Highlights
-
•
In this article, reporting on the case of a huge 10 levels spontaneous spinal subdural hematoma treated with decompressive thoracic no-instrumented laminectomy in a 45-year-old woman with good neurological recovery, we would like to underline the importance of a timely surgical decompression as the mainstay option in the management of strongly symptomatic spontaneous idiopathic acute spinal subdural hematomas.
-
•
To our knowledge, 10 levels thoracic laminectomy for a SSDH removal have never been described. We performed “conservative” laminectomy by sparing of articular processes with no need to posterior fixation also considering the intrinsic stability of thoracic chest.
Abstract
Introduction
Spontaneous idiopathic acute spinal subdural hematoma (SSDH) is a rare cause of acute back pain followed by signs and symptoms of nerve root and/or spinal cord compression, frequently associated with coagulopathies, blood dyscrasias and arterio-venous malformations. Standard management includes non-operative treatment and timely (within 24 h) surgical decompression.
Presentation of case
We report on the case of a huge 10 levels SSDH treated with decompressive thoracic no-instrumented laminectomy in a 45-year-old woman with good neurological recovery (from ASIA A to D).
Discussion
Spontaneous SSDHs without detectable structural lesion or anticoagulant therapy are very rare. Among 26 cases documented the literature harbouring SSDHs, the thoracic spine was found to be the preferred site, and the compression was usually extending over several vertebral levels. Nonoperative treatment for SSDH may be justified in presence of minimal neurologic deficits, otherwise, early decompressive laminectomy along with evacuation of hematoma are considered the treatment of choice in presence of major deficits.
Conclusion
To our knowledge, the present case is the most extensive laminectomy for a SSDH removal never described before. No postoperative instability occurs in 10 levels thoracic laminectomy in case the articular processes are spared. When major neurological deficits are documented, early decompressive laminectomy with evacuation of hematoma should be considered the best treatment for SSDH.
1. Introduction
Spontaneous idiopathic acute spinal subdural hematoma (SSDH) is a rare cause of back pain, associated with high morbidity. Neurological symptoms are usually severe and timely diagnosis with Magnetic Resonance Imaging (MRI) is mandatory [1,2].
Frequently the onset of symptoms is acute with a severe, often radiating, back pain followed by the stigmata of nerve root and/or spinal cord compression, developing from minutes to days later. The true etiology of SSDHs still remains unknown, but associations with some predisposing conditions, such as coagulopathies, blood dyscrasias and arteriovenous malformations, have been reported [1,3]. Whether surgical evacuation is necessary or not is still a matter of debate.
We report on the case of a 45 year-old female who underwent ten levels laminectomy and durotomy within 24 h from progressively severe paraparesis caused by a spontaneous acute SSDH. A subtotal recovery was documented at 36 months follow up.
Huge thoracic decompressive laminectomy is an uncommon procedure to dealing with multilevel thoracic spine pathology; no more extended decompressive procedures have been described so far, according to the literature review.
2. Case report
A 45 year old woman (HIV+ and HCV+) with history of drug abuse, was admitted to our Institution (Catholic University of Medicine of Rome) with an acute and rapidly progressive onset of sensory/motor deficits involving the trunk and the lower limbs. Laboratory exams did not show any coagulopathy neither blood dyscrasias. No anticoagulant therapy was ongoing. During the previous 20 h the patient complained bowel and bladder dysfunctions along with intense back pain, poorly responsive to common analgesic therapy. The neurological examination in emergency showed total anaesthesia from Th2 level, paraplegia, deep and superficial areflexia at the lower limbs and the trunk. According to the American Spinal Injury Association (ASIA Scale) the clinical status was scored A.
