Highlights
-
•
Recurrence of breast cancer at the site of a prior core needle biopsy after definitive surgery is very rare—only 13 cases have been reported.
-
•
The majority are recurrent invasive cancers forming palpable masses in dermis and subcutaneous tissue at the skin puncture site of the core needle biopsy.
-
•
Diagnosis can be delayed if a lesion is not initially recognized as being at a needle biopsy site.
-
•
We report the first case of needle puncture site recurrence as Paget disease of the epidermis (ductal carcinoma in situ).
-
•
It is important to document the site of the skin puncture of a needle biopsy to aid in early detection, and recognition, of needle track recurrences.
Keywords: Breast cancer, Paget disease, Needle biopsy, Needle track recurrence
Abstract
Introduction
Core needle biopsy has become the preferred method of diagnosing breast carcinomas prior to definitive surgery. The possibility of displacing tumor cells into the needle track is a concern.
Presentation of case
A 38 year old woman was diagnosed with right breast ductal carcinoma in situ (DCIS) with microinvasion by core needle biopsy. Bilateral skin sparing mastectomies with immediate autologous reconstruction were performed. One and a half years later the patient noted erythema and a scaling crust on the skin of the right breast that progressed over several months. Punch biopsy revealed Paget disease restricted to the epidermis. Subsequent comparison to initial clinical photographs confirmed the cancer was associated with the skin puncture site of the needle biopsy. The patient underwent complete excision with skin grafting and remains free of disease three years later.
Discussion
Only 13 cases of needle track recurrences have been reported. The majority presented as invasive carcinoma forming a subcutaneous mass. In the current case, detection was delayed due to not initially noting that a skin lesion was at the puncture site of the original needle biopsy. This is the only case of recurrence as tumor limited to the epidermis.
Conclusion
Although recurrence in a needle track occurs very infrequently, clinicians should be aware of this phenomenon and investigate any changes, particularly when occurring at a needle biopsy site. Recording the skin puncture site can aid in early detection of recurrences. Recognition of a recurrence is important for prompt treatment and optimal prognosis.
1. Introduction
Needle biopsies have become the preferred method for the initial evaluation of breast lesions. Patients with benign findings are spared surgical excision and patients with malignant findings can have definitive surgery planned. There has been a long-standing concern about the possibility of needle track seeding leading to local breast cancer recurrence [1]. Fortunately, recurrences in the needle track are exceedingly rare. We report the case of a woman treated for ductal carcinoma in situ DCIS and one focus of microinvasion with mastectomy and autologous reconstruction, who recurred with DCIS involving the epidermis (Paget disease of the skin) at the skin puncture site of the original core needle biopsy.
2. Case report
A 38 year old woman at high risk of breast cancer due to prior radiation for Hodgkin disease was discovered to have an area of calcifications in the lower inner quadrant of the right breast on her first screening mammogram. No skin lesions were present and the nipple was normal in appearance. A 10 gauge vacuum-assisted core needle biopsy successfully sampled the calcifications and a marking clip was placed. The needle entered the breast at approximately 5:00 (Fig. 1). The biopsy revealed high grade DCIS with comedo necrosis and calcifications. A single focus was suspicious for microinvasion. The DCIS was negative for estrogen receptor (ER), showed 1–4% positivity for progesterone receptor (PR), and was positive for HER2 (3+). Breast MRI showed a corresponding 2.5 × 1.0 cm area of enhancement in the area of the DCIS, located 5.3 cm away from the nipple.
Fig. 1.
