Abstract
Objective
To examine whether pain sensitivity and pain catastrophizing are associated with persistent pain and disability after lumbar spine surgery.
Design
Prospective observational cohort study.
Setting
Academic medical center.
Participants
Patients (N = 68, mean ± SD age = 57.9 ± 13.1 years, N female = 40 (58.8%)) undergoing spine surgery for a degenerative condition from March 1, 2012 to April 30, 2013 were assessed 6 weeks, 3 months, and 6 months after surgery.
Interventions
Not applicable.
Main Outcome Measure(s)
The main outcome measures were persistent back pain intensity, pain interference, and disability. Patients with persistent back pain intensity, pain interference, or disability were identified as those patients reporting Brief Pain Inventory scores ≥ 4 and Oswestry Disability Index scores ≥ 21 at all postoperative time points.
Results
From 6 weeks to 6 months after surgery, approximately 12.9%, 24.2%, and 46.8% of patients reported persistent back pain intensity, pain interference, or disability, respectively. Increased pain sensitivity at 6 weeks was associated with having persistent back pain intensity (OR = 2.0, 95% CI = 1.0; 4.1) after surgery. Increased pain catastrophizing at 6 weeks was associated with having persistent back pain intensity (OR = 1.1, 95% CI = 1.0; 1.2), pain interference (OR = 1.1, 95% CI = 1.0; 1.2), and disability (OR = 1.3, 95% CI = 1.1; 1.4). An interaction effect was not found between pain sensitivity and pain catastrophizing on persistent outcomes (p > 0.05).
Conclusion(s)
Findings suggest the importance of early postoperative screening for pain sensitivity and pain catastrophizing in order to identify patients at-risk for poor postoperative pain intensity, interference, and/or disability outcomes. Future research should consider the benefit of targeted therapeutic strategies for patients with these postoperative prognostic factors.
Keywords: Catastrophization, low back pain, lumbar stenosis, pain threshold, prognosis, rehabilitation
INTRODUCTION
Degenerative lumbar spine conditions, such as spinal stenosis, are common in the general population.1 These conditions are the most frequent indication for spine surgery in adults.1–4 In the United States (US), spine surgery continues to have the fastest growth among major musculoskeletal procedures.5, 6 Total medical expenditure for lumbar spine surgery in the US exceeds $1 billion annually and is estimated to increase.7, 8 Despite increases in the utilization of lumbar spine surgery, pain-related outcomes after surgery are poor with up to 40% of individuals experiencing chronic pain and pain-related disability.9, 10
Enhanced pain sensitivity responses reflect alterations in pain processing and have the potential to explain variability in chronic postoperative pain outcomes.11–17 Preoperative pain sensitivity has been studied in various patients undergoing surgery,18–26 with some evidence to support an association with postoperative pain following shoulder and knee replacement surgery. However, the role of early postoperative pain sensitivity has not been widely studied. 27, 28 Katz29 has suggested that preoperative factors are influential in the transition from acute to chronic pain after surgery, while early postoperative factors may be integral to the maintenance of ongoing postoperative pain. Early postoperative factors may be useful for the identification of patients with persistent pain-related complaints occurring after surgery. Furthermore, early postoperative assessment is relevant to rehabilitation practitioners who initiate care for patients after surgery. Maladaptive pain processes occurring after surgery may be early indicators of negative, long-term outcome that can signify the necessity of additional postoperative therapeutic strategies to optimize recovery.30
Psychological factors are widely considered as key determinants of postoperative outcome.27 Pain catastrophizing, specifically, is one of the most consistent risk factors for chronic pain development and reflects an individual’s “psychological vulnerability.”27, 28, 31 Papaioannou et al.31 showed that preoperative pain catastrophizing was a unique predictor of acute postoperative pain and analgesic use after lumbar fusion surgery. Additionally, Abbott et al. 32 found preoperative pain catastrophizing predicted pain intensity and disability 2–3 years after lumbar fusion surgery. Similar to studies on pain sensitivity, much of the evidence on the role of pain catastrophizing has focused on the preoperative time point. Recent studies have shown early postoperative psychosocial factors are predictive of longer term postoperative lumbar spine outcome.33–35 For example, Archer et al.33, 34 and Seebach and colleagues35 found that negative pain beliefs at 6 weeks after spine surgery were predictive of longer term pain, disability, and general health in patients with cervical and lumbar degenerative conditions.
To date, few studies have considered both postoperative pain sensitivity and pain catastrophizing as independent predictors of persistent pain outcomes after musculoskeletal surgery.22, 36 Together, pain sensitivity and pain catastrophizing may indicate a potential vulnerability profile for postoperative patients at risk for poor outcomes, especially persistent pain.37 Examining multiple factors including pain sensitivity and pain catastrophizing for determining risk is consistent with the multidimensional nature of pain.38 There is evidence to suggest that these factors are not redundant constructs and can be used together to explain additional variance in clinical outcome.39, 40 The combined information from both factors could lead to a better assessment of postoperative risk or assist in directing postoperative management for improved outcomes.
Therefore, the primary aim of the current study was to determine whether pain sensitivity and pain catastrophizing are associated with persistent pain and disability after lumbar spine surgery. Our hypothesis was that higher levels of postoperative pain sensitivity and pain catastrophizing would identify individuals with persistent back pain intensity, pain interference, and disability up to 6 months after surgery.