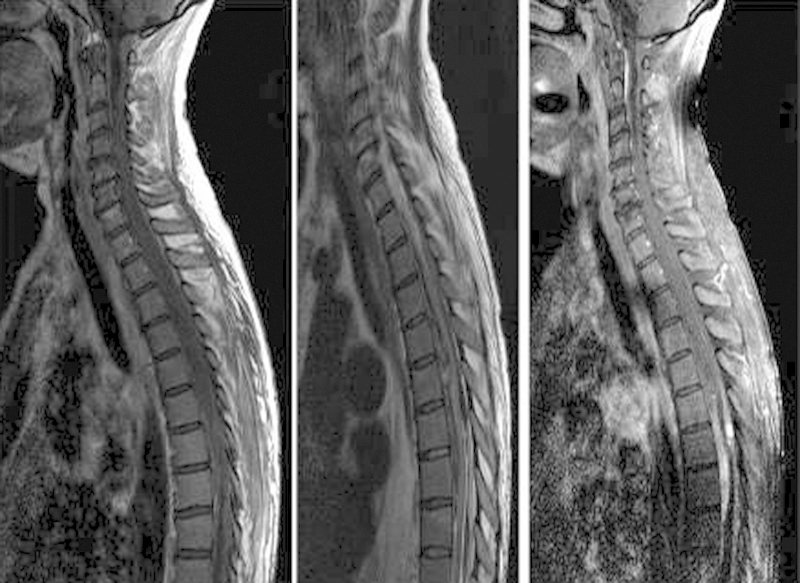
The pre-operative spinal MRI documented an extramedullary lesional pattern anterior and posterior to the cord, spanning from T1 to T10 (Fig. 1) with areas of hypointensity in T2-weighted images and an hyperintense spot at Th6 Tr long images consistent with SSDH. The spinal cord was compressed, mainly from Th5 to Th8 with T2 hyperintense swelling signal without intramedullary contrast enhancement.
Fig. 1.
Sagittal T1 (left) T2 (middle) and Tr long images (right) MR reconstructions. Areas of hypo-intensity in T1, hyper-intensity in T2 along with an hyperintense spot at Th6 in Tr Long images are consistent with a Th 1–Th 10 SSDH with onset before 24 h.
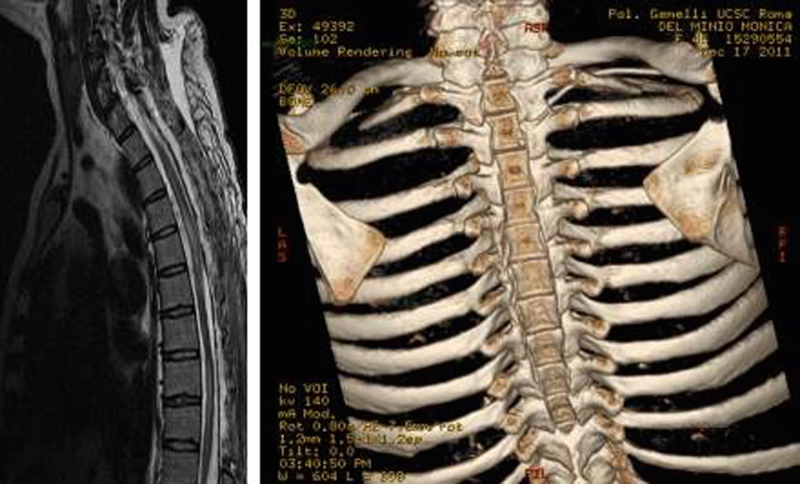
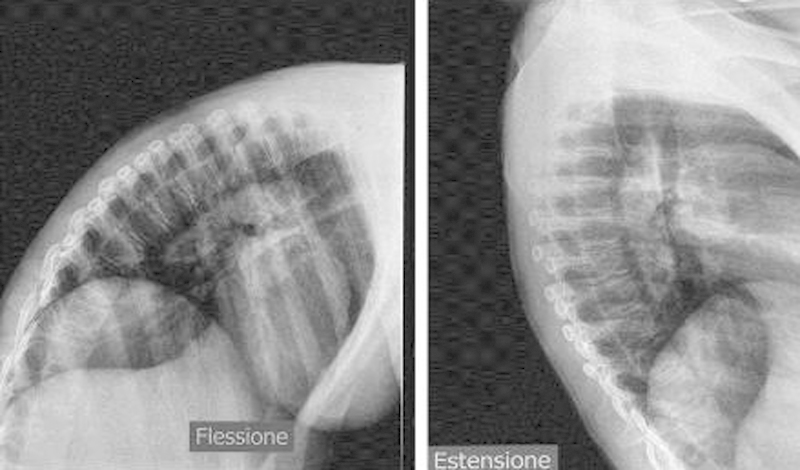
The patient underwent surgical decompression within 24 h from the onset of the symptoms by means of Th1–Th10 conservative laminectomy; the intersomatic joints and the two posterior interapophyseal articulations were spared. The d9ura was opened for the entire length of the exposition and the SSDH was completely evacuated without apparent medullary damage. Post-operative MRI and 3 D CT scan confirmed the extension and the effectiveness of the surgical procedure, along with the spinal cord lesional pattern (Fig. 2). At 36 months follow up, dynamic spine X-ray confirmed the stability of the thoracic spine; the ASIA Score improved to D (Fig. 3).