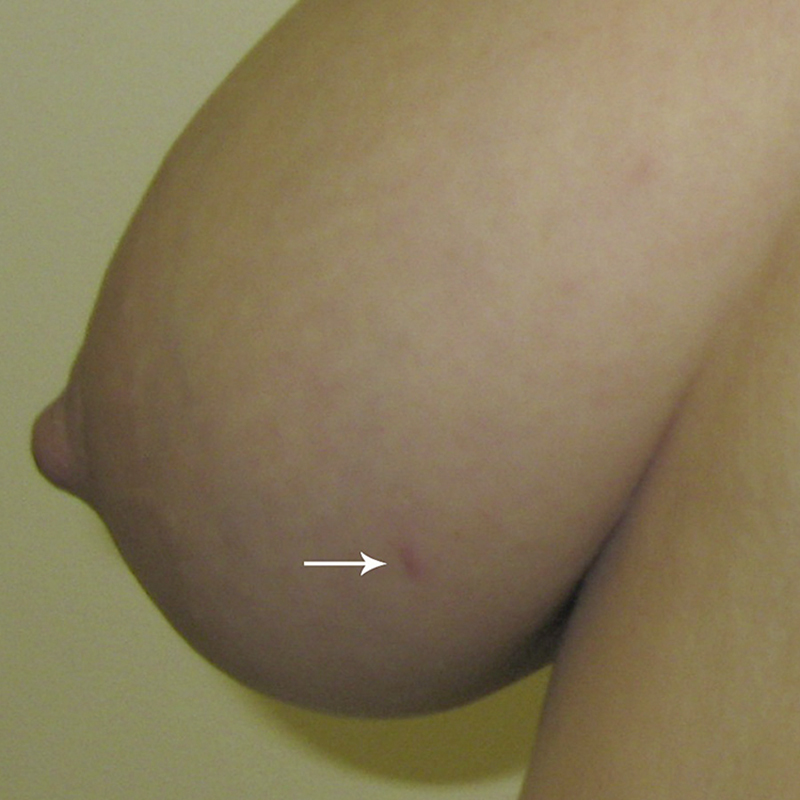
This photograph was taken by the plastic surgeon as part of routine pre-surgical planning for breast reconstruction. The diagnostic core needle biopsy skin entrance site is visible in the lower inner quadrant of the right breast (arrow). The site is located away from the patient's nipple, which is normal in appearance, and no other skin lesions are present. It was fortuitous that the photograph was taken shortly after the core needle biopsy before the skin puncture site had healed.
The patient opted to undergo bilateral skin sparing mastectomies with immediate reconstruction using pedicled transverse rectus abdominus myocutaneous (TRAM) flaps. The nipple and areolar complexes were removed, but the skin in the area of the prior needle biopsy site was preserved. The right mastectomy revealed DCIS with 2 foci of microinvasion over 3–4 cm. The skin, nipple, superficial skin flap margin, and deep margin were free of carcinoma, as were 3 sentinel nodes.
One and a half years after the mastectomy, the patient noted an area of skin change in the lower inner aspect of the right reconstructed breast (Fig. 2). This area was not close to the mastectomy scar or the reconstructed nipple. The lesion measured 1.2 cm but gradually increased in size and was treated by her primary care physician with topical antifungal medication. The skin lesion continued to enlarge and was brought to the attention of her breast surgical oncologist three years after her mastectomy. The erythematous lesion now measured 2.5 × 2.0 cm and appeared eroded (Fig. 3). Photographs taken by her plastic surgeon prior to her mastectomy and reconstruction fortuitously showed the skin puncture site of the original core needle biopsy (Fig. 1). It was apparent that the skin lesion was at this same site. A skin punch biopsy was performed and revealed tumor cells restricted to the epidermis (Paget disease).
Fig. 2.
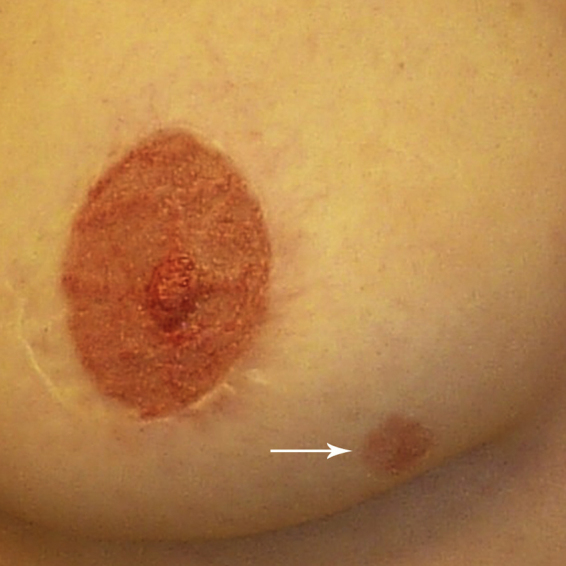
One and a half years after mastectomy, the patient developed an erythematous skin lesion. Over the next several months, this lesion continued to increase in size (arrow). The nipple has been reconstructed.
Fig. 3.
