682. http://doi.org/10.1177/0883073811435682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Khatibi K, Hill SS, Siegel B, Arroyo MS, Dowling AF, Nagarajan SS. Children with autism show reduced somatosensory response: an MEG study. Autism Research: Official Journal of the International Society for Autism Research. 2012;5(5):340–351. doi: 10.1002/aur.1247. http://doi.org/10.1002/aur.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory - a unifying theory of the neurobiology of autism. Frontiers in Human Neuroscience. 2010;4:224. doi: 10.3389/fnhum.2010.00224. http://doi.org/10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Nagamine T, Nakamura K, Shibasaki H. Attention modulates both primary and second somatosensory cortical activities in humans: a magnetoencephalographic study. Journal of Neurophysiology. 1998;80(4):2215–2221. doi: 10.1152/jn.1998.80.4.2215. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Fujii E, Saijo T, Mori K, Hashimoto T, Kagami S, Kuroda Y. Short-latency somatosensory evoked potentials in infantile autism: evidence of hyperactivity in the right primary somatosensory area. Developmental Medicine and Child Neurology. 2007;49(1):13–17. doi: 10.1017/s0012162207000059.x. http://doi.org/10.1111/j.1469-8749.2007.0059a.x. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pryweller JR, Schauder KB, Anderson AW, Heacock JL, Foss-Feig JH, Newsom CR, Cascio CJ. White matter correlates of sensory processing in autism spectrum disorders. NeuroImage Clinical. 2014;6:379–387. doi: 10.1016/j.nicl.2014.09.018. http://doi.org/10.1016/j.nicl.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RAE. Impaired tactile processing in children with autism spectrum disorder. Journal of Neurophysiology. 2014;111(9):1803–1811. doi: 10.1152/jn.00890.2013. http://doi.org/10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey AJ, Lord C. The social communication questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Schauder KB, Muller CL, Veenstra-VanderWeele J, Cascio CJ. Genetic Variation in Serotonin Transporter Modulates Tactile Hyperresponsiveness in ASD. Research in Autism Spectrum Disorders. 2015;10:93–100. doi: 10.1016/j.rasd.2014.11.008. http://doi.org/10.1016/j.rasd.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LMT, Schalock M, Gabrielsen KR, Budden SS, Buenrostro M, Horton G. Early Intervention with a Parent-Delivered Massage Protocol Directed at Tactile Abnormalities Decreases Severity of Autism and Improves Child-to-Parent Interactions: A Replication Study. Autism Research and Treatment. 2015;2015:904585. doi: 10.1155/2015/904585. http://doi.org/10.1155/2015/904585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrandies W. Global field power and topographic similarity. Brain Topography. 1990;3(1):137–141. doi: 10.1007/BF01128870. [DOI] [PubMed] [Google Scholar]
- Sörös P, Knecht S, Imai T, Gürtler S, Lütkenhöner B, Ringelstein EB, Henningsen H. Cortical asymmetries of the human somatosensory hand representation in right- and left-handers. Neuroscience Letters. 1999;271(2):89–92. doi: 10.1016/s0304-3940(99)00528-5. [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Auyeung B, Murphy LC, Baron-Cohen S, Chakrabarti B. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Molecular Autism. 2012;3(1):6. doi: 10.1186/2040-2392-3-6. http://doi.org/10.1186/2040-2392-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res. 2007;1154:116–23. doi: 10.1016/j.brainres.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos AC, Pelphrey KA, Kaiser MD. Autistic traits are associated with diminished neural response to affective touch. Social Cognitive and Affective Neuroscience. 2013;8(4):378–386. doi: 10.1093/scan/nss009. http://doi.org/10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LR, Patten E, Baranek GT, Poe M, Boyd BA, Freuler A, Lorenzi J. Differential Associations Between Sensory Response Patterns and Language, Social, and Communication Measures in Children With Autism or Other Developmental Disabilities. Journal of Speech, Language, and Hearing Research. 2011;54(6):1562–1576. doi: 10.1044/1092-4388(2011/10-0029). http://doi.org/10.1044/1092-4388(2011/10-0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WASI: Wechsler Abbreviated Scale of Intelligence. San: Harcourt Assessment, Inc; 1999. [Google Scholar]
- Zopf R, Giabbiconi CM, Gruber T, Müller MM. Attentional modulation of the human somatosensory evoked potential in a trial-by-trial spatial cueing and sustained spatial attention task measured with high density 128 channels EEG. Brain Research Cognitive Brain Research. 2004;20(3):491–509. doi: 10.1016/j.cogbrainres.2004.02.014. http://doi.org/10.1016/j.cogbrainres.2004.04.006. [DOI] [PubMed] [Google Scholar]