Abstract
Aims
To investigate the short-term outcome of treatment of chronic osteomyelitis where management was based on a refined host stratification system.
Methods
A retrospective review of 109 adult patients with chronic osteomyelitis.
Results
At a minimum follow-up of 12 months (range 12–36) we observed an overall success rate of 89.9% (95% CI: 82.7–94.9%). There was no statistically significant difference in success rates by host status (p-value = 0.201).
Conclusion
By integrating the redefined host status and treatment strategy, we were able to achieve comparable short-term outcomes in both low and high-risk cases while maintaining a low rate of amputation.
Keywords: Osteomyelitis, Chronic, Classification, HIV, Management
1. Introduction
There are currently no evidence-based guidelines in terms of the treatment of chronic osteomyelitis.1 Achieving remission is notoriously difficult, with some studies reporting failure rates of 20–60%.2,3 In essence the aim is to improve quality of life through either a curative or a palliative treatment strategy. Curative management strategies, aimed at limb salvage, usually comprise of a combination of complex surgical procedures and tailored adjuvant antibiotic therapy.4 On the other hand, palliative treatment strategies are less invasive and typically involve to the use of chronic suppressive antibiotic therapy.5 The decision to embark on either a curative or palliative treatment strategy requires consideration of several factors, principle amongst which is the host's physiological status. Furthermore, in cases where a curative treatment strategy is employed the host status also influences the clearance margin that is required during surgical debridement.6
Recognizing the importance of considering the host's physiological status during formulation of a treatment plan, Cierny and Mader revolutionized our approach to chronic osteomyelitis through the publication of their clinical staging system in 1985 (Table 1).7 According to this classification system A- and B-hosts could be considered for a curative treatment protocol. To justify the considerable demands and risks associated with limb salvage, the expected outcome should, however, offer distinct advantages over an amputation or palliation. In cases where treatment aimed at remission is contraindicated or deemed excessive, as a result of the risks it entails, a patient should be classified as a C-host and offered palliation.8,9 Amputation should be considered in cases where limb salvage or palliation is deemed to be neither safe nor feasible.10
Table 1.
Cierny and Mader clinical staging system for adult chronic osteomyelitis.7
| Anatomic type | |
| I | Medullary osteomyelitis |
| II | Superficial osteomyelitis |
| III | Localized osteomyelitis |
| IV | Diffuse osteomyelitis |
| Physiological Class | |
| A | Good immune system and delivery |
| B | Compromised locally (BL) or systemically (BS) |
| C | Requires suppressive or no treatment; minimal disability; treatment worse than disease; not a surgical candidate |
| Clinical Stage | |
| Type + Class = Clinical stage | |
The choice between curative or palliative treatment strategies may however be particularly problematic. This results from the absence of precisely defined criteria according to which a C-host should be defined. Unfortunately no discreet objective criteria exist to guide the decision-making process. Originally, Cierny and Mader defined a C-host as any patient in whom treatment or the result of treatment will be more compromising to the patient than the disability caused by the disease itself.7 The main shortcoming of this definition is that it is subjective in nature and susceptible to widely varying interpretation depending on the experience of the surgeon.
In this study we set out to determine the short term outcome of treatment in a cohort of adult patients with chronic osteomyelitis where management strategy selection was based on a modified classification system.
2. Patients and methods
A retrospective review was performed of patients with chronic osteomyelitis treated at our tertiary referral center from 2011 to 2013. Patient notes, blood tests and radiographs were reviewed pre- or post-treatment. For the purposes of this study chronic osteomyelitis was defined as a bone infection characterized by the presence of necrotic bone (sequestrum) or host reparative reaction (involucrum) and/or duration of at least 6 weeks.1 All patients, 18 years or older, treated for chronic osteomyelitis with a minimum follow-up of twelve months were included in the study. Cases involving atypical organisms, acute postoperative infection where the fracture was expected to unite, periprosthetic joint infection with retained implants and hand sepsis were excluded from the study.
Following clinical, radiological and biochemical evaluation, patients were classified according to a modified version of the Cierny and Mader classification system (Table 2).7 The characterization of the host's physiological status was modified in order to provide a more pragmatic definition of a C-host. A patient was classified as a C-host if one major risk factor or three (or more) minor risk factors were present (Table 3). Risk factors were selected following systematic review of existing data and consideration of previously published classification systems.11–25 One of the aims of the modified classification system was to emphasize host optimization prior to surgical intervention. Resultantly the majority of major risk factors are modifiable which places appropriate emphasis on risk factor modification prior to surgery.