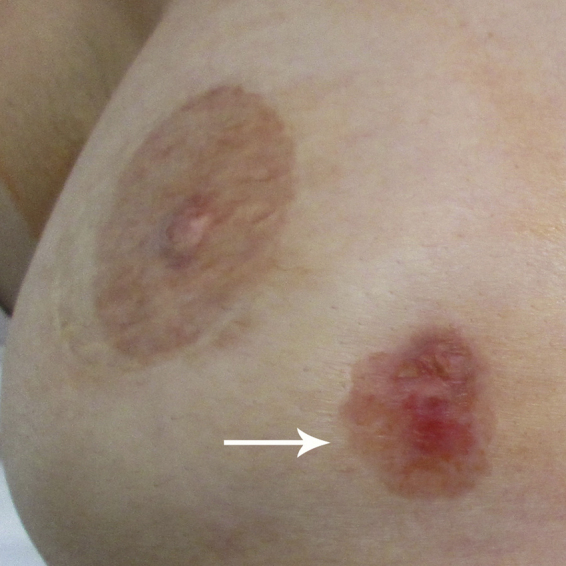
Three years after mastectomy, the lesion had increased to a size of 2 cm and now appeared eroded (arrow). It was noted at this time that the lesion was present at the site of the prior core needle biopsy based on the clinical photograph in Fig. 1. A skin punch biopsy was performed.
The entire area of involved skin was excised to negative margins. Because of the size and location of the lesion, full thickness skin grafting was necessary for cosmetic closure. The carcinoma was restricted to the epidermis over a 1.7 cm area (Fig. 4). The carcinoma was negative for ER, showed low levels of PR, and was strongly positive for HER2. No invasion was present. No evidence of an ectopic nipple, Toker cells, residual breast tissue, or apocrine glands was seen. Therefore the most likely source of the tumor cells was iatrogenic displacement of tumor cells from the breast into the epidermis from the prior core needle biopsy. The patient is without evidence of carcinoma, 3 years after the skin excision and 6 years after mastectomy.
Fig. 4.
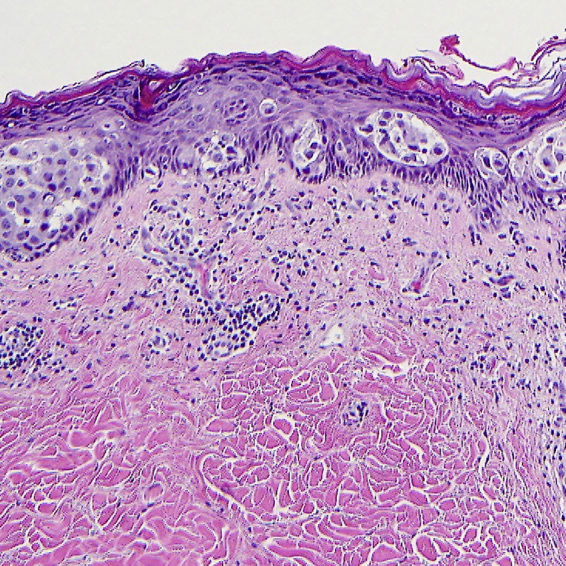
The skin punch biopsy and subsequent complete skin excision revealed tumor cells involving the epidermis. No invasion of the dermis was seen. There was no evidence of an ectopic nipple, apocrine glands, or eccrine gland involvement.
3. Discussion
Adenocarcinoma between an intact basement membrane and an overlying normal epithelium is classified as Paget disease. The most common cause in the breast is DCIS extending from breast ducts into the epidermis of the nipple areolar complex. In the current case, the Paget disease was located away from the nipple and other etiologies had to be considered. Paget disease can arise from an ectopic nipple [2,3]. However, the location was far from the normal milk line along which ectopic nipples usually occur and a previously existing nipple was not observed. Extramammary Paget disease is thought to arise from apocrine glands, as it occurs in the genital, perianal, and axillary areas [4,5]. However, the recurrence was far from the axilla and no apocrine glands were present. Very rare cases of “ectopic” extramammary Paget disease occur in skin without apocrine glands and may arise from eccrine glands [6]. This disease most commonly involves the trunk of elderly males. No involvement of eccrine glands was seen in the current case.
The only likely remaining mechanism for this case of Paget disease is displacement of tumor cells from the original DCIS to the epidermis from tumor seeding the needle track. This possibility is supported by the identical histologic appearance and tumor marker expression of the original and recurrent carcinomas and the location at the core needle biopsy site.