Fig. 2.
(Left) Sagittal T2 MR reconstruction (late follow up) showing T1–T10 cord decompression along with diffuse lesional pattern signal from Th3 to Th 10. (Right) 3D CT scan reconstruction showing the extension of laminectomy (C7) Th1–Th 10.
Fig. 3.
Dynamic (Right Flexion–Flessione Left Extension–Estensione) Thoracic spine X-ray exam excluding postoperative instability.
3. Discussion
SSDH is a rare condition determining spinal cord compression (less than 1%). Spontaneous SSDHs without detectable structural lesions or anticoagulant therapy are further rare. Among 26 cases harbouring SSDHs, documented in the literature, the thoracic spine was found to be the preferred site and the compression was usually spanning over several vertebral elements[1–4] (Table 1).
Table 1.
Review, 26 cases of SSDH reported in literature.
| Author | Year | Age, sex | Hematoma location | Bleeding cause, risk factors | Preop neuro deficit | Angio | Treatment (spinal) | Recovery |
|---|---|---|---|---|---|---|---|---|
| Swann [22] | 1984 | 46, F | TL junction | Unknown | Transient mild paraparesis | Yes | Lumbar puncture | Complete recovery |
| Kalina [10] | 1995 | 60, F | T7–S2 anterior | Unknown, polycythemia vera | Mild paraparesis | No | Conservative | Complete recovery |
| Kang [11] | 2000 | 49, F | T5–L3, anterior | Unknown | Transient mild paraparesis | No | Conservative | Complete recovery |
| Küker [14] | 2000 | 81, M | Mid T spine | Unknown | Paraparesis (M3/5) | No | Surgery | Complete recovery |
| Küker [14] | 2000 | 56, F | Thoraco-lumbar | Unknown | Paraparesis (M1–3/5) | Yes | Surgery | Good recovery |
| Kirsch [2] | 2000 | 47, M | T4–L5, antero-lateral | Unknown | Paraparesis | Yes | Laminectony T11-L1 | Improved |
| Kirsch [2] | 2000 | 42, M | CCJ–L3, around SC | Unknown | Paraplegia | No | Laminectomy T2-5 | No recovery |
| Kirsch [2] | 2000 | 34, M | T1–4, around SC | Unknown | Only pain and paresthesia | Yes | Conservative | Complete recovery |
| Yamada [23] | 2003 | 38, F | T1–7, anterior | Unknown | Mild paraparesis | Yes | Conservative | Complete recovery |
| Konitsiotis [13] | 2003 | 60, F | T3–L5, anterior-lateral | Unknown, essential thrombocythaemia | Only pain | No | Conservative | Pain subsided |
| Cha [6] | 2005 | 72, F | T3–T6, posterior-lateral | Unknown, aspirin + low molecular heparin | Paraplegia | Yes | Laminectomy T3-5 | No relevant recovery |
| Kyriakides [15] | 2007 | 44, M | T2–T6, anterior | Unknown | Paraplegia | No | Laminectomy T2-6 | Subtotal recovery |
| Kim SD [12] | 2008 | 48, F | T1–4, mainly anterior | Unknown | FMDParaplegia | No | Laminectomy T1-4 | No recovery |
| Ozdemir [21] | 2008 | 50, M | T4–T8, anterior | Unknown | Paraparesis (M3–4) | No | Laminectomy T4-6 | Complete recovery |
| Kakitsubata [3] | 2009 | 66, M | T11/12, anterior-lateral | Unknown | Only pain | No | Conservative | Pain subsided |
| Oh [20] | 2009 | 59, F | C3–C6, posterior-lateral | Unknown | Left-sided hemiparesis | No | Conservative | Complete recovery |
| Badge [5] | 2009 | 78, F | T3–T12 posterior | Anticoagulant therapy | Neuro-deficit in lower limb | No | Laminectomy L5 | Good Recovery |
| Panciani [18] | 2009 | 79, F | C5–T6 | Unknow | Paraplegia and urinary retention | No | Conservative | Improvement |
| Payer [19] | 2010 | 59, M | T2–T9 anterior | Anticoagulant therapy | Acute paraparesis, sphincter dysfunction | No | Conservative | Complete recovery |
| Dampeer [8] | 2010 | 68, M | T6–T7 anterior | Anticoagulant therapy | Paraplegia with paresthesia, urinary retention | No | Laminectomy T6-7 | Improved |
| Alpoim [4] | 2011 | 57, F | T4–T9 | Anticoagulant therapy | Dorsal pain, paresthesias and paraparesis | No | Laminectomy T4-5 | Complete recovery |
| Na-rae Yang [24] | 2011 | 55, F | C2–T6 | Hypertension, diabetes | Back pain and progressive paraplegia | No | Conservative | Complete recovery |
| Na-rae Yang [24] | 2011 | 38, M | C6–T5 antero-lateral | Unknow | Chest and back pain, acute urinary retention | No | Conservative | Complete recovery |
| Haji Mohd Yasin [9] | 2012 | Unknow | Unknow | Warfarin and fluoxetine | Acute neurological abnormalities of the limbs | Unknown | Unknow | Unknow |
