Highlights
-
•
Hemangiopericytoma can present with abdominal metastases manifested by abdominal pain and subacute obstruction.
-
•
Laparoscopic resection is a feasible treatment strategy for intraperitoneal metastases from hemangiopericytoma.
Abbreviations: CT, computed tomography; HPC, hemangiopericytoma; WHO, World Health Organization
Keywords: Hemangiopericytoma, Intra-abdominal solid tumor metastasis, Intraperitoneal metastasis, Laparoscopy, Mesenchymal tumor
Abstract
Introduction
Hemangiopericytoma (HPC) is a rare mesenchymal tumor derived from capillary and postcapillary pericytes that often has an indolent course and occasionally presents with abdominal metastasis.
Presentation of case
Twenty-three years after the initial resection of an intracranial HPC located in the right frontoparietal region and left lateral ventricle, a 63-year-old man experienced dull abdominal pain and early satiety and had a palpable epigastric mass. Computed tomography indicated a suspected metastasis of HPC to the left upper abdomen. On laparoscopic exploration, the tumor was found in the falciform ligament and was excised laparoscopically per request of the patient. He had a fast recovery and experienced good relief of his pain and satiety. The patient had 2 additional metastases at his 12-month follow-up, both in the right retroperitoneum, and he again underwent laparoscopic resection. At his next annual follow-up, new metastases were identified in his liver, small-bowel mesentery, and peritoneal surface, prompting a trial of systemic chemotherapy. Because of progress of a left lower abdominal preperitoneal metastasis on follow-up at 3 years, the patient underwent a further successful laparoscopic exploration. Postoperatively, systemic chemotherapy was maintained.
Discussion
We report the recurrent laparoscopic resection of peritoneal metastases of primary intracranial HPC with good symptom control and fast recovery. Both the patient and the referring physician requested a minimally invasive surgical approach.
Conclusion
Laparoscopic resection is a feasible treatment strategy for intraperitoneal metastases and is effective in symptom palliation.
1. Introduction
Originally described in 1942 by Stout and Murray [1], hemangiopericytoma (HPC) is a rare mesenchymal tumor derived from pericytes, the cells of capillary walls and postcapillary venule walls [2]. This tumor type accounts for less than 1% of all sarcomas and can occur at any location in the body, including intra-abdominally [3]. Although most HPC has an indolent course usually, poorly differentiated HPC frequently can have an aggressive tumor biology and a poor prognosis [4–6]. Intracranial HPC is rare, estimated to account for 0.4% of primary intracranial neoplasms; the ideal treatment regimen has yet to be delineated [1,6,7]. Gross total resection is the only therapy with a proven survival benefit for central nervous system HPC [8–10], though adjuvant radiation therapy leads to longer tumor-free survival [6,11] and multimodal therapy has become the standard of care [12].
Recurrence rates have been reported as high as 42–90%, with time of recurrence at a median of 5 years after initial resection but as late as 27 years [6,9,13]. Extracranial metastases occur in 20% of cases, through both hematogenous and lymphatic routes [6,7,14]. Commonly reported areas of extracranial metastases include bone, liver, lung, and skin [1,6,14].
We report the case of a patient with primary intracranial HPC that metastasized to the peritoneal cavity.
2. Presentation of case
A laparoscopic resection found metastatic HPC in a 63-year-old man. He had received a diagnosis of primary intracranial HPC at age 40 years. Initially, 2 lesions were found: a 3-cm mass in the base of the right frontoparietal region and a 1-cm mass in the left lateral ventricle. Pathologic analysis indicated the lesions were World Health Organization (WHO) grade II. Fourteen years after his initial surgery, the patient had a new lesion in the right frontal lobe, which was resected and identified as a WHO grade III recurrent HPC. After this second operation, he underwent adjuvant external beam radiation therapy.
Five years later, a pathologic fracture of his right femur occurred, and imaging showed a likely metastatic HPC. The HPC was treated with surgical resection and fixation, and pathologic evaluation confirmed WHO grade III metastatic HPC. In addition, a metastatic work-up consisting of computed tomography (CT) of the chest, abdomen, and pelvis showed 2 additional intra-abdominal lesions: a 6-cm lesion in the epigastric area and a 2-cm lesion in the right retroperitoneum. At that time, the patient was asymptomatic, and thus, the lesions were observed.