Carcinoma recurring in a needle track as Paget disease of the skin has not been reported previously. There are only 13 other cases of breast cancer recurrence in a needle track after definitive surgery and a disease- free interval (Table 1) [7–13]. Eleven of the cases were invasive cancers, one was DCIS, and one was not specified. The time to recurrence ranged from 1 to 6 years (average 2.5 years). Three patients who underwent breast conservation recurred as invasive carcinoma in the breast and 1 recurred in the skin. Seven of 9 patients who underwent mastectomy recurred as invasive carcinomas involving the skin and subcutaneous tissue. One patient recurred as invasive carcinoma in skeletal muscle. The site of recurrence was not specified for 1 patient. All but 1 patient had carcinoma limited to the core needle site. This patient had multiple foci of carcinoma in the dermis in the same quadrant [10]. She had presented with 3 invasive carcinomas and had undergone 3 core needle biopsies.
Table 1.
Cases of recurrent cancer in the needle track after definitive surgical treatment.
| Study | Biopsy type | Primary carcinoma | ER | HER2 | Surgery | XRT | Systemic treatment | Time to recurrence | Recurrent carcinoma | Location of recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Thurfjell et al. [7] | FNA | Adenoid cystic | Unknown | Unknown | BCT | No | Unknown | 4.1 years | Adenoid cystic | Breast |
| FNA | Invasive mucinous | Unknown | Unknown | BCT | No | Unknown | 2.5 years | Invasive mucinous | Breast | |
| FNA | Invasive tubuloductal | Unknown | Unknown | BCT | No | Unknown | 2.1 years | Invasive tubuloductal | Breast | |
| Chao et al. [8] | 14G stereo CNB | Invasive ductal | − | Unknown | M | No | Unknown | 1.4 years | Invasive ductal | Subcutaneous |
| 14G CNB | Invasive ductal | + | Unknown | M | No | AC, tam | 1 year | Invasive ductal | Unknown | |
| Intra et al. [9] | FNA CNB |
DCIS | + | + | M | No | No | 2 years | Invasive ductal | Muscle |
| Uriburu et al. [10] | 14G stereo CNB | Invasive mucinous | + | Unknown | M SS | No | CMF | 1.9 years | Invasive with mucin | Dermis and subcutaneous |
| 14G stereo CNB | Invasive ductal | + | − | M SS | No | Unknown | 1.8 years | Invasive ductal | Dermis and subcutaneous | |
| 14G stereo CNB | Invasive ductal | + | + | M SS | No | Unknown | 1.3 years | Invasive ductal | Subcutaneous | |
| Kwo and Grotting [11] | CNB | Not specified | Unknown | Unknown | M SS | No | No | 6 years | Not specified | Skin |
| Brouwer et al. [12] | 14G CNB | Invasive ductal | + | + | M SS | No | No | 1.1 years | Invasive ductal | Skin and dermis |
| Invasive ductal/lobular | + | − | M SS | No | No | 1.2 years | Invasive | Skin and dermis | ||
| Kawasaki et al. [13] | 16G CNB | Well differentiated neuroendocrine tumor | + | − | BCT | No | Aromatase inhibitor | 3.9 years | Well differentiated neuroendocrine tumor | Skin and subcutaneous tissue |
| Current study | 10G stereo CNB | DCIS with microinvasion | − | + | M SS | No | No | 1.5 years | Paget disease (DCIS) | Epidermis |
FNA, fine needle aspiration; stereo CNB, stereotactic core needle biopsy; DCIS, ductal carcinoma in situ; ER, estrogen receptor; BCT, breast conserving therapy; M, mastectomy; SS, skin sparing; XRT, radiation therapy; CMF, cyclophosphamide, methotrexate, and 5-fluorocuracil; AC, adriamycin, cytoxan; tam, tamoxifen.
Epithelial displacement into needle tracks immediately after the procedure has been shown to be quite common [1,14]. How then, does one explain the scarcity of clinically evident recurrences? The likelihood of finding cells in the needle track diminishes over time, supporting that, in many cases, these cells do not persist, perhaps due to lack of a developed blood supply [15]. It is also possible that radiation therapy plays a role, as none of the patients with needle track recurrences received this therapy. The possible role of systemic therapy is less clear. Two patients received chemotherapy, 6 did not, and this information is not provided for the other 5 patients (Table 1). Finally, removal of the needle track as part of the intended surgical resection or as a separate excision will eliminate any residual cells, though the utility of such a minor risk-reducing maneuver at the expense of likely cosmetic compromise may be debatable.
There are no definite risk factors for needle track recurrences. Needle track seeding has not been clearly associated with the type and size of needles, the number of needle passes, or the duration of the procedure [1]. Recurrences have been associated with both fine needle aspirations and core needle biopsies (Table 1). Although 5 of the cancers were of special histologic types, this association may be related to the decision not to radiate rather than the type of cancer.