| Panciani [17] | 2013 | 79, F | C5–T6 | Unknow | Paraparesis, anesthesia from mammillary line, sphincter dysfunction | No | Delayed surgery: T5 hemilaminectomy | Significant improvement |
| Chung [7] | 2014 | 66, F | C7–T4 | Unknow | Headache of sudden onset and neck stiffness | Yes | Conservative | Improvement |
3.1. Pathophysiology
SSDHs often result from major or minor spine trauma or from spine puncture, including spinal anesthesia. “Spontaneous” acute SSDHs are even more rare and have mostly been observed in conjunction with coagulopathies or anticoagulant therapy, intraspinal tumor and vascular anomalies such as aneurysms or spinal dural arteriovenous fistulas [2,3,15].
The physiopathology of spontaneous idiopathic SSDHs is little understood [1,12,14]. Rupture of valveless radiculo-medullary veins in the subarachnoid space after increased intra-abdominal or intra-thoracic pressure or from minor trauma are some possible mechanisms [2]. This hypothesis could explain clinical signs of subarachnoid hemorrhage (SAH) in many patients with SSDH, the reported combination of SSDH and SAH and the potential dilution of such hematomas by the cerebrospinal fluid (CSF) [2,3,21]. Conversely, SSDH has been thought to arise from the few thin, delicate extra-arachnoidal vessels located on the inner dural surface and then breaking through the arachnoid into the subarachnoid space: it is usually impossible to determine the origin [3,12,25]. In either case, the diluting effect of the CSF prevents clot formation, unless the hematoma is sufficiently large to block CSF flow [15,26].
In idiopathic SSDHs, therapy is limited to the hematoma management, as there is no underlying pathology to face with surgically [2,3,21]. Platelet dysfunction has been shown to be associated with SSDH as shown in Table 1 [10]. Discontinuation of anti-aggregating therapy, however, must be weighed against potential thrombotic complications, and depends on the individual indication of such a treatment.
3.2. Clinical presentation
The clinical presentation of SSDH is characteristic of a sudden onset of severe back or neck pain around the involved vertebrae with radiating pain around the corresponding dermatomes. The initial symptoms are usually vague and the hematoma is difficult to identify until the patient displays symptoms of cord compression hours or days after the onset of pain. Ascending numbness, radicular paresthesia, bowel and bladder dysfunction and progressive paraparesis can be prodromic to permanent neurologic deficits or even death [2,3,15].
3.3. Diagnosis
Although in the past computed tomographic myelography (CT) was largely used for SSDH diagnosis, nowadays MRI is the gold standard imaging modality for recording and recognizing the temporal changes of the hemorrhage, thus facilitating both monitoring of treatment. Indeed the MRI findings of SSDH vary based on the clot, age and oxygenation: within the first 24 h after symptom onset, the hematoma shows isointensity on T1WI and hyperintensity on T2WI. After 24 h, it appears as a high signal on T1WI and as a low signal on T2WI. After injection of gadolinium, peripheral enhancement of the lesion is found frequently; otherwise the central enhancement is found only occasionally. The early MRI findings of our case confirmed the hypothesis of the onset of the SSDH within 24 h. Although T2 hyperintense signals suggesting intramedullary edema are frequently related with a poor prognosis, our patient presented a good postoperative recovery. The effects of the primary mechanical injury and the development of a secondary injury are frequently coexisting, but the vascular impairment seems to be the epiphenomenon leading to the final outcome. Possible reversal of the differential pressure resulting in slow vascular drainage of the cord could explain the secondary vascular anomalies as well as the damage of the neural tissue as observed in our case. The major involvement of the central grey matter, compared to the less compromised peripheral white matter, as seen on the axial MRI imaging, could be explained by the greater sensitivity of the more metabolically active grey matter to venous congestion due to the AVDF.