METHODS
Participants
This study is a prospective observational cohort study of patients who have chronic pain and are undergoing surgery for a lumbar degenerative condition. Patients were consecutively enrolled into this study from the Department of Orthopaedic Surgery at Vanderbilt University Medical Center from March 1, 2012 to April 30, 2013. To be eligible, patients needed to be 21 years of age or older, English-speaking, have back and/or leg pain complaints greater than 6 months, and undergoing surgical laminectomy with or without arthrodesis (single or multiple levels) for a lumbar degenerative condition (e.g., spinal stenosis, spondylosis with or without myelopathy, degenerative spondylolisthesis). The choice of fusion approach (i.e., posterolateral, anterolateral, transforaminal) was at the discretion of the treating surgeon. Patients were excluded if they met any of the following: (1) spinal deformity as the primary indication for surgery; (2) surgery secondary to pseudarthrosis, trauma, infection, or tumor; (3) history of neurological disorder or disease resulting in moderate to severe movement dysfunction; (4) presence of schizophrenia or other psychotic disorder; (5) surgery under workman’s compensation claim; and (6) unable to return to clinic for standard follow-up visits with the surgeon. Patients who had undergone microsurgery or experienced radicular symptoms caused by a prolapsed or sequestered disc were also excluded from the study. The study protocol was approved by the Institutional Review Board at Vanderbilt University Medical Center.
Procedures
Eligible participants were approached for study consent during a preoperative clinic visit. Written informed consent was obtained and patients completed a preoperative demographic and clinical questionnaire that collected data on age, sex, ethnicity, race, marital status, education level, smoking status, pain duration, and prior surgery. At a standard 6 week postoperative visit, participants completed a questionnaire assessing pain catastrophizing and underwent pressure pain threshold (PPT) testing. Participants also completed patient-reported outcome measures that assessed pain intensity, pain interference, and disability. These same outcome measures were completed at 3 and 6 months after surgery.
Measures
Pressure Pain Threshold
A hand-held pressure algometer (Wagner Instruments, Greenwish, CT) with 1-cm diameter tip was used for PPT testing. A trained examiner applied a pressure stimulus to each side of the L1 spinous process at a rate of 1 kg/s. This anatomical site was chosen as a standardized site for testing of all participants. Participants were instructed to report the moment when the pressure sensation first becomes painful by saying the word “pain”. The amount of pressure in kilograms was recorded. A total of three measurements were obtained on each side of the L1 region and the average of PPT measurements was computed.41 The test-retest reliability of PPT measurements has been established in previous studies.42 PPT assessment has shown to be a useful and discriminative measure of pain hypersensitivity in patients with chronic low back pain.43
Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) is a 13-item questionnaire that assesses the degree to which individuals have different thoughts and feelings when in pain.44 Each item is scored on a 5-point rating scale where 0 is “not at all” and 5 is “all the time”. Total scores for the PCS can range from 0 to 52 with higher scores reflecting higher levels of pain catastrophizing. The PCS is a reliable and valid measure.45, 46 PCS scores have been shown to be influential on pain-related outcomes including disability in patients with degenerative lumbar spine conditions.47, 48
Brief Pain Inventory
The Brief Pain Inventory (BPI) was used to measure back pain intensity and pain interference with activity.49 The 4-item pain intensity subscale assesses current, worst, least, and average back pain. The 7-item pain interference subscale assesses general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life. Both subscales use an 11-point numerical rating scale with 0 representing “no pain” or “does not interfere” and 10 representing “pain as bad as you can imagine” or “completely interferes”. BPI scores for pain intensity and pain interference were computed as the average of the 4-item and 7-item subscales, respectively. The BPI has proven both reliable and valid in both surgical patients and patients with chronic low back pain.50
Oswestry Disability Index
The 10-item Oswestry Disability Index (ODI) measures condition-specific disability.51 Individual items of pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling are each scored on a 0 (high functioning) to 5 (low functioning) scale. Total scores are divided by the total possible score and multiplied by 100 to create a percentage of disability. Higher scores on the ODI indicate higher levels of disability. The ODI has demonstrated strong test-retest reliability and validity, and good internal consistency in both patients after spine surgery and with chronic low back pain.52, 53
Persistent Pain and Disability Classification
Patients were classified as having persistent back pain intensity or interference if BPI subscale scores were greater than 4 or persistent disability if ODI scores were greater than 21 at all postoperative time-points (e.g., 6 week, 3 month, and 6 month). These cut-off scores have been used to indicate moderate-to-severe complaints.53, 54 Patients who did not report moderate-to-severe complaints at all postoperative time points were classified as non-persistent.
Sample Size
The minimum acceptable sample size was determined based on the guideline of 10–15 cases per individual predictor (n = 4) for conducting regression analyses.55, 56 In this case, the minimum total sample size needed was 60 participants.
Data Analysis
Data were analyzed with IBM SPSS Statistics for Windows, version 22 (SPSS, Inc., Chicago, IL). Alpha was set at an a-priori level of 0.05 or less for statistical significance. Descriptive data were generated for categorical and continuous variables. Comparisons of PCS and PPT scores were made based on persistent and non-persistent classification using a t-test or Mann-Whitney U test.