The rarity of clinically diagnosed needle track recurrences supports that routine excision of the skin and needle track is not required, especially for women undergoing radiation therapy. It has been suggested that it would be helpful to document the site of the biopsy with a diagram or photograph in the patient's medical record [12]. The location of the skin puncture site for a needle biopsy depends on the location of the lesion, the imaging modality used, and the size of the breast and cannot be determined with certainty unless recorded before the site heals. The need to document biopsy sites may be questioned due to the low number of reported recurrences. However, in the absence of knowing the location of the skin puncture, recurrences may be underreported. Documentation can also aid in early detection of recurrences. In the reported case, diagnosis was delayed for over a year as the skin lesion was mistaken for an infection. Fortunately, the carcinoma was detected while still in situ. Invasion can occur from Paget disease and the delay in diagnosis could have resulted in development of invasive carcinoma and a poorer prognosis [16,17].
The reported recurrences have been successfully treated with local excision and adjuvant treatment. Although follow-up is short, no cases have been reported with distant metastasis. It is important to recognize a skin recurrence as an isolated needle track recurrence, as this type of recurrence may not have the same dire prognosis for recurrent invasive carcinoma elsewhere in the chest wall [18,19].
4. Conclusions
Needle track recurrences after diagnosis and treatment of carcinoma are rare and have not been reported in patients receiving radiation therapy. For patients not receiving radiation, and whose needle track will not be removed by the planned surgery, documentation of the location of the skin puncture site in the patient's chart may be of value. This can aid in the early detection of a recurrence as a subcutaneous mass or as changes in the skin. Early detection, especially for recurrences as DCIS as in the described case, can avoid possible poor clinical outcomes.
Conflict of interest
There are no conflicts of interest related to the contents of the submitted case report.
Funding
There are no sources of funding.
Ethical approval
This case report concerns one patient who is under the personal care of two of the authors (KZC and LG). The patient has provided written consent for publication of this case report and the accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of the journal on request.
Authors contribution
Katherina Zabicki Calvillo, MD: Surgeon, consenting patient, reviewing and editing drafts of manuscript.
Lifei Guo, MD, PhD: Surgeon, clinical photographs, reviewing and editing drafts of manuscript.
Valerie Brostrom, MD: Review of pathology, reviewing and editing drafts of manuscript.
Stuart J Schnitt, MD: Review of pathology, reviewing and editing drafts of manuscript.
Xuefei Hong, MD: Review of pathology, reviewing and editing drafts of manuscript.
Sughra Raza, MD: Review of imaging information, reviewing and editing drafts of manuscript.
Susan C Lester: Review of pathology, literature review, preparation of manuscript.
Guarantor
Susan C. Lester, MD, PhD.
Acknowledgements
The authors wish to thank the patient for allowing her case to be presented and to Peter van der Meer for translation of a reference.
References
- 1.Loughran C.F., Keeling C.R. Seeding of tumour cells following breast biopsy: a literature review. Br. J. Radiol. 2011;84:869–874. doi: 10.1259/bjr/77245199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao G.F., Graham J.H., Helwig E.B. Paget's disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J. Cutan. Pathol. 1986;13:59–66. doi: 10.1111/j.1600-0560.1986.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 3.Decaussin M., Laville M., Mathevet P., Frappart L. Paget's disease versus Toker cell hyperplasia in a supernumerary nipple. Virchows Archiv. 1998;432:289–291. doi: 10.1007/s004280050167. [DOI] [PubMed] [Google Scholar]
- 4.El Khoury M., Lalonde L., David J., Issa-Chergui B., Peloquin L., Trop I. Paget's disease of the axilla arising from an underlying accessory mammary tissue. Clin. Radiol. 2011;66:575–577. doi: 10.1016/j.crad.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira A., Sanches M., Selores M. Axillary Paget's disease associated with breast carcinoma in an elderly patient. EJD. 2011;21:102–103. doi: 10.1684/ejd.2011.1161. [DOI] [PubMed] [Google Scholar]