After 4 more years and at 23 years after his initial tumor resection, the patient presented with dull abdominal pain, early satiety, and a palpable epigastric mass. CT showed a considerable increase in size of the epigastric lesion to 10 cm in maximum diameter (Fig. 1). He was taken to the operating room for laparoscopic resection. The tumor was identified in the peritoneal space attached to the falciform ligament. This mass was removed successfully with ultrasonic dissection. The patient did well and was discharged 2 days after the operation without complication.
Fig. 1.
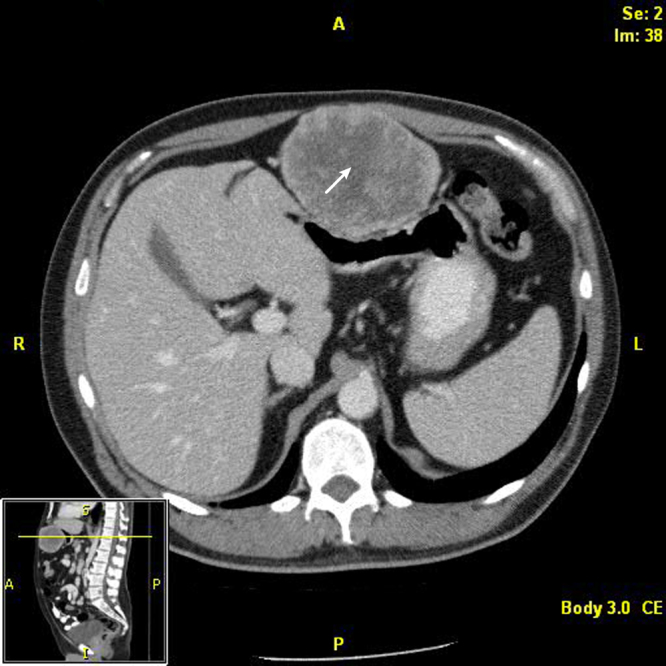
Computed tomographic scan showing large metastasis in the falciform ligament. The arrow indicates the metastasis.
At his 12-month follow-up, 2 additional asymptomatic lesions were noted on CT. The right retroperitoneal mass had substantially increased in size since previous scans, measuring 10 cm in maximum diameter, and the patient had a new 1-cm metastasis in the retroperitoneal attachments of the hepatic flexure of the right colon (Fig. 2). He again underwent a laparoscopic resection of his tumor recurrences. The smaller mass was removed easily. The right colon then was mobilized, allowing visualization and subsequent dissection of the right retrorenal mass with an ultrasonic dissection (Fig. 3). The patient tolerated the procedure well and was discharged 3 days after the operation. However, subsequent annual follow-up showed new metastases to the liver, small-bowel mesentery, and several peritoneal nodules on CT (Fig. 4). Given his advanced intra-abdominal disease, the patient underwent a trial of chemotherapy. This treatment was partially successful at slowing his disease; however, 3 years later, left lower abdominal preperitoneal metastases became progressively symptomatic, and the patient underwent further laparoscopic exploration and resection. In this interval, he also had pathologic fractures in the right femur and left humerus secondary to metastatic disease, which were treated by curettage and fixation, as well as an L1 vertebral metastasis that was treated with radioablation and vertebroplasty. His treatment continued as varying regimens of systemic chemotherapy, including temozolomide, bevacizumab, pazopanib, gemcitabine, and doxorubicin in the intervals.
Fig. 2.