Hierarchical multivariable logistic regression analyses with maximum likelihood estimation were performed to determine the unique contribution of PPT and PCS on persistent pain and disability, after controlling for a-priori factors of age and sex. Prior to multivariable analyses, PPT values were reflected so that higher values indicated enhanced pain sensitivity. Pearson correlation coefficients were used to assess correlation values between independent variables. A value of 0.7 or greater indicated multicollinearity.56 Based on this criterion, there was a lack of multicollinearity between independent variables (data not presented). In each of the a priori determined hierarchical models, age, sex, and PCS were entered into the first step, PPT was entered into the second step, and an interaction term of PCS and PPT (e.g., the product of PCS and PPT) was entered into the third step. The interaction of PCS and PPT was added to further examine the joint effects of the two variables and whether the influence of PCS or PPT was dependent on the value of the other factor.57 Since interaction terms pose a threat for multicollinearity, independent variables were centered prior to analyses.58 Model statistics including likelihood ratio (LR) tests and classification accuracy were examined for each model step. Relative individual predictor strength and significance was assessed with odds ratios and 95% confidence intervals.
RESULTS
Patients
Table 1 and 2 summarize socio-demographic and clinical characteristics of the sample and outcome scores across all postoperative time points. Sixty-eight patients (40 female (58.8%), 28 male (41.2%)) with mean age (SD) of 57.9 (13.1) years were enrolled into this study. The majority of patients were non-Hispanic, white, non-smokers with some college experience at a minimum, and underwent fusion surgery. The mean (SD) PCS score and PPT at 6 weeks after surgery were 12.6 (11.1) and 4.0 (2.3) kg, respectively. No differences in PCS and PPT scores at 6 weeks were noted between patients with or without fusion surgery (p < 0.05).
TABLE 1.
Socio-demographic and clinical characteristics of participants (N = 68).
| Mean ± SD or Median [IQR] | N (%) | |
|---|---|---|
| Demographic Characteristics | ||
| Age in years | 57.9 ± 13.1 | |
| Sex, N (%) female | 40 (58.8) | |
| Ethnicity, N (%) not Hispanic or Latino | 66 (97.1) | |
| Race, N (%) white | 56 (82.4) | |
| Marital status, N (%) married | 45 (66.2) | |
| Education, N (%) more than high school | 49 (72.1) | |
| Smoking status, N (%) current smoking | 13 (19.1) | |
| Clinical Characteristics | ||
| Duration in months of preoperative pain | 24.8 ± 27.5 | |
| Prior spine surgery, N (%) yes | 25 (36.8) | |
| Fusion, N (%) yes | 47 (69.1) | |
| Number of fusion levels | 1 [1 – 2] | |
| Number of laminectomy levels | 2 [1 – 3] | |
TABLE 2.
Summary of descriptive statistics for postoperative clinical outcomes.
| Mean ± SD | Min - Max | Median | |
|---|---|---|---|
| Back pain intensity | |||
| 6 week | 2.8 ± 2.1 | 0.0 – 8.0 | 2.5 |
| 3 month | 2.8 ± 2.4 | 0.0 – 9.0 | 1.8 |
| 6 month | 2.1 ± 2.2 | 0.0 – 9.0 | 1.4 |
| Pain interference | |||
| 6 weeks | 3.3 ± 2.9 | 0.0 – 9.4 | 2.6 |
| 3 month | 3.0 ± 3.2 | 0.0 – 9.3 | 1.4 |
| 6 month | 2.5 ± 2.8 | 0.0 – 9.6 | 1.0 |
| Disability | |||
| 6 weeks | 35.8 ± 17.9 | 0.0 – 72.0 | 34.0 |
| 3 month | 28.3 ± 20.1 | 0.0 – 76.0 | 26.0 |
| 6 month | 23.5 ± 19.8 | 0.0 – 74.0 | 20.0 |
Identification of Persistent Pain and Disability
Sixty-two patients (91.2%) completed all follow-up assessments up to 6 months after surgery. Over the course of postoperative period, eight patients (12.9%) reported persistent back pain intensity, 15 participants (24.2%) reported persistent pain interference, and 29 participants (46.8%) reported persistent disability. Based on these data, twenty-one patients (33.9%) reported persistent disability without persistent back pain intensity. Figure 1 illustrates the mean value for back pain intensity, pain interference, and disability at 6 weeks, 3 months, and 6 months for persistent and nonpersistent groups.
FIGURE 1.
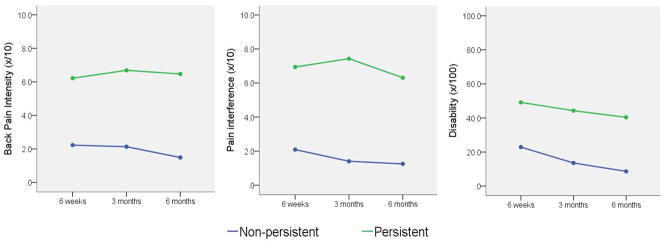
Outcome score comparison of persistent and non-persistent groups.