- 6.Sawada Y., Bito T., Kabashima R., Yoshiki R., Hino R., Nakamura M., Shiraishi M., Tokura Y. Ectopic extramammary Paget's disease: case report and literature review. Acta Dermato Venereol. 2010;50:2–505. doi: 10.2340/00015555-0892. [DOI] [PubMed] [Google Scholar]
- 7.Thurfjell M.G., Jansson T., Nordgren H., Bergh J., Lindgren A., Thurfjell E. Local breast cancer recurrence caused by mammographically guided punctures. Acta Radiol. 2000;41:435–440. doi: 10.1080/028418500127345884. [DOI] [PubMed] [Google Scholar]
- 8.Chao C., Torosian M.H., Boraas M.C., Sigurdson E.R., Hoffman J.P., Eisenberg B.L., Fowble B. Local recurrence of breast cancer in the stereotactic core needle biopsy site: case reports and review of the literature. Breast J. 2001;7:124–127. doi: 10.1046/j.1524-4741.2001.007002124.x. [DOI] [PubMed] [Google Scholar]
- 9.Intra M., Mazzarol G., Rietjens M., Diaz Brito J.A., Gennari R., Soteldo J., Rodriguez J., Bassani G., Bassi F. Extramammary recurrence of DCIS after total mastectomy: an iatrogenic displacement following needling procedures? Breast J. 2005;11:297–300. doi: 10.1111/j.1075-122x.2005.21695.x. [DOI] [PubMed] [Google Scholar]
- 10.Uriburu J.L., Vuoto H.D., Cogorno L., Isetta J.A., Candas G., Imach G.C., Bernabo O.S. Local recurrence of breast cancer after skin-sparing mastectomy following core needle biopsy: case reports and review of the literature. Breast J. 2006;12:194–198. doi: 10.1111/j.1075-122X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 11.Kwo S., Grotting J.C. Does stereotactic core needle biopsy increase the risk of local recurrence of invasive breast cancer? Breast J. 2006;12:191–193. doi: 10.1111/j.1075-122X.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer O.R., Donker M., Woerdman L.A., Vrancken Peeters M.J. Local recurrence after skin-sparing mastectomy [Article in Dutch] Ned. Tijdschr. Geneeskd. 2012;156:1256–1259. [PubMed] [Google Scholar]
- 13.Kawasaki T., Ishida M., Tada T., Matsuya H., Saitoh M. Well-differentiated neuroendocrine tumor of the breast with recurrence due to needle tract seeding. Virchows Archiv. 2015;466:479–481. doi: 10.1007/s00428-014-1704-5. [DOI] [PubMed] [Google Scholar]
- 14.Uematsu T., Kasami M. The use of positive core wash cytology to estimate potential risk of needle tract seeding of breast cancer: directional vacuum-assisted biopsy versus automated core needle biopsy. Breast Cancer. 2010;17:61–67. doi: 10.1007/s12282-009-0109-9. [DOI] [PubMed] [Google Scholar]
- 15.Diaz L.K., Wiley E.J., Venta L.A. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR. 1999;173:1303–1313. doi: 10.2214/ajr.173.5.10541110. [DOI] [PubMed] [Google Scholar]
- 16.Duan X., Sneige N., Gullett A.E., Prieto V.G., Resetkova E., Andino L.M., Wu Y., Gilcrease M.Z., Bedrosian I., Dawood S., Arun B., Albarracin C.T. Invasive paget disease of the breast: clinicopathologic study of an underrecognized entity in the breast. Am. J. Surg. Pathol. 2012;36:1353–1358. doi: 10.1097/PAS.0b013e318259ef7f. [DOI] [PubMed] [Google Scholar]
- 17.Sanders M.A., Dominici L., Denison C., Golshan M., Wiecorek T., Lester S.C. Paget disease of the breast with invasion from nipple skin into the dermis: an unusual type of skin invasion not associated with an adverse outcome. Arch. Pathol. Lab. Med. 2013;137:72–76. doi: 10.5858/arpa.2011-0611-OA. [DOI] [PubMed] [Google Scholar]
- 18.Wapnir I.L., Anderson S.J., Mamounas E.P., Geyer J., r C.E., Jeong J.-H., Tan-Chiu E., Fisher B., Wolmark N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five national surgical adjuvant breast and bowel project node-positive adjuvant breast cancer trials. J. Clin. Oncol. 2006:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 19.Aebi S., Gelber S., Anderson S.J., Lang I., Robidoux A., Martin M., Nortier J.W., Paterson A.H., Rimawi M.F., Canada J.M., Thurlimann B., Murray E., Maounas E.P., Geyer C.E., Jr., Price K.N., Coates A.S., Gelber R.D., Rastogi P., Wolmark N., Wapnir I.L. CALOR investigators. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomized trial. Lancet Oncol. 2014;15:156–163. doi: 10.1016/S1470-2045(13)70589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