Table 2.
Modified classification system.
| Physiology | |
| Type A host | No risk factors |
| Type B host | Less than three minor risk factors |
| Type C host | One major and/or three or more minor risk factors |
| Pathoanatomy | |
| I - Medullary (stable) | No cortical sequestration |
| II - Cortical (stable) | Direct contiguous involvement of cortex only |
| III - Combined (stable) | Both cortex and medullary regions involved |
| IV - Combined (unstable) | As for III plus unstable prior to debridement |
Table 3.
Major and minor risk factors used during host stratification. A patient with one major or three (or more) minor risk factors was considered to be a C-host.
| Major risk factors | Minor systemic risk factors | Minor local risk factors |
|---|---|---|
| CD4 count <350 cells/mm3 | HIV infection | Poor soft tissues requiring flap |
| Albumin <30 g/L | Anemia | Chronic venous insufficiency |
| HbA1C ≥ 8% | Smoking | Peripheral vascular disease |
| Cellulitis or abscess formation | Diabetes mellitus | Previous radiation therapy |
| Malignancy at site of infection | Rheumatoid Arthritis | Surgery will result in instability |
| Pathological fracture | Chronic lung disease | Adjacent joint stiff/arthritic |
| Chronic cardiac failure | Heterotopic ossification | |
| Common variable immune deficiency | Segmental resection of ≥6 cm required to achieve cure | |
| Paraplegia/Quadriplegia | ||
| Drug or substance abuse | ||
| Chronic corticosteroid use | ||
| Active tuberculosis | ||
| Ischemic heart disease | ||
| Cerebrovascular disease |
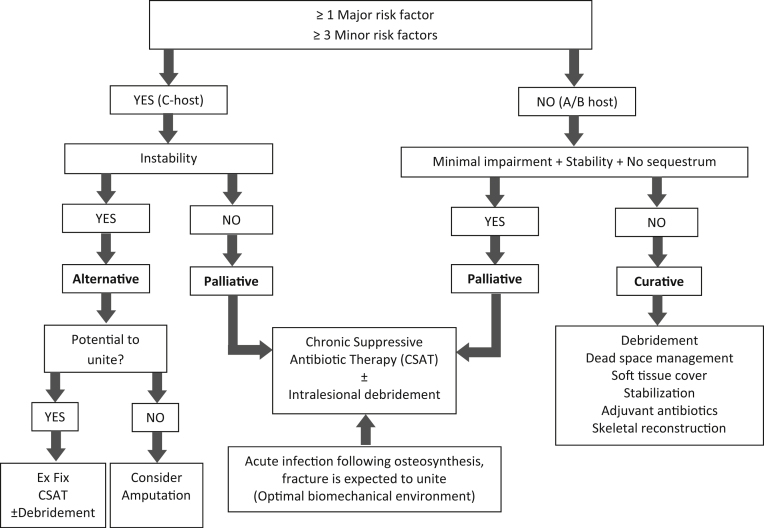
Palliative treatment was instituted in all C-hosts without skeletal instability. A- or B-hosts with minimal impairment, no sequestrum and no skeletal instability, were also managed palliatively (Fig. 1). All remaining A- and B-hosts were treated curatively. C-hosts with skeletal instability were managed through the implementation of alternative treatment strategies that involved either amputation (if union was unlikely to occur) or chronic suppressive antibiotic therapy in combination with external fixation, with or without debridement.
Fig. 1.
Treatment selection algorithm. (i) CSAT: Chronic suppressive antibiotic therapy; (ii) Ex Fix: Circular External Fixation.