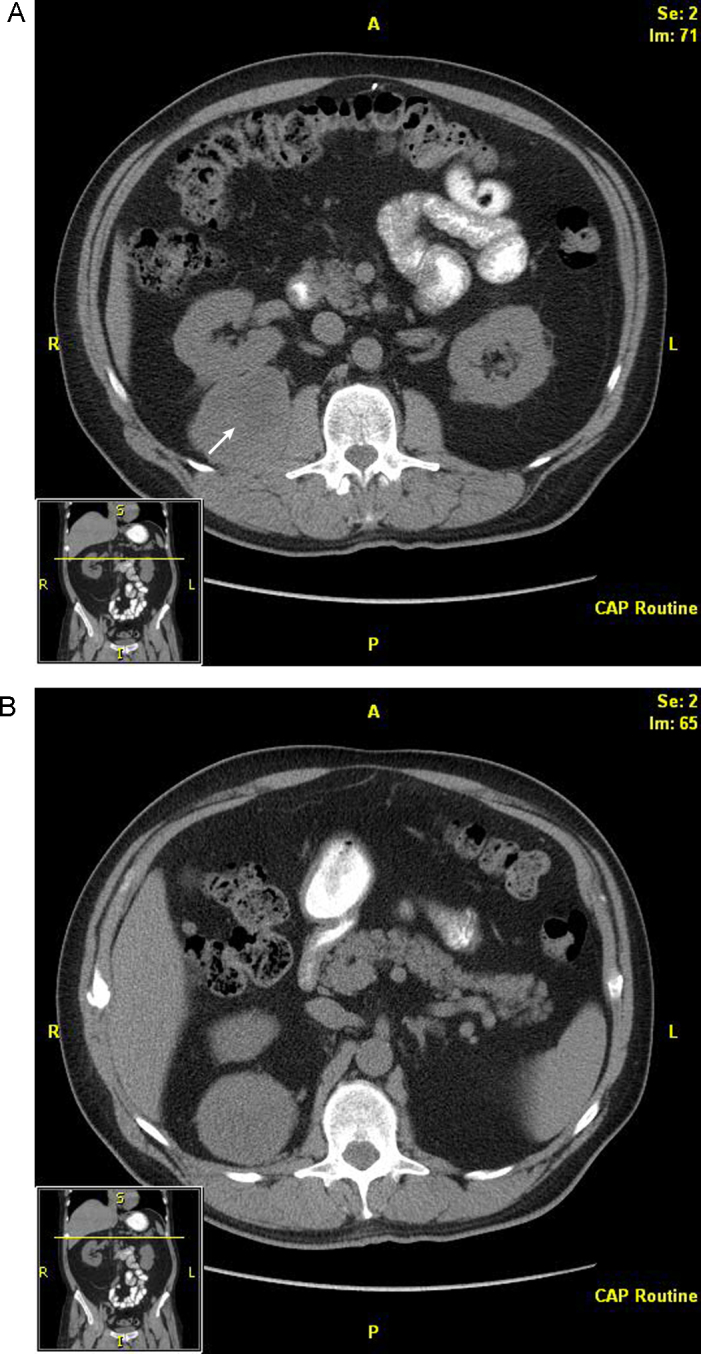
Computed tomographic scan showing metastases in the (A) right retrorenal retroperitoneum and (B) hepatic flexure attachments of the right colon. The arrow indicates the retrorenal metastasis.
Fig. 3.
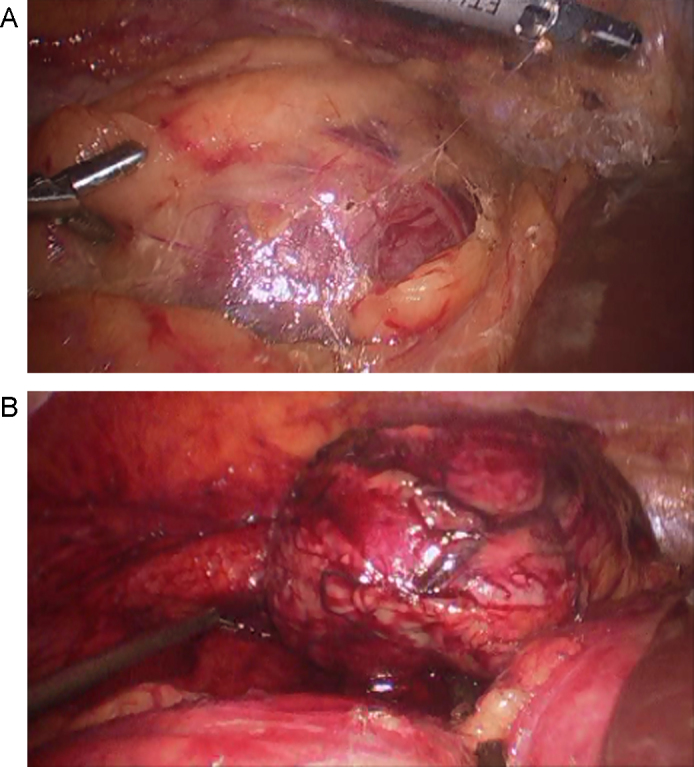
Laparoscopic resection using ultrasonic dissection. (A) Tumor is visible in tissue. (B) Tumor after it was dissected free.
Fig. 4.