Persistent and Non-persistent Group Comparisons
Figure 2 illustrates PPT and PCS differences between persistent and non-persistent groups. PPTs were significantly different between persistent and non-persistent back pain intensity (mean difference [95% CI] = −1.8 [−3.5; −0.04]), pain interference (mean difference [95% CI] = −1.5 [−2.8; −0.1]), and disability groups (mean difference [95% CI] = −1.4 [−2.5; −0.2]). Participants with persistent complaints had lower PPTs reflecting higher pain sensitivity. PCS scores were different between persistent and non-persistent back pain intensity (mean difference [95% CI] = 15.0 [7.2; 22.7]), pain interference (mean difference [95% CI] = 13.4 [7.6; 19.3]), and disability groups (mean difference [95% CI] = 14.3 [9.8; 18.8]). Participants with persistent complaints demonstrated higher levels of pain catastrophizing.
FIGURE 2.
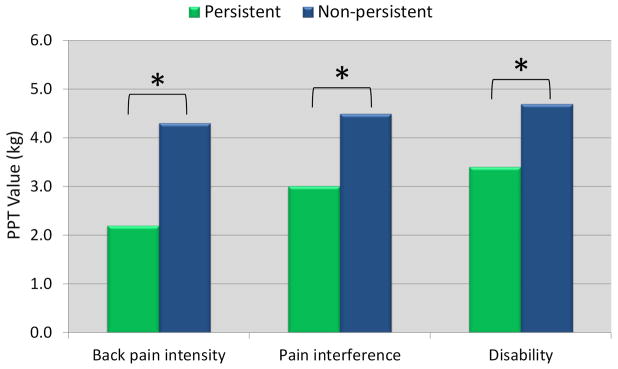
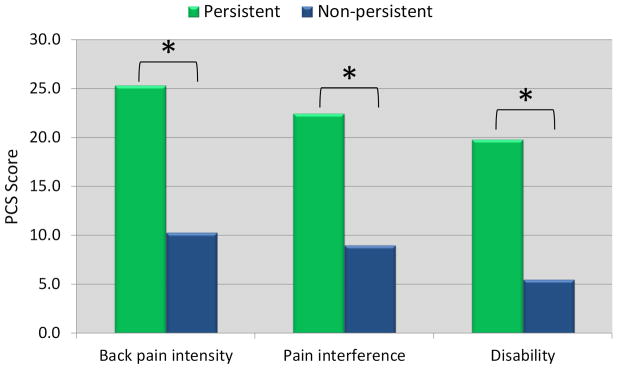
Comparison of a) pain catastrophizing (PCS) and b) pressure pain thresholds (PPT) between persistent and non-persistent groups. * indicates significant difference between groups (p < 0.05).
Multivariable Association of PPT and PCS with Persistent Outcome
Table 3 displays the hierarchical regression models for each of the three persistent outcomes. After accounting for pain catastrophizing, higher pain sensitivity was associated with higher odds of persistent back pain intensity (OR [95% CI] = 2.0 [1.0; 4.1]). Likewise, after accounting for pain sensitivity, higher PCS scores were associated with higher odds of persistent back pain intensity (OR [95% CI] = 1.1 [1.0; 1.2]), pain interference (OR [95% CI] = 1.1 [1.0; 1.2]), and disability (OR [95% CI] = 1.3 [1.1; 1.4]). The interaction between PPT and PCS was not associated with persistent outcome (p > 0.05) (results of model step not shown).
TABLE 3.
Hierarchical regression predicting persistent back intensity, interference, and disability.
| Odds Ratio (OR) | 95% OR | LR test | p | % Accuracy | ||
|---|---|---|---|---|---|---|
| Persistent Back Pain Intensity | ||||||
| Step 1 | 11.01 | < 0.05 | 85.5 | |||
| Age | 0.99 | 0.92; 1.06 | ||||
| Sex | 2.22 | 0.32; 15.19 | ||||
| PCS | 1.11* | 1.03; 1.20 | ||||
| Step 2 | 5.31 | < 0.05 | 95.2 | |||
| PCS | 1.12* | 1.03; 1.21 | ||||
| PPT | 2.03* | 1.02; 4.07 | ||||
| Persistent Pain Interference | ||||||
| Step 1 | 16.51 | < 0.05 | 82.3 | |||
| Age | 0.97 | 0.92; 1.03 | ||||
| Sex | 1.70 | 0.37; 7.65 | ||||
| PCS | 1.12* | 1.05; 1.20 | ||||
| Step 2 | 3.84 | 0.05 | 83.9 | |||
| PCS | 1.11* | 1.04; 1.18 | ||||
| PPT | 1.56 | 0.93; 2.60 | ||||
| Persistent Disability | ||||||
| Step 1 | 40.18 | < 0.05 | 82.3 | |||
| Age | 0.94* | 0.88; 1.00 | ||||
| Sex | 3.75 | 0.71; 20.00 | ||||
| PCS | 1.28* | 1.13; 1.45 | ||||
| Step 2 | 1.72 | 0.19 | 82.3 | |||
| PCS | 1.27* | 1.12; 1.44 | ||||
| PPT | 1.32 | 0.83; 2.08 | ||||
p < 0.05,
Note: LR test is change in -2LL from the previous step. For step 1, LR test is change from the null model (e.g., Step 0). PPT values were reflected prior to conducting regression so higher values indicate higher pain sensitivity. Abbreviations: LR = likelihood ratio; n.s. = non-significant; PCS = pain catastrophizing scale; PPT = pressure pain threshold
DISCUSSION
This study examined whether pain sensitivity and pain catastrophizing are associated with persistent pain and disability after lumbar spine surgery. After accounting for pain catastrophizing, postoperative pain sensitivity was predictive of persistent back pain intensity, but not pain interference or disability. Postoperative pain catastrophizing was a consistent predictor of all persistent pain-related outcomes. These findings suggest pain sensitivity and pain catastrophizing may be useful indicators early after lumbar spine surgery for identifying individuals at risk for persistent pain problems.