Curative treatment involved debridement, dead space management, provision of bony stability, soft tissue reconstruction and/or skeletal reconstruction, in conjunction with pathogen directed adjuvant antibiotics for a period of six weeks. The extent of the debridement was determined by the host status and the anatomic nature of the infection. Resection margins were defined according to the guidelines previously published by Simpson et al.6 In B-hosts we strived to obtain a wide clearance margin, as long is it did not compromise skeletal stability. In type I, II and III lesions this was achieved by direct debridement (tangential excision with high speed burr) and/or indirect debridement (medullary reaming). In cases with pre-operative skeletal instability (type IV lesions) segmental resection was performed and stability provided by circular external fixation. Dead space management techniques were also tailored to anatomic nature of the pathology [Table 2]. Continuous irrigation, as popularized by Lautenbach, was used in type I (medullary) post-operative infections.26,27 Dead space management in type II lesions was achieved through soft tissue flaps. In type III lesions gentamycin impregnated polymethylmethacrylate (PMMA) beads (Septopal® Merck, Darmstadt Germany) were utilized and removed at six to eight weeks. Dead space following debridement of type IV lesions were dealt with through the use of physician-directed antibiotic-impregnated PMMA spacers, as described by Masquelet.28 The PMMA spacers were constructed from Palacos R + G® bone cement (Heraeus Medical, Hanau Germany) containing 500 mg Gentamycin per 40 mg of PMMA powder, mixed with 2 g of Vancomycin powder per 40 mg of PMMA. Post-operatively all patients were treated with generic parenteral antibiotics, in the form of Vancomycin and Meropenem, until the seven day microscopy, culture and sensitivity (MCS) results became available. Oral antibiotic therapy, tailored according to the culture and sensitivity, was then commenced and continued for a period of six weeks. Following this period, reconstruction of segmental bone defects in Cierny and Mader type IV lesions were undertaken; if clinical and biochemical evaluation confirmed the absence of active infection. The size of the bone defect determined the nature of the skeletal reconstruction procedure. Defects less than 1–2 cm in magnitude were managed by acute shortening. Defects between 2 and 4 cm in size were managed utilizing the Masquelet technique, involving autogenous bone grafting into an induced membrane. Gaps in excess of 4 cm were treated through the use of bone transport through the induced membrane.
Palliative treatment, aimed at suppression of infection, was provided as a three to six months course of chronic suppressive antibiotic therapy (CSAT) in the form of trimethoprim-sulfamethoxazole (800 mg/160 mg twice daily) and rifampicin (600 mg daily). If suppression was successfully achieved following three to six months of therapy the treatment was stopped and the patient followed-up for recurrence. In the case of recurrence CSAT was restarted and continued for a further 6 months before cessation. If symptoms of infection again returned following 12 months of treatment, permanent CSAT was instituted.29 Patients with cellulitis or abscess formation received culture directed pre-operative antibiotic therapy. In this scenario palliation was aimed at resolution of the local compromising factors that prohibited the performance of definitive surgical procedures. Alternative treatment strategies involved either amputation of the limb or antibiotic therapy combined with external fixation and/or intralesional debridement (minimally invasive surgical procedure involving drainage of abscess and/or removal of large sequestra/obviously necrotic bone). Patients refusing amputation where managed with long term CSAT.
Following a minimum follow-up period of 12 months the outcome was determined in respect of the success or failure of the treatment of infection. Success was defined as achievement of remission through a curative treatment strategy or suppression (or better) in patients treated palliatively. Remission was defined as the absence of clinical evidence of infection.30 Suppression was defined as resolution of symptoms and signs of infection to the extent that it did not interfere with activities of daily living (ADL). For the purposes of this study failure of treatment was defined by failure of the initial treatment plan to achieve the predetermined goal (remission or suppression); recurrence of infection; the necessity for unplanned reoperation; and/or failure to achieve patient satisfaction with the outcome of treatment. If suppression was not successfully achieved following six months of CSAT the case was classified as a treatment failure.
Data were process and analyzed using Stata 13.0 SE (StataCorp, 2013). Standard t-tests were used to identify significant mean differences in continuous explanatory variables. For non-normal distributed continuous data the Wilcoxon rank-sum test was used. Categorical explanatory variables were cross-tabulated against C-host status or treatment failure and significant association was identified using the standard Pearson's chi-square (χ2) test. If an expected cell count in the cross tabulation was less than 5 (sparse numbers) then the Fishers exact test was preferred. Ninety-five percent confidence intervals (CI) were constructed around proportions using binomial exact limits.
Ethical approval was obtained from a national level ethics review board prior to commencement of the study.
3. Results
A total number of 123 cases were enrolled. Fourteen patients were excluded from the study: nine patients were lost to follow-up before 12 months; two cases were excluded on the basis of the involvement of atypical organisms (Cryptococcus neoformans and Actinomyces israelii) and three patients, who presented with acute post-operative infection, were also excluded. The final sample utilised in this analysis thus comprised 109 patients. The mean follow-up period was 18.6 months (standard deviation [SD]: 6.8; range: 12–36 months). The mean age was 39.8 years (SD: 13.8; range: 18–78 years).
3.1. Pathology
Post-traumatic infection (following compound fractures) was the most common cause of chronic osteomyelitis, involving 53% (n = 58) of cases. Contiguous post-operative infection involved 30% (n = 33), while hematogenous chronic osteomyelitis accounted for 15% (n = 16) of cases. In two cases chronic osteomyelitis resulted from direct contiguous spread from ulcers on the lower leg. In terms of the causative organisms, methicillin-sensitive Staphyllococcus aureus was the most commonly isolated organism in patients with hematogenous chronic osteomyelitis. Enterococcus, Serratia, Proteus, Pseudomonas, Enterobacter and Klebsiella spp., as well as methicillin-resistant S. aureus where identified as to most prevalent pathogens in the chronic post-traumatic group. Multiple organisms were involved in 31% of contiguous (post-operative or post-traumatic) cases. In 15% of cases the causative organism could not be isolated using routine culturing techniques. The tibia was to most commonly affected bone, involving 52% of cases (n = 57). The femur was the second most common site at 23% (n = 25), followed by the foot (5%), pelvis and forearm (4% each). The remainder of infections involved the ankle, knee, hip, fibula, humerus and clavicle.
3.2. Host stratification
The majority of patients in this study were classified as C-hosts (46.8%; n = 51), followed by B-host classification in 41.3% of cases (n = 45) (Table 4). The mean albumin and haemoglobin levels were 35.8 g/L (SD: 5.5) and 13.0 g/dL (SD: 2.0) respectively. HIV infection was present in 30% (n = 33) of cases, with a median CD4 count of 336 cells/mm3 (Interquartile range [IQR]: 307–507; min–max 13–1034).Fifty five percent of these patients were not on antiretroviral therapy at the onset of treatment. These patients were either newly diagnosed cases or did not qualify for treatment according to the national guidelines.31 Antiretroviral treatment was initiated in all patients with a CD4 count below 350 cells/mm3 as prescribed by the national policy. With regards to the C-hosts, 45% (n = 23) were designated on the basis of the presence of a major risk factor, while 55% (n = 28) were classified as C-hosts on the basis of the presence three or more minor risk factors.
Table 4.
Descriptive statistics of the most common risk factors, the host classification according to the modified classification system and treatment strategies employed.
| Variable | n | Summary measure | Range |
|---|---|---|---|
| Risk factors | |||
| Age: Mean (SDa) | 109 | 39.8 (13.8) | 18–78 |
| HIV positive | 33 | 30% | |
| CD4 count: Median (IQRb) | 33 | 336 (307–509) | 13–1034 |
| Albumin: Mean (SDa) | 109 | 35.8 (5.5) | 22–48 |
| Hemoglobin: Mean (SDa) | 109 | 13.0 (2.0) | 6.6–18.6 |
| Poor soft tissue necessitating flap | 48 | 44.0% | |
| Current smoker | 42 | 38.5% | |
| Diabetes mellitus | 10 | 9.2% | |
| Anemia | 9 | 8.3% | |
| Final host status | |||
| A | 13 | 11.9% | |
| B | 45 | 41.3% | |
| C | 51 | 46.8% | |
| Palliative treatment | 47 | 43.1% | |
| Chronic suppressive antibiotic therapy (CSAT) | 45 | ||
| Intralesional debridement plus CSAT | 2 | ||
| Curative treatment | 46 | 42.2% | |
| Stable lesions | |||
| Direct debridement (high speed burr) | 15 | ||
| Indirect debridement (medullary reaming) | 5 | ||
| Unstable lesions | |||
| Debridement without reconstruction | 6 | ||
| Debridement and external fixation | 3 | ||
| Segmental resection, acute shortening | 2 | ||
| Segmental resection, Masquelet bonegraft | 5 | ||
| Segmental resection, bone transport | 10 | ||
| Alternative treatment | 16 | 14.7% | |
| Amputation | 6 | ||
| Debridement, external fixation, CSAT | 10 | ||
Standard deviation;
Interquartile range.
3.3. Treatment strategies
A curative management strategy was employed in 42% (n = 46) of patients, while a palliative strategy was selected in 43% (n = 47) of cases. In the palliative group two patients required additional intralesional debridement involving simple sequestrectomy and/or drainage of an abscess.6 The specific therapeutic interventions employed in the curative group are listed listed in Table 4. An alternative treatment strategy was required in 15% (n = 16) of patients. This involved debridement and/or circular external fixation followed by CSAT in ten cases. Primary amputation was performed in the 5% (n = 6) of patients.
3.4. Success rate
We observed an overall success rate of 89.9% (95% CI: 82.7–94.9%). There was no statistically significant difference in failure rates by host status (Fishers exact p-value = 0.201) (Table 5). Zero failures occurred among A-hosts, with a possible one sided 97.5% CI for the success rate in this group of 81.5–100%. The success rate among B-hosts was 93.3% (95% CI: 81.7–98.6%) and 84.3% (95% CI: 71.4–93.0%) among C-hosts. In terms of the management strategy, success was achieved in 93.5% of patients treated curatively, 87.2% of patients treated palliatively and 87.5% in the alternative treatment group. Remission was achieved in 62% of patients in whom the aim of treatment was disease suppression, through the use of CSAT as part of a palliative (n = 47) or an alternative treatment strategy (n = 16). Fifty three percent (n = 25) of patients treated palliatively required more than six months of antibiotic treatment in order to achieve suppression. Approximately half (52%, n = 13) of these patients required chronic suppressive antibiotic therapy on a permanent basis.
Table 5.
Host status versus treatment outcome.
| Host status | Treatment strategy (n) | Success | Failure | p-valuea |
|---|---|---|---|---|
| A | 100% (n = 13) | 0% (n = 0) | 0.201 | |
| Curative | 12 | |||
| Palliative | 1 | |||
| Alternative | – | |||
| B | 93.3% (n = 42) | 6.7% (n = 3) | ||
| Curative | 34 | |||
| Palliative | 11 | |||
| Alternative | – | |||
| C | 84.3% (n = 43) | 15.7% (n = 8) | ||
| Curative | – | |||
| Palliative | 35 | |||
| Alternative | 16 | |||
Fishers Exact test.
Overall, the success rate in HIV positive patients was 84.8% or 28/33 (95%CI: 68.1–94.9%) compared to 92.1% or 70/76 among HIV negative patients (95%CI: 83.6–97.0). The success rate did not significantly vary by HIV status (p-value = 0.248). All treatment failures, in the HIV positive group, occurred in patients who fulfilled the World Health Organization immunological criteria for advanced HIV infection (CD4 count < 350 cells/mm3).32 It is important to note that the majority of HIV positive patients were treated through either a palliative or alternative treatment strategy. Curative treatment was however successful in all four of the HIV positive patients in whom it was employed.
Eight of the eleven treatment failures occurred in C-hosts (Table 6). Several additional risk factors, which were not considered during initial host stratification, were identified in the failure group. These include prior attempts at limb reconstruction, poor motivation and compliance, age, involvement of the adjacent joint, and foot or pelvic involvement.
Table 6.
Description of cases in which treatment failure occurred.
| Age | Host status | Etiology | Site | Anatomic nature | Risk factors | Management strategy | Treatment |
|---|---|---|---|---|---|---|---|
| 44 | B | Contiguous post-traumatic | Tibia | IV | Compliance and motivation | Curative | Wide resection, Masquelet bone transport |
| 34 | C | Contiguous post-traumatic | Ankle | IV | HIV infection (CD4 <350 cells/mm3), smoking, joint involved | Alternative | Patient refused amputation, marginal debridement and acute shortening |
| 25 | C | Contiguous post-traumatic | Tibia | IV | HIV infection (CD4 <350 cells/mm3) | Alternative | Circular fixation, fibula osteotomy, CSATa |
| 30 | C | Contiguous post-traumatic | Midfoot | IV | Poor soft tissue, joint involved, foot, local extent | Palliative | CSATa |
| 49 | C | Contiguous post-traumatic | Tibia | III | HIV infection (CD4 <350 cells/mm3), poor soft tissue, local extent, debridement will result in instability | Palliative | CSATa |
| 26 | B | Contiguous post-traumatic | Proximal tibia | IV | Failed reconstruction elsewhere, joint involved, poor soft tissues | Curative | Wide resection, classic Masquelet |
| 45 | C | Contiguous post-operative | Proximal femur | III | Chronic venous insufficiency, joint involved, local extent, debridement will result in instability | Palliative | CSATa |
| 65 | C | Contiguous post-operative | Femur | III | Ischemