Highlights
-
•
Obesity itself is an independent risk factor for hiatal hernia.
-
•
The mechanisms responsible for the development of a de novo hiatal hernia are difficult to identify.
-
•
The dissection of the angle of his during creation of the gastric tube may also increase the risk of herniation.
Keyswords: Bariatric surgery, Laparoscopic complications, Sleeve gastrectomy, Hiatal hernia
Abstract
Introduction
Sleeve gastrectomy (SG) is a frequently used surgical procedure for the treatment of morbid obesity. Several complications of SG have been described; however, de novo hiatal hernia of the gastric tube, as a complication of SG, has not been described in the literature.
Presentation of case
Here, we report a case of a hiatal hernia 2 years after SG. In the case reported here, the hiatal hernia was associated with weight regain. The mechanisms responsible for the herniation of the pouch are difficult to identify. Conversion from sleeve gastrectomy to Roux-en-Y gastric bypass is an effective treatment for this complication. Its management is safe and effective.
Discussion
Obesity itself is an independent risk factor for hiatal hernia, found preoperatively in more than half of the morbidly obese patients. This predisposition is explained by higher intra-gastric pressure due to intra-abdominal or visceral fat, reduced inferior oesophageal sphincter pressure, and oesophageal motility problems.
Conclusion
To our knowledge, this is the first described case of hiatal hernia of the gastric tube after SG.
1. Introduction
Sleeve gastrectomy (SG) became the leading bariatric procedure in France in 2011 [1]. Although several complications of SG have been identified, de novo hiatal hernia of the gastric tube is yet to be detailed. Here, we report the case of a patient with herniation of the gastric tube through the hiatal orifice, complicating the SG 2 years after surgery. We describe the laparoscopic repair and discuss the mechanisms leading to this rare complication.
2. Presentation of case
A 57-year-old female patient presented to the department of surgery with mild intermittent gastric pain and reflux. Two years previously, she had undergone a SG for morbid obesity (BMI 50). Preoperative studies of upper gastro intestinal (UGI) >series and gastroscopy were normal. Peroperatively, the left diaphragmatic crus was seen, the greater curvature of the stomach was released and the whole fundus was excised, no hiatal hernia was identified. A postoperative UGI showed a narrow gastric tube with no other abnormalities (Fig. 1). The patient lost around 45 kg 1 year postoperatively. Two years later, the patient was complaining of mild epigastric pain. An enhanced CT scan showed a trans-hiatal hernia of the gastric tube (Fig. 2). Taking into consideration her symptoms and a moderate weight regain, a decision to convert the SG to a gastric bypass was validated in a multidisciplinary team meeting [2]. This conversion to a gastric bypass was performed with reduction of the gastric pouch, closure of the posterior crural orifice with interrupted non absorbable sutures, fashioning of a 30 ml gastric pouch, and a manual gastrojejunal anastomosis. Her postoperative course was uncomplicated. The patient had lost 28 kg at 6 months postoperatively.
Fig. 1.
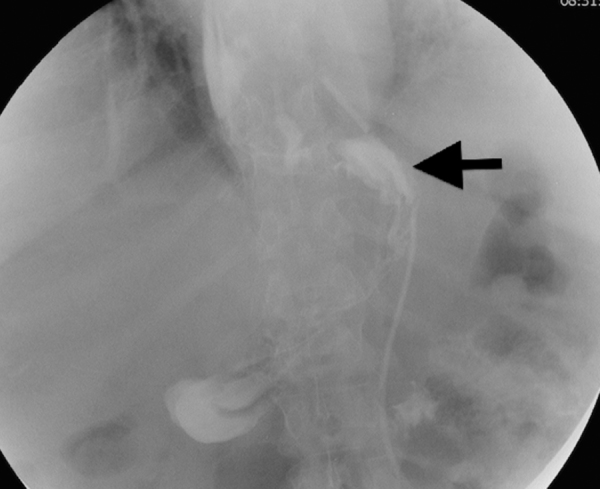
Postoperative oeso-gastroduodenal transit studies.
Fig. 2.
Gastric volumetry by gastric tomography. Arrow: pouch hiatal hernia.