Pain sensitivity responses are proxy measures of pain processing and reflect alterations such as peripheral and central sensitization.59 Due to pain processing changes that remain after the removal of a nociceptive stimulus,60 sensitization is considered a risk factor for the development and maintenance of chronic pain. Previous research has shown enhanced pain sensitivity at the site of pain (e.g., lumbar spine) to be a good indicator of sensitization.43 As hypothesized, these findings suggest that enhanced sensitization is a contributing factor in persistent pain intensity levels, even after controlling for pain catastrophizing. Conversely, these data did not support the hypothesis that postoperative pain sensitivity was related to persistent pain interference and disability. Local pain sensitivity as measured by PPTs at the site of pain may not be an indicator of persistent functional outcome after spine surgery, but rather a risk measure related to the sensory aspects of the pain experience (e.g., pain intensity). Similarly findings supporting the association between pain sensitivity and clinical pain have been found in patients with spine pain and knee osteoarthritis.61–63
To date, this is the first study to examine postoperative pain sensitivity and its association with persistent pain outcomes after lumbar spine surgery. Kim and colleagues64, 65 recently found an association between preoperative self-reported sensitization, as measured by the Pain Sensitivity Questionnaire (PSQ), and postoperative lumbar spine outcomes. In contrast to our findings, Kim et al.66 found self-reported pain sensitivity to be associated with both pain intensity and disability. It is possible the method of assessing sensitization (e.g., PSQ vs. psychophysical testing) may explain the differing results with the Kim et al.66 paper having potential for shared method variance to account for the association with disability. Our findings add to the growing body of literature examining the clinical utility of pain sensitivity measurement, especially in relation to predicting persistent pain. Pain sensitivity measures are considered useful tools for examining pain mechanisms.13 Heightened pain sensitivity suggests alterations in peripheral and central processes involved in the transmission and modulation of nociceptive information. Based on findings from the current study, early pain sensitivity alterations after spine surgery may suggest deficits in a patient’s pain modulation capacity. This is a potential explanation for why some patients did not show recovery in back pain intensity.
The association of postoperative pain catastrophizing and persistent pain outcomes after lumbar spine surgery is in agreement with the literature on pain catastrophizing and chronic pain.67, 68 Pain catastrophizing is a consistent predictor of pain-related outcomes, even after controlling for other influential factors such as anxiety and depression.31, 69 Currently, there is limited evidence for the role of catastrophizing in patients with degenerative lumbar spine conditions undergoing surgery. Kim et al. 48 found pain catastrophizing to be associated with pain intensity and disability in patients with lumbar spine stenosis at an initial evaluation visit. Additionally, Papaioannou et al.31 reported a longitudinal association between preoperative pain catastrophizing and acute (e.g., 1–2 days) postoperative pain intensity. In the current study, pain catastrophizing was consistently associated with persistent pain and disability over a course of 6 months after lumbar spine surgery. Interestingly, an interaction between pain catastrophizing and pain sensitivity was not found. This finding suggests there is not an additive effect and pain catastrophizing and pain sensitivity can be viewed as separate prognostic factors. Consistent with this, previous research has also shown similar psychological profiles including PCS scores in patients with spine pain who had either low or high pain sensitivity, further supporting the distinction in measurement of pain sensitivity and pain catastrophizing.61
Although the mechanisms by which pain sensitivity and/or pain catastrophizing influence clinical outcomes remain unknown,14 therapeutic strategies to address higher levels of these risk factors are available. Specifically, multi-modal postoperative management is an integral part of a personalized pain management approach and appears to be warranted in patients after spine surgery with both persistent disability and back pain. For pain sensitivity, interventions to induce hypoalgesia are useful adjuncts to include within a postoperative management scheme. These interventions include both pharmacological and non-pharmacological options. For example, Duloxetine has been shown to be an efficacious pain inhibitor in multiple conditions showing heightened pain sensitivity including chronic low back pain.70–72 Cognitive-behavioral therapy (CBT) has been employed to address elevated levels of pain catastrophizing in select patient populations.27 There is evidence demonstrating the clinical benefits of cognitive-behavioral based approaches for patients after lumbar spine surgery.73–75 Abbott et al.73 demonstrated superior outcomes with a combined biocognitive and exercise approach after lumbar spine fusion. Similarly, Monticone et al.75 reported superior clinical benefits up to one year after lumbar fusion in patients who received CBT in addition to standardized physical therapy exercises. However, neither of these studies targeted the CBT intervention to patient subgroups with elevated pain catastrophizing. Future research should examine the effectiveness of cognitive-based interventions for improving outcomes in patients with higher levels of pain catastrophizing.