Spinal angiography is generally considered to be the gold standard for demonstrating spinal artery aneurysms, arteriovenous malformations, spinal dural arteriovenous fistulas or other pathologies that can cause spinal subarachnoid hemorrhage. Nevertheless a clear infarction or hemorrhage are extremely rare especially in cases of spinal DAVF [25]. In our case spinal angiography had not been performed for two reasons: the unavailability of angiographic room in emergency and a rapid progression of symptoms; therefore we preferred to remove the cause of spinal cord compression promptly, although the cause of bleeding was not assessed previously.
By reviewing the literature, angiography has been used to rule out vascular malformations and was performed in 6 of the 26 cases described in the literature; it still remains a case to case decision based on availability, degree of neurosurgical emergency and suspicion level of vascular malformation (see Table 1).
3.4. Treatment and follow up
Among the similar cases reported in the literature (12/26 patients: 46.15%) nonoperative treatment may be justified in presence of minimal neurologic deficits. In presence of major deficits, or a rapidly deteriorating clinical and radiological (CT, MRI) patterns, patients are usually likely to benefit from drainage or surgery [18]. [10,18,20,23]. In such cases, early decompressive laminectomy with evacuation of hematoma are considered the best treatment for SSDH, as performed in our case (Table 1). On the other hand a case of a 79-year-old female undergone a surgical evacuation of a spontaneous SCSH one year after the diagnosis has been described [17]. She presented with a severe paraparesis and showed a considerable improvement in sensory-motor performances after surgery. Consequently the treatment of spontaneous SCSH is not well defined and universally accepted. Early surgery is mandatory in cases presenting with severe deficits and an aggressive approach should be considered as a viable option in cases of spontaneous SSDH even after a long lasting spinal cord compression [17].
Although the outcome predominantly depends on the clinical status and the levels of the lesion, our case showed a satisfactory late follow up, despite he presented severe neurological deficits and he underwent 10 levels laminectomy; such finding seems related to the short interval (within the 24 h) between the onset of the symptoms and the surgical decompression [27–29]. Moreover it is also known that patients with paraplegia and bowel and bladder dysfunction present the poorest prognosis regardless of surgical or conservative treatment [18,27–29].
3.5. Levels laminectomy
Our case is the first reported in the literature harbouring 10 levels thoracic SSDH treated with conservative 10 levels laminectomy; consequently posterior fixation was not performed because unnecessary. In adult patients conservative laminectomy at thoracic level does not necessarily affect spinal stability, since thoracic chest “per se” supports local stability. Conversely, post-laminectomy kyphosis is expected in most of pediatric patients and up to 100% when it is performed at cervical level [30].
We did not decide to perform laminectomy at alternate levels because in this way a complete access to the hematoma and its total removal, could not been completely allowed. Furthermore we did not perform neither laminoplasty nor posterior fixation since the intrinsic stability of the thoracic chest does not require anatomic reconstruction of the posterior elements at this level and the operative time would be excessively prolonged for an urgent procedure. Surprisingly thoracic kyphosis has been reported as complication after laminoplasty [31].
4. Conclusion
-
•
Fast clinical and neuroradiological identification along with urgent (within 24 h from the onset of the symptoms) surgical management are mandatory in SSDHs in order to achieve satisfactory clinical results.
-
•
Extensive operative strategy can be necessary.
-
•
Ten levels thoracic laminectomy is safe and effective, as long as conservative. No postoperative instability occurs in 10 levels thoracic laminectomy since articular processes are spared.
Conflict of interest statement
None.
Funding
None.
Consent
We have obtained written consent from the patient before starting the study.
Author contributions
All authors of this paper have directly participated in the planning, execution, or analysis of this study. All authors of this paper have read and approved the final version submitted.