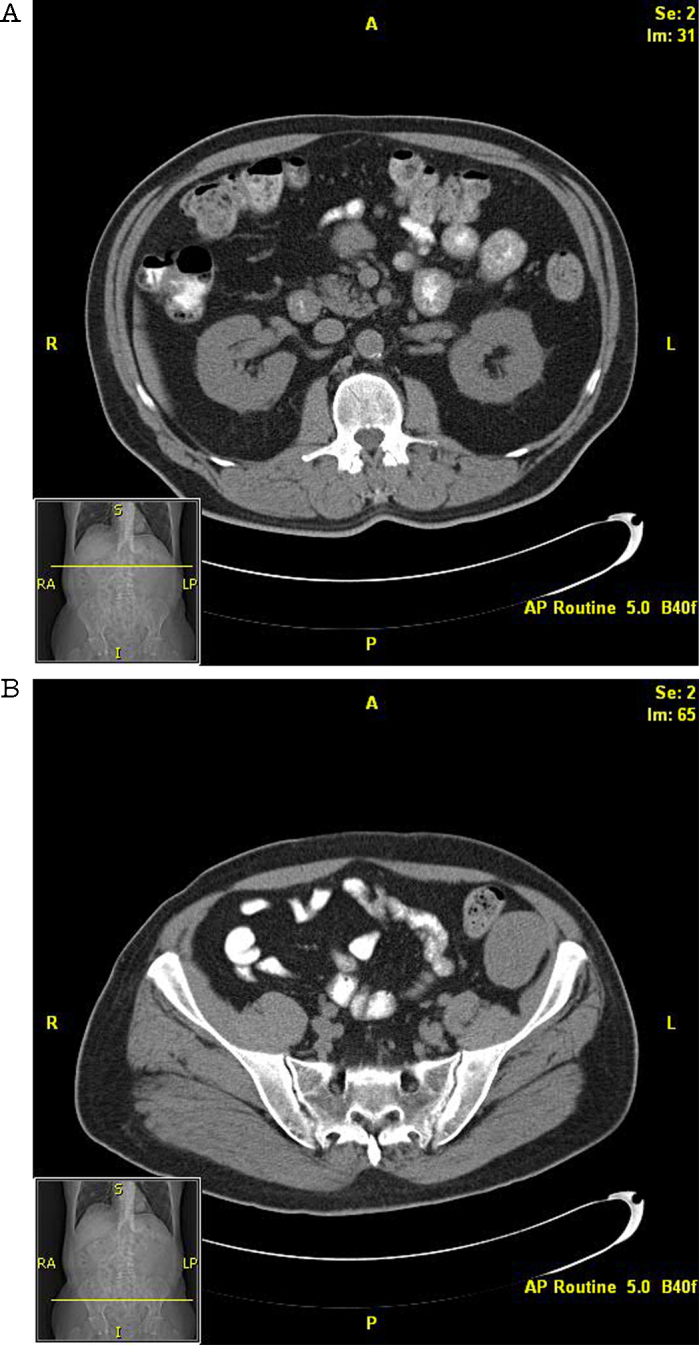
Computed tomographic scans (A and B) show metastases to small-bowel mesentery.
3. Discussion
Our case report confirms the potentially aggressive nature and metastatic potential of malignant HPC. Late recurrences have been reported in the literature as long as 27 years after the initial surgical resection [2,13]. Little is known about the course of HPC metastatic disease. The current best treatment is surgical resection of all known disease; an optimal management strategy for recurrent and metastatic disease has yet to be fully elucidated [1,12].
The patient in this case presented with local recurrence and delayed bony metastasis, a commonly reported development in HPC. However, he subsequently had additional metastases that presented in a delayed manner and occurred in the peritoneal cavity and retroperitoneum. His peritoneal and retroperitoneal disease was treated using a laparoscopic technique in accordance with the patient’s request, and the benefits of laparoscopic resection can be seen in his uneventful postoperative course.
Though initially resected to negative margins, the patient’s metastatic disease eventually progressed beyond what could be treated surgically.
4. Conclusion
Laparoscopic resection of symptomatic HPC metastases is feasible and effective in relieving symptoms. Repeat resections can be safely performed laparoscopically.
Conflict of interest
The authors have no conflicts of interest to report.