Study Limitations
There are several limitations in this study. Generalizability may be limited to the select patients enrolled from a single academic medical center. Inclusion of a larger and potentially broader sample involving multiple clinical sites should be considered in future studies. Similarly, outcomes are limited to the 6-month time period after surgery. These findings cannot be extended to longer periods after surgery. PPT at the local region only was used as sensitization measure primarily for its administrative ease within a busy clinical setting. Neziri et al.43 suggested that local PPT responses are among the most useful measures of hypersensitivity in patients with low back pain complaints. However, the method of assessing pain sensitivity in the current study was limited to a static PPT measurement and it is undetermined whether different pain sensitivity measurement procedures would result in similar findings. Future studies in patients with lumbar spine surgery may want to consider multiple site assessment, different experimental stimuli, or inclusion of more time-intensive protocols (e.g., dynamic pain sensitivity) for determining a comprehensive pain sensitivity profile.76, 77 Pain catastrophizing was the only psychological factor included in this study. This study cannot account for the potential influence of other factors such as depression, anxiety, or fear. Preoperative pain sensitivity and pain catastrophizing data were not included in this study, thus it is not possible to determine whether these responses are a result of surgery or reflect a preoperative state.
Clinical Implications
Clinically, the findings of the current study suggest that measurement of pain sensitivity and pain catastrophizing in the early postoperative period can be an indicator of poor outcomes. The relative clinical importance of pain catastrophizing seems greater as pain catastrophizing was associated with persistent pain and disability, while pain sensitivity was only associated with persistent pain intensity. Measurement of postoperative PPT and pain catastrophizing requires minimal training and effort and can be easily embedded as a screening procedure at a standard postoperative visit. This screening process has potential to influence postoperative decision-making as individuals with heightened pain sensitivity or pain catastrophizing may benefit from directed postoperative management.
CONCLUSION
Early postoperative pain sensitivity and pain catastrophizing were associated with persistent pain-related complaints after lumbar spine surgery. Specifically, pain sensitivity predicted persistent back pain intensity, while pain catastrophizing predicted back pain intensity, pain interference, and disability. These findings suggest the importance of early postoperative screening for pain sensitivity and pain catastrophizing in order to identify patients at-risk for poor outcomes following lumbar spine surgery.
Acknowledgments
This research was supported by a grant award from the National Institute of Arthritis and Musculoskeletal Skin Diseases (NIAMS) of the National Institutes of Health (NIH) (R21AR062880) and the Magistro Family Foundation grant through the Foundation for Physical Therapy. The authors wish to acknowledge Shannon Mathis, PhD and Erin Van Hoy for assistance with data collection and Chigozie Nkemka for proofreading this manuscript prior to submission. The authors report no conflicts of interest.
LIST OF ABBREVIATIONS
- BPI
Brief Pain Inventory
- CBT
cognitive-behavioral therapy
- OD
Oswestry Disability Index
- PCS
Pain Catastrophizing Scale
- PPT
pressure pain threshold
- PSQ
Pain Sensitivity Questionnaire
- US
United States
Footnotes
Data from this study will be presented at the American Pain Society Annual Scientific Meeting in Palm Springs, CA, May 13 – 16, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. The spine journal : official journal of the North American Spine Society. 2009;9(7):545–50. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. The spine journal : official journal of the North American Spine Society. 2010;10(7):625–7. doi: 10.1016/j.spinee.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Issack PS, Cunningham ME, Pumberger M, Hughes AP, Cammisa FP., Jr Degenerative lumbar spinal stenosis: evaluation and management. The Journal of the American Academy of Orthopaedic Surgeons. 2012;20(8):527–35. doi: 10.5435/JAAOS-20-08-527. [DOI] [PubMed] [Google Scholar]
- 4.Katz JN. Lumbar spinal fusion. Surgical rates, costs, and complications. Spine. 1995;20(24 Suppl):78S–83S. [PubMed] [Google Scholar]
- 5.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30(12):1441–5. doi: 10.1097/01.brs.0000166503.37969.8a. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 6.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 7.Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine. 2013;38(11):916–26. doi: 10.1097/BRS.0b013e3182833e7c. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA : the journal of the American Medical Association. 2010;303(13):1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17(1):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA : the journal of the American Medical Association. 2008;299(6):656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen CS, Staud R, Price DD. Individual differences in pain sensitivity: measurement, causation, and consequences. The journal of pain : official journal of the American Pain Society. 2009;10(3):231–7. doi: 10.1016/j.jpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114(3):315–9. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. The journal of pain : official journal of the American Pain Society. 2009;10(6):556–72. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Coghill RC, Keefe FJ. Quantitative sensory testing in predicting persistent pain after joint replacement surgery: promise and challenges. Pain. 2015;156(1):4–5. doi: 10.1016/j.pain.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 15.Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Current opinion in anaesthesiology. 2009;22(3):425–30. doi: 10.1097/ACO.0b013e32832a40e1. [DOI] [PubMed] [Google Scholar]
