Highlights
-
•
Different biomaterials have been used for breast augmentation, including paraffin, dimethilpolysiloxane (liquid silicon), polyacrylammyde hydrogel and hyaluronic acid.
-
•
In many countries, unregulated manufactured materials are available for biomedical use.
-
•
Permanent filler to the breast can lead to severe complications.
-
•
Complete removal of permanent fillers is challenging and require open surgical procedure.
-
•
Female patients who underwent permanent injection to the breast should avoid breast feeding.
Abbreviations: PAAG, polyacrylammide hydrogel; SDS, sodium dodecyl sulfate; PMMA, polymethyl-methacrylate; EMA, ethyl-methacrylate; HEMA, hydroxyethyl-methacrylathe; FDA, food and drug administration
Keywords: Breast injection, Breast augmentation, Methacrylathe, Synthetic biomaterial, Synthetic polymer
Abstract
Introduction
Several alloplastic biomaterials are available for injection to the breast, nevertheless not all of them are approved for biomedical use. Although in North America and Western Europe experience with synthetic biomaterials for breast augmentation is very limited, migratory streams might expose physicians worldwide to manage the related complications of these procedures. The aim of this study was to share with other surgeons the case of a patient presenting complications after breast augmentation with an unknown synthetic substance containing methacrylate.
Presentation of case
A 33-years old Asian woman presented to our Institution with breast deformities, lumps and chest pain. The patient referred previous breast injection “with hospital fat” performed in China six years before. She was not aware about the details of the procedure, and language barriers limited communication. Clinical examination and ultrasounds revealed the irregular distribution of an unknown substance in both breasts. The material was surgically removed and replaced in the same session with polyurethane implants. Chemical analysis revealed the presence of methacrylate.
Discussion
With a growing demand for non-invasive cosmetic surgery, has been reported a growing population of untrained and unlicensed personnel performing cosmetic surgery in many countries where there are no laws that restrict the use of cosmetic procedures to physicians with appropriate training and with approved materials. Surgical removal of this substances can be extremely challenging and an open procedure with surgical debridement is recommended.
Conclusion
Breast augmentation with non-absorbable biomaterials can lead to severe complications, in particular for patients intending to breastfeed.
1. Introduction
In recent years, breast augmentation with non-invasive approaches such as autologous fat grafting become more popular [1]. Several synthetic materials have been used for breast augmentation, either resorbable, like hyaluronic acid [2], or non-resorbable, like polyacrylammide hydrogel (PAAG) [3]. However, biomaterial injections for large volume breast augmentation are limited as they presents several limitations involving patient safety and high costs.
Currently, many efforts have been performed in the field of tissue engineering and regenerative medicine for the realization of an ideal biomaterial to be used as scaffold in combination with stem cells for breast augmentation. Nevertheless, to date no synthetic scaffold has been approved to be combined with stem cells for breast augmentation in humans. Current techniques for breast augmentation and reconstruction include implant positioning, autologous tissue transfer and fat grafting.
However, several synthetic substances are available on the market, and not all of them are approved for medical use. The aim of this study was to share with other plastic surgeons the case of a patient presenting complications after breast augmentation with an unknown synthetic substance containing methacrylate.
2. Case presentation
A 33 years old Chinese patient came to our Institution presenting breast deformities, lumps, and chest pain (Fig. 1). Symptoms started about four months before, during lactation after her first pregnancy. The initial manifestation was pain, swelling and right breast leakage of a yellow material. A thorough examination of the patient’s history revealed previous breast augmentation performed in China six years before through bilateral breast injections with an unknown substance. She referred several times to the procedure as “lipofilling with hospital fat” but when asked, she affirmed to have received neither liposuction nor fat excision because she were too slim. The patient was not aware about the details of the procedure, and language barriers further limited the communication.
Fig. 1.

Pre-operative pictures show breast asymmetry and displaceable lumps. In the lower quadrants of the right breast, a scar shows the previous site of methacrylate leakage.
On examination, breasts resulted asymmetric with irregular contours and visible lumps. The right breast appeared deflated and smaller then the left one, and presented an hyperchromic scar above the infra-mammary fold indicated by patient as the site of previous breast discharge. The left breast was larger then the right one, presenting edema, firmness and visible lumps. The most noticeable was located medially, showing gel migration toward sternal region (Fig. 1). Palpation of the right breast did not detect any particular sign, while in the left breasts it produced intense pain. Under pressure palpation lumps could be displaced producing a sense of fluctuation, and the indentation persists after releasing the pressure. Before operative procedure, the implanted material was localized with ultrasounds.