References
- 1.Domenicucci M., Ramieri A., Ciappetta P. Nontraumatic acute spinal subdural hematoma: report of five cases and review of the literature. J. Neurosurg. 1999;91(1 Suppl):65–73. [PubMed] [Google Scholar]
- 2.Kirsch E.C., Khangure M.S., Holthouse D., McAuliffe W. Acute spontaneous spinal subdural haematoma: MRI features. Neuroradiology. 2000;42(8):586–590. doi: 10.1007/s002340000331. [DOI] [PubMed] [Google Scholar]
- 3.Kakitsubata Y., Theodorou S.J., Theodorou D.J., Miyata Y., Ito Y., Yuki Y., Honbu K., Maehara T. Spontaneous spinal subarachnoid hemorrhage associated with subdural hematoma at different spinal levels. Emerg. Radiol. 2010;17(1):69–72. doi: 10.1007/s10140-008-0792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpoim B., Rodrigues M., Silva P., Carvalho B., Pereira P., Vaz R. Spontaneous spinal subdural hematoma. Acta Med. Port. 2011;24(3 Suppl 3):725–728. [PubMed] [Google Scholar]
- 5.Badge R., Chan D. Spinal subdural haematoma in association with anticoagulant therapy, an unusual presentation: a case report and review of literature. Cases J. 2009;12(2):151. doi: 10.1186/1757-1626-2-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha Y.H., Chi J.H., Barbaro N.M. Spontaneous spinal subdural hematoma associated with low-molecular-weight heparin. Case report. J. Neurosurg. Spine. 2005;2(5):612–613. doi: 10.3171/spi.2005.2.5.0612. [DOI] [PubMed] [Google Scholar]
- 7.Chung J., Park I.S., Hwang S.H., Han J.W. Acute spontaneous spinal subdural hematoma with vague symptoms. J. Korean Neurosurg. Soc. 2014;56(3):269–271. doi: 10.3340/jkns.2014.56.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dampeer R.A. Spontaneous spinal subdural hematoma: Case study. Am J Crit Care. 2010;19(2):191–193. doi: 10.4037/ajcc2009982. [DOI] [PubMed] [Google Scholar]
- 9.Haji Mohd Yasin N.A., Donato-Brown D., Taha A. Non-traumatic spontaneous spinal subdural haematoma. N. Z. Med. J. 2012;125(1363):77–80. [PubMed] [Google Scholar]
- 10.Kalina P., Drehobl K.E., Black K., Woldenberg R., Sapan M. Spinal cord compression by spontaneous spinal subdural haematoma in polycythemia vera. Postgrad. Med. J. 1995;71(836):378–379. doi: 10.1136/pgmj.71.836.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang H.S., Chung C.K., Kim H.J. Spontaneous spinal subdural hematoma with spontaneous resolution. Spinal Cord. 2000;38(Mar. (3)):192–196. doi: 10.1038/sj.sc.3100967. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.D., Park J.O., Kim S.H., Lee Y.H., Lim D.J., Park J.Y. Spontaneous thoracic spinal subdural hematoma associated with fibromuscular dysplasia. J. Neurosurg. Spine. 2008;8(5):478–481. doi: 10.3171/SPI/2008/8/5/478. [DOI] [PubMed] [Google Scholar]
- 13.Konitsiotis S., Glantzouni A., Argyropoulou M.I., Tsapoga T., Elisaf M., Efremidis S.C. Acute spontaneous spinal subdural haematomas in a patient with essential thrombocythaemia. J. Neurol. 2003;250(9):1109–1111. doi: 10.1007/s00415-003-0125-1. [DOI] [PubMed] [Google Scholar]
- 14.Küker W., Thiex R., Friese S., Freudenstein D., Reinges M.H., Ernemann U., Kringes T., Skalej M. Spinal subdural and epidural haematomas: diagnostic and therapeutic aspects in acute and subacute cases. Acta Neurochir. (Wien) 2000;142(7):777–785. doi: 10.1007/s007010070092. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakides A.E., Lalam R.K., El Masry W.S. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine. 2007;32(21):619–622. doi: 10.1097/BRS.0b013e318154c618. [DOI] [PubMed] [Google Scholar]