Funding
Dr Bingener’s time in this research was supported in part by NIDDK K23 DK093553. This study had no funding sources. The investigators’ sponsors (for other projects) had no role in collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to submit this manuscript for publication.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- 1.Stout A.P., Murray M.R. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg. 1942;116(July (1)):26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkowski M.J., Jian B.J., Bloch O., Chen C., Sughrue M.E., Tihan T. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012;118(March (6)):1628–1636. doi: 10.1002/cncr.26411. Epub 2011 Aug 11. [DOI] [PubMed] [Google Scholar]
- 3.Slattery L.R., Aronson S.G., Lowman E.W. Hemangiopericytoma review of abdominal cases. Am. J. Surg. 1956;91(June (6)):985–990. doi: 10.1016/0002-9610(56)90332-4. [DOI] [PubMed] [Google Scholar]
- 4.Leowardi C., Hinz U., Hormann Y., Wente M.N., Mechtersheimer G., Willeke F. Malignant vascular tumors: clinical presentation, surgical therapy, and long-term prognosis. Ann. Surg. Oncol. 2005;12(December (12)):1090–1101. doi: 10.1245/ASO.2005.09.002. Epub 2005 Nov 1. [DOI] [PubMed] [Google Scholar]
- 5.Wushou A., Miao X.C., Shao Z.M. Treatment outcome and prognostic factors of head and neck hemangiopericytoma: meta-analysis. Head Neck. 2014;(June) doi: 10.1002/hed.23812. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Melone A.G., D'Elia A., Santoro F., Salvati M., Delfini R., Cantore G. Intracranial hemangiopericytoma: our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg. 2014;81(March–April (3–4)):556–562. doi: 10.1016/j.wneu.2013.11.009. Epub 2013 Nov 13. [DOI] [PubMed] [Google Scholar]
- 7.Damodaran O., Robbins P., Knuckey N., Bynevelt M., Wong G., Lee G. Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J. Clin. Neurosci. 2014;21(August (8)):1310–1314. doi: 10.1016/j.jocn.2013.11.026. Epub 2014 Apr 13. [DOI] [PubMed] [Google Scholar]
- 8.Rutkowski M.J., Sughrue M.E., Kane A.J., Aranda D., Mills S.A., Barani I.J. Predictors of mortality following treatment of intracranial hemangiopericytoma. J. Neurosurg. 2010;113(August (2)):333–339. doi: 10.3171/2010.3.JNS091882. [DOI] [PubMed] [Google Scholar]
- 9.Leowardi C., Hinz U., Hormann Y., Wente M.N., Mechtersheimer G., Willeke F. Malignant vascular tumors: clinical presentation, surgical therapy, and long-term prognosis. Ann. Surg. Oncol. 2005;12(December (12)):1090–1101. doi: 10.1245/ASO.2005.09.002. Epub 2005 Nov 1. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishna R., Rostomily R., Sekhar L., Rockhill J., Ferreira M. Hemangiopericytoma: radical resection remains the cornerstone of therapy. J. Clin. Neurosci. 2014;21(April (4)):612–615. doi: 10.1016/j.jocn.2013.08.006. Epub 2013 Aug 27. [DOI] [PubMed] [Google Scholar]
- 11.Olson C., Yen C.P., Schlesinger D., Sheehan J. Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat gamma knife surgery. J. Neurosurg. 2010;112(January (1)):133–139. doi: 10.3171/2009.3.JNS0923. [DOI] [PubMed] [Google Scholar]
- 12.Chen L.F., Yang Y., Yu X.G., Gui Q.P., Xu B.N., Zhou D.B. Multimodal treatment and management strategies for intracranial hemangiopericytoma. J. Clin. Neurosci. 2015;22(April (4)):718–725. doi: 10.1016/j.jocn.2014.11.011. Epub 2015 Mar 3. [DOI] [PubMed] [Google Scholar]
- 13.Schiariti M., Goetz P., El-Maghraby H., Tailor J., Kitchen N. Hemangiopericytoma long-term outcome revisited: clinical article. J. Neurosurg. 2011;114(March (3)):747–755. doi: 10.3171/2010.6.JNS091660. Epub 2010 Jul 30. [DOI] [PubMed] [Google Scholar]
- 14.Kumar N., Kumar R., Kapoor R., Ghoshal S., Kumar P., Salunke P.S. Intracranial meningeal hemangiopericytoma: 10 years experience of a tertiary care Institute. Acta Neurochir. (Wien) 2012;154(September (9)):1647–1651. doi: 10.1007/s00701-012-1442-x. Epub 2012 Jul 12. [DOI] [PubMed] [Google Scholar]