- 16.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 17.Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010;112(6):1494–502. doi: 10.1097/ALN.0b013e3181dcd5a0. [DOI] [PubMed] [Google Scholar]
- 18.Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. The Journal of bone and joint surgery British volume. 2011;93(4):498–502. doi: 10.1302/0301-620X.93B4.25054. [DOI] [PubMed] [Google Scholar]
- 19.Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. The Journal of bone and joint surgery British volume. 2008;90(2):166–71. doi: 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]
- 20.Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesthesia and analgesia. 2007;105(3):815–21. doi: 10.1213/01.ane.0000278091.29062.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156(1):55–61. doi: 10.1016/j.pain.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 22.Valencia C, Fillingim RB, Bishop M, Wu SS, Wright TW, Moser M, et al. Investigation of central pain processing in postoperative shoulder pain and disability. The Clinical journal of pain. 2014;30(9):775–86. doi: 10.1097/AJP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100(1):115–9. doi: 10.1097/00000542-200401000-00020. discussion 5A. [DOI] [PubMed] [Google Scholar]
- 24.Wilder-Smith OH, Tassonyi E, Crul BJ, Arendt-Nielsen L. Quantitative sensory testing and human surgery: effects of analgesic management on postoperative neuroplasticity. Anesthesiology. 2003;98(5):1214–22. doi: 10.1097/00000542-200305000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Wylde V, Palmer S, Learmonth ID, Dieppe P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(9):1253–6. doi: 10.1016/j.joca.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Wylde V, Sayers A, Lenguerrand E, Gooberman-Hill R, Pyke M, Beswick AD, et al. Preoperative widespread pain sensitizatoin and chronic pain after hip and knee replacement: a cohort analysis. Pain. 2015;156(1):47–54. doi: 10.1016/j.pain.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns JW, Moric M. Psychosocial factors appear to predict postoperative pain: interesting, but how can such information be used to reduce risk. Tech Reg Anesth Pain Manag. 2011;15:90–9. [Google Scholar]
- 28.Khan RS, Ahmed K, Blakeway E, Skapinakis P, Nihoyannopoulos L, Macleod K, et al. Catastrophizing: a predictive factor for postoperative pain. American journal of surgery. 2011;201(1):122–31. doi: 10.1016/j.amjsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Katz J. One man's risk factor is another man's outcome: difference in risk factor profiles for chronic postsurgical pain maintenance vs transition. Pain. 2012;153(3):505–6. doi: 10.1016/j.pain.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Wilder-Smith OH, Arendt-Nielsen L. Postoperative hyperalgesia: its clinical importance and relevance. Anesthesiology. 2006;104(3):601–7. doi: 10.1097/00000542-200603000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Papaioannou M, Skapinakis P, Damigos D, Mavreas V, Broumas G, Palgimesi A. The role of catastrophizing in the prediction of postoperative pain. Pain medicine. 2009;10(8):1452–9. doi: 10.1111/j.1526-4637.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 32.Abbott AD, Tyni-Lenne R, Hedlund R. Leg pain and psychological variables predict outcome 2–3 years after lumbar fusion surgery. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2011;20(10):1626–34. doi: 10.1007/s00586-011-1709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer KR, Seebach CL, Mathis SL, Riley LH, 3rd, Wegener ST. Early postoperative fear of movement predicts pain, disability, and physical health six months after spinal surgery for degenerative conditions. The spine journal : official journal of the North American Spine Society. 2014;14(5):759–67. doi: 10.1016/j.spinee.2013.06.087. [DOI] [PubMed] [Google Scholar]
- 34.Archer KR, Wegener ST, Seebach C, Song Y, Skolasky RL, Thornton C, et al. The effect of fear of movement beliefs on pain and disability after surgery for lumbar and cervical degenerative conditions. Spine. 2011;36(19):1554–62. doi: 10.1097/BRS.0b013e3181f8c6f4. [DOI] [PubMed] [Google Scholar]
- 35.Seebach CL, Kirkhart M, Lating JM, Wegener ST, Song Y, Riley LH, 3rd, et al. Examining the role of positive and negative affect in recovery from spine surgery. Pain. 2012;153(3):518–25. doi: 10.1016/j.pain.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Grosen K, Vase L, Pilegaard HK, Pfeiffer-Jensen M, Drewes AM. Conditioned pain modulation and situational pain catastrophizing as preoperative predictors of pain following chest wall surgery: a prospective observational cohort study. PloS one. 2014;9(2):e90185. doi: 10.1371/journal.pone.0090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123(3):226–30. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nature reviews Rheumatology. 2013;9(6):340–50. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. The journal of pain : official journal of the American Pain Society. 2011;12(1):133–40. doi: 10.1016/j.jpain.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coronado RA, Simon CB, Valencia C, Parr JJ, Borsa PA, George SZ. Suprathreshold heat pain response predicts activity-related pain, but not rest-related pain, in an exercise-induced injury model. PloS one. 2014;9(9):e108699. doi: 10.1371/journal.pone.0108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walton DM, Levesque L, Payne M, Schick J. Clinical pressure pain threshold testing in neck pain: comparing protocols, responsiveness, and association with psychological variables. Physical therapy. 2014;94(6):827–37. doi: 10.2522/ptj.20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. The Clinical journal of pain. 2007;23(9):760–6. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 43.Neziri AY, Curatolo M, Limacher A, Nuesch E, Radanov B, Andersen OK, et al. Ranking of parameters of pain hypersensitivity according to their discriminative ability in chronic low back pain. Pain. 2012;153(10):2083–91. doi: 10.1016/j.pain.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assessment. 1995;7(4):524–32. [Google Scholar]