3. Surgical procedure
The patient was informed that complete removal of the substance would likely be not possible in one session, therefore she was encouraged to come back if symptoms would persist or represent. An open procedure under general anesthesia was undertaken, and surgical drainage was performed through hemi-periareolar incisions. In the right breast the infiltrated subcutaneous and glandular tissues were dissected. In the left breast the injected substance were encapsulated. One capsule was excided en-block (Fig. 2) while the rest of the material was drained and squeezed out (Fig. 3). It had a yellow, granular and semisolid aspect. After surgical removal, it was sent to the laboratory for chemical analysis. Cavities were subjected to curettage and irrigation. Immediate breast reconstruction was performed with polyurethane prosthesis. The implant pocket was realized in the no gel plane, and implants were placed in the submuscular plane. Negative pressure drains were placed in both breasts. After surgery, the patient was invited to multiple follow-up controls including clinical examinations and breast ecography. Patient’s recovery was uneventful, with no recurrence during 30 months of follow-up (Fig. 4).
Fig. 2.

Intra-operative pictures. In the left breast part of the injected material was encapsulated and excised en-block.
Fig. 3.
Intra-operative pictures. The not-encapsulated material had a yellow aspect with a granular and semisolid consistence.
Fig. 4.
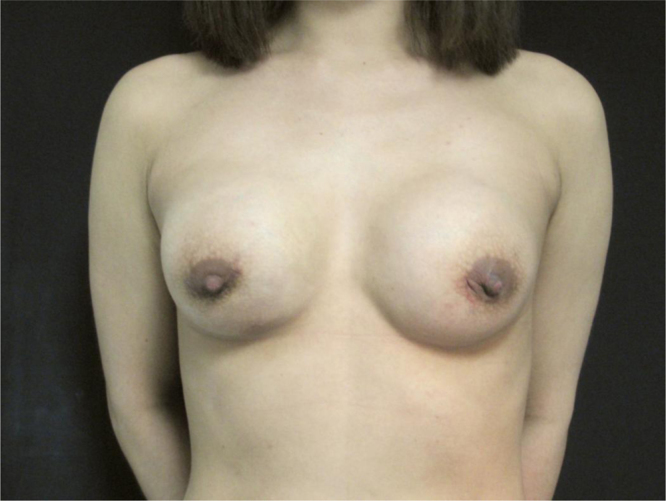
Post-operative picture: 30-months follow-up results.
4. Chemical analysis
The dispersion was centrifuged and the precipitate was subjected to solubility test in organic solvents, re-suspended in acetone and n-hexane, boiled in sodium dodecyl sulfate (SDS) in the presence of β-mercaptoethanol, and, after centrifugation, the precipitate was treated with sulfuric acid. Chemical analysis revealed that no protein band and peptide were detectable and that the material had an organic nature not constituted by the silicone polymer. Methacrylate was the only identified material.
5. Discussion
Over the past decades, plastic surgeons have searched for an ideal biological or synthetic substance for breast augmentation [4]. Several non-absorbable materials are available on the market and few of those contain acrylates, a family of polymers made from acrylate monomers, including acrylammide (in the form of polyacrylammide hydrogel PAAG) or methacrylate (in the form of polymethyl-methacrylate PMMA, ethyl-methacrylate EMA or hydroxyethyl-methacrylathe HEMA) [5]. Products containing methacrylate are commercialized as ArteFill® (Suneva Medical, San Diego, USA), Dermalive® (Dermatech, France), Metacrill® (private lab, Rio de Janeiro, Brazil), and Rhegecoll® (Dermabiol Institute of Kuhra Vital GmbH, Lucerne, Switzerland); among those only ArteFill® is approved by FDA for nasolabial folds [6]. Nevertheless, several unapproved manufactured materials can be available for injections in many regions like China, Latin America or Eastern Europe, and often their components remain unknown.