3. Discussion
Sleeve gastrectomy is a surgical technique to treat obesity by both restrictive effect and hormonal action, through reduction in the levels of ghrelin in the plasma, the appetite stimulating hormone. Postoperative complications include gastro-oesophageal reflux (20–30%), ulcerations (2–15%), fistula and stenosis (2%) [3]. The differential diagnosis of abdominal pain in a patient who has undergone surgery for SG is based on the OGD transit examination, enhanced computer tomography and upper GI endoscopy.
Obesity itself is an independent risk factor for hiatal hernia, found preoperatively in more than half of the morbidly obese patients. The preoperative study did not reveal a hiatal hernia in our patient. This predisposition is explained by higher intra-gastric pressure due to intra-abdominal or visceral fat, reduced inferior oesophageal sphincter pressure, and oesophageal motility problems. Defective motility can be explained by the production of cytokines by visceral fat, reduced elastin in the diaphragmatic crura, and abnormal changes in the extra cellular matrix and collagen [4]. In our case, the preoperative and postoperative OGD studies were normal.
To conclude, the mechanisms responsible for the development of a de novo hiatal hernia are difficult to identify. Rapid weight loss following the SG may lead to increased risk of hernia mainly due to the enlargement of natural orifices such as the hiatal orifice, and it might have affected the pillars of the diaphragm because of the muscle depletion, which appeared hypotonic. The dissection of the angle of His and the left pillar during creation of the gastric tube may also increase the risk of herniation. The weight regain, the dilatation of the whole gastric tube and the persistence of the pylorus in the SG might also be a factor because of the increase in intra-gastric pressure. The weight regain after SG might be attributed to stomach tissue dilatation due to technical problems, and it could be also related to patient's psychological problems or negligence in following the post-surgical diet recommendations, especially for patients known to be sweet eaters. We believe that these factors, technical and not, are often both involved in the process of weight regain after LSG. For example, an incomplete section of the gastric fundus will not decrease the secretion of ghrelin, which can explain the incapacity of the patient to follow diet recommendations, therefore permitting stomach dilatation and weight regain [5].
4. Conclusion
Trans-hiatal herniation of the gastric tube should be added to the list of possible complications post SG and bariatric surgeons need to be aware of this rare complication and how to manage it. To our knowledge, this is the first described case of hiatal hernia of the gastric tube after SG. Laparoscopic repair of the hiatal orifice and conversion to Roux en Y gastric Bypass can be safely performed.
Conflict of interests
The authors declare no conflict of interest.
Funding
None.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Imed Ben Amor Tarek Debs Radwan KASSIR: writing.
Rodolphe Anty, Virginie Ben Amor, Jean Gugenheim reviewed and revised the paper.
References
- 1.http://www.atih.sante.fr/.
- 2.Amor I.B., Debs T., Martini F., Elias B., Kassir R., Gugenheim J. Laparoscopic conversion of a sleeve gastrectomy to the Roux-en-Y gastric bypass. Obes. Surg. 2015;25(August (8)):1556–1557. doi: 10.1007/s11695-015-1749-9. [DOI] [PubMed] [Google Scholar]
- 3.Toro J.P., Lin E., Patel A.D., Davis S.S., Jr., Sanni A., Urrego H.D., Sweeney J.F., Srinivasan J.K., Small W., Mittal P., Sekhar A., Moreno C.C. Association of radiographic morphology with early gastroesophageal reflux disease and satiety control after sleeve gastrectomy. J. Am. Coll. Surg. 2014;(May (9)) doi: 10.1016/j.jamcollsurg.2014.02.036. pii: S1072–7515(14)00377–9. [DOI] [PubMed] [Google Scholar]
- 4.Curci J.A., Melman L.M., Thompson R.W., Soper N.J., Matthews B.D. Elastic fiber depletion in the supporting ligaments of the gastroesophageal junction: a structural basis for the development of hiatal hernia. J. Am. Coll. Surg. 2008;207(2):191–196. doi: 10.1016/j.jamcollsurg.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Noel P., Nedelcu M., Nocca D., Schneck A.S., Gugenheim J., Iannelli A., Gagner M. Revised sleeve gastrectomy: another option for weight loss failure after sleeve gastrectomy. Surg. Endosc. 2014;28:1096–1102. doi: 10.1007/s00464-013-3277-9. [DOI] [PubMed] [Google Scholar]