- 45.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittman L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. Journal of behavioral medicine. 2000;23(4):351–65. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 46.George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40(4):197–205. doi: 10.2519/jospt.2010.3298. [DOI] [PubMed] [Google Scholar]
- 47.Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. American journal of epidemiology. 2002;156(11):1028–34. doi: 10.1093/aje/kwf136. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Cho CH, Kang KT, Chang BS, Lee CK, Yeom JS. The significance of pain catastrophizing in clinical manifestations of patients with lumbar spinal stenosis: mediation analysis with bootstrapping. The spine journal : official journal of the North American Spine Society. 2014 doi: 10.1016/j.spinee.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 50.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. The journal of pain : official journal of the American Pain Society. 2004;5(2):133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25(24):3115–24. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 52.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Physical therapy. 2001;81(2):776–88. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 53.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–52. doi: 10.1097/00007632-200011150-00017. discussion 52. [DOI] [PubMed] [Google Scholar]
- 54.Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. British journal of anaesthesia. 2011;107(4):619–26. doi: 10.1093/bja/aer195. [DOI] [PubMed] [Google Scholar]
- 55.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66(3):411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 56.Field AP. Discovering statistics using SPSS. 3. Los Angeles i.e. Thousand Oaks, Calif. ; London: SAGE Publications; 2009. [Google Scholar]
- 57.Fitzmaurice G. The meaning and interpretation of interaction. Nutrition. 2000;16(4):313–4. doi: 10.1016/s0899-9007(99)00293-2. [DOI] [PubMed] [Google Scholar]
- 58.Aiken LS, West SG, Reno RR. Multiple regression : testing and interpreting interactions. Newbury Park, Calif: Sage Publications; 1991. [Google Scholar]
- 59.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nature reviews Rheumatology. 2010;6(10):599–606. doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 60.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 61.Coronado RA, Bialosky JE, Robinson ME, George SZ. Pain sensitivity subgroups in individuals with spine pain: potential relevance to short-term clinical outcome. Physical therapy. 2014;94(8):1111–22. doi: 10.2522/ptj.20130372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King CD, Sibille KT, Goodin BR, Cruz-Almeida Y, Glover TL, Bartley E, et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(9):1243–52. doi: 10.1016/j.joca.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Kim HJ, Lee JI, Kang KT, Chang BS, Lee CK, Ruscheweyh R, et al. Influence of Pain Sensitivity on Surgical Outcomes after Lumbar Spine Surgery in Patients with Lumbar Spinal Stenosis. Spine. 2014 doi: 10.1097/BRS.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 65.Kim HJ, Park JH, Kim JW, Kang KT, Chang BS, Lee CK, et al. Prediction of Postoperative Pain Intensity after Lumbar Spinal Surgery Using Pain Sensitivity and Preoperative Back Pain Severity. Pain medicine. 2014 doi: 10.1111/pme.12578. [DOI] [PubMed] [Google Scholar]
- 66.Kim HJ, Suh BG, Lee DB, Lee GW, Kim DW, Kang KT, et al. The influence of pain sensitivity on the symptom severity in patients with lumbar spinal stenosis. Pain physician. 2013;16(2):135–44. [PubMed] [Google Scholar]
- 67.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. The Clinical journal of pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, et al. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis research & therapy. 2012;14(5):R231. doi: 10.1186/ar4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clinical orthopaedics and related research. 2010;468(3):798–806. doi: 10.1007/s11999-009-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain medicine. 2007;8 (Suppl 2):S63–74. doi: 10.1111/j.1526-4637.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 71.Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253–60. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 72.Skljarevski V, Desaiah D, Liu-Seifert H, Zhang Q, Chappell AS, Detke MJ, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine. 2010;35(13):E578–85. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 73.Abbott AD, Tyni-Lenne R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine. 2010;35(8):848–57. doi: 10.1097/BRS.0b013e3181d1049f. [DOI] [PubMed] [Google Scholar]
- 74.Christensen FB, Laurberg I, Bunger CE. Importance of the back-cafe concept to rehabilitation after lumbar spinal fusion: a randomized clinical study with a 2-year follow-up. Spine. 2003;28(23):2561–9. doi: 10.1097/01.BRS.0000097890.96524.A1. [DOI] [PubMed] [Google Scholar]
- 75.Monticone M, Ferrante S, Teli M, Rocca B, Foti C, Lovi A, et al. Management of catastrophising and kinesiophobia improves rehabilitation after fusion for lumbar spondylolisthesis and stenosis. A randomised controlled trial. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014;23(1):87–95. doi: 10.1007/s00586-013-2889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–5. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 77.O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Association between a composite score of pain sensitivity and clinical parameters in low-back pain. The Clinical journal of pain. 2014;30(10):831–8. doi: 10.1097/AJP.0000000000000042. [DOI] [PubMed] [Google Scholar]