To the best of our knowledge, this is the first case reporting the use of methacrylate for large volume injections to the breast: in the medical literature several studies describing breast injection with permanent fillers are available, including PAAG, dimethylpolisiloxane (liquid silicon) and paraffin, but no data on the use of methacrylate for breast augmentation have been previously published. The only reported breast application of methacrylate consisted on small volume injections to the nipple in order to improve its projection after breast reconstruction [7].
One of the most challenging aspects for a surgeon facing this cohort of patients is the inability to completely remove the injected material [8]. Some authors argue that permanent fillers in the breast, like PAAG, can be easily removed by aspiration with a suction cannula [9]. Nevertheless, we considered blunt aspiration extremely difficult because of the semisolid consistence of methacrylate and also unpredictable as it might leaves residual material in the breast tissues, therefore an open procedure with surgical debridement is recommended.
The patient remained asymptomatic until she started breastfeeding. The inflammation of mammary ducts during lactation in patients with permanent fillers has been previously reported after PAAG injection, and it has been postulated that the blocked lactiferous ducts may lead to inflammation and galactocele formation [10]. Furthermore, we hypothesize that the mechanical suction performed by the child during lactation can cause material displacement in the breast tissue and rupture of the capsule that surrounds it, causing material leakage and inflammation. Therefore, asymptomatic patients who underwent breast injection with permanent biomaterials should avoid breastfeeding.
6. Conclusion
Injection of synthetic non resorbable biomaterials to the breast can causes irreversible damages, necessitating debridement procedures and breast reconstruction.
With a growing demand for non-invasive cosmetic surgery, a growing population of untrained and unlicensed personnel performing cosmetic surgery has been reported in many countries where there are no laws that restrict the use of cosmetic procedures to physicians with appropriate training and with approved materials. In North America and Western Europe experience with non-resorbable filler for breast augmentation is very limited. However, migratory streams might expose surgeons without any experience with these injections to manage patients with the related complications.
Conflict of interest
None.
Funding
None.
Ethical approval
N/A.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
All the authors contributed on the realization of the manuscript.
Guarantor
Prof. Francesco D’Andrea.
References
- 1.Coleman S.R. Structural fat grafting: more than a permanent filler. Plast. Reconstr. Surg. 2006;118(September (Suppl. 3)):108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 2.Hedén P., Sellman G. Body shaping and volume restoration: the role of hyaluronic acid. Aesthet. Plast. Surg. 2009;33(May (3)):274–282. doi: 10.1007/s00266-008-9303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z., Li S. Polyacrylamide hydrogel injection for breast augmentation: another injectable failure. Med. Sci. Monit. 2012;18(June (6)) doi: 10.12659/MSM.882910. CR399–408. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Alster T.S., West T.B. Human-derived and new synthetic injectable materials for soft-tissue augmentation: current status and role in cosmetic surgery. Plast. Reconstr. Surg. 2000;105(7):2515–2525. doi: 10.1097/00006534-200006000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Yang N., Muradali D. The augmented breast: a pictorial review of the abnormal and unusual. AJR Am. J. Roentgenol. 2011;196(4):W451–60. doi: 10.2214/AJR.10.4864. [DOI] [PubMed] [Google Scholar]
- 6.http://www.accessdata.fda.gov/cdrh_docs/pdf2/p020012b.pdf.
- 7.McCarthy C.M., VanLaeken N. The efficacy of artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10(January (4)):e7. [PMC free article] [PubMed] [Google Scholar]
- 8.Patlazhan G., Unukovych D. Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesthet. Plast. Surg. 2013;37:312–320. doi: 10.1007/s00266-012-0045-5. [DOI] [PubMed] [Google Scholar]
- 9.Cheng N.X., Wang Y.L., Wang J.H. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthet. Plast. Surg. 2002;26:375–382. doi: 10.1007/s00266-002-2052-4. [DOI] [PubMed] [Google Scholar]
- 10.Lin W.C., Hsu G.C., Hsu Y.C., Hsu H.H., Li C.S., Chen T.Y., Huang G.S. A late complication of augmentation mammaplasty by polyacrylamide hydrogel injection: ultrasound and magnetic resonance imaging findings of huge galactocele formation in a puerperal woman with pathological correlation. Breast J. 2008;14:584–587. doi: 10.1111/j.1524-4741.2008.00652.x. [DOI] [PubMed] [Google Scholar]