- 17.Panciani P.P., Cornali C., Agnoletti A., Esposito G., Ronchetti G., Fontanella M. Recovery after delayed surgery in a case of spinal subdural hematoma. Case Rep. Neurol. Med. 2013:310854. doi: 10.1155/2013/310854. Epub 2013 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panciani P.P., Forgnone S., Fontanella M., Ducati A., Lanotte M. Unusual presentation of a spontaneous spinal epidural haematoma. Acta Neurol. Belg. 2009;109(2):146–148. [PubMed] [Google Scholar]
- 19.Payer M., Agosti R. Spontaneous acute spinal subdural hematoma: spontaneous recovery from severe paraparesis? Case report and review. Acta Neurochir. (Wien) 2010;152(Nov. (11)):1981–1984. doi: 10.1007/s00701-010-0758-7. Review. [DOI] [PubMed] [Google Scholar]
- 20.Oh S.H., Han I.B., Koo Y.H., Kim O.J. Acute spinal subdural hematoma presenting with spontaneously resolving hemiplegia. J. Korean Neurosurg. Soc. 2009;45(6):390–393. doi: 10.3340/jkns.2009.45.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozdemir O., Calisaneller T., Yildirim E., Caner H., Altinors N. Acute spontaneous spinal subdural hematoma in a patient with bilateral incarcerated inguinal hernia. Joint Bone Spine. 2008;75(3):345–347. doi: 10.1016/j.jbspin.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Swann K.W., Ropper A.H., New P.F., Poletti C.E. Spontaneous spinal subarachnoid hemorrhage and subdural hematoma. Report of two cases. J. Neurosurg. 1984;61(5):975–980. doi: 10.3171/jns.1984.61.5.0975. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K., Nakahara T., Yamamato K., Muranaka T., Ushio Y. Nontraumatic spinal subdural haematoma occurring in a postpartum period. Acta Neurochir. (Wien) 2003;145(2):151–155. doi: 10.1007/s00701-002-1045-z. [DOI] [PubMed] [Google Scholar]
- 24.Yang N.R., Kim S.J., Cho Y.J., Cho do S. Spontaneous resolution of nontraumatic acute spinal subdural hematoma. J. Korean Neurosurg. Soc. 2011;50(Sep. (3)):268–270. doi: 10.3340/jkns.2011.50.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch C., Gottschalk S., Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J. Neurosurg. 2004;100(Apr. (4 Suppl Spine)):385–391. doi: 10.3171/spi.2004.100.4.0385. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y.H., Cho K.T., Chung C.K., Kim H.J. Idiopathic spontaneous spinal subarachnoid hemorrhage. Spinal Cord. 2004;42(9):545–547. doi: 10.1038/sj.sc.3101620. [DOI] [PubMed] [Google Scholar]
- 27.Lenehan B., Fisher C.G., Vaccaro A., Fehlings M., Aarabi B., Dvorak M.F. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976) 1976;35(21 Suppl):35. doi: 10.1097/BRS.0b013e3181f32a44. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi-Movaghar V., Haghnegahdar A., Niakan A., Omidvar A., Barzideh E., Baghban F., Jamali M., Mohebali N., Yazdanpanah H., Fallahi S.M., Salimi Sotoudeh M., Sharifirad M.R. Primary report for a randomized controlled trial of traumatic spinal cord injured patients from T1 to L1—description of the surgical decompression in two groups of before 24 hours and 24–72 hours. J. Inj. Violence Res. 2012;4(3 Suppl 1) [Google Scholar]
- 29.Visocchi M., Di Rocco F., Meglio M. Subacute clinical onset of postraumatic myelopathy. Acta Neurochir. (Wien Austria) 2003;145:799–804. doi: 10.1007/s00701-003-0082-6. [DOI] [PubMed] [Google Scholar]
- 30.Yasuoka S., Peterson H.A., MacCarty C.S. Incidence of spinal column deformity after multilevel laminectomy in children and adults. CSJ Neurosurg. 1982;57(4):441–445. doi: 10.3171/jns.1982.57.4.0441. [DOI] [PubMed] [Google Scholar]
- 31.Amhaz H.H., Fox B.D., Johnson K.K., Whitehead W.E., Curry D.J., Luerssen T.G., Jea A. Postlaminoplasty kyphotic deformity in the thoracic spine: case report and review of the literature. Pediatr. Neurosurg. 2009;45(2):151–154. doi: 10.1159/000209655. [DOI] [PubMed] [Google Scholar]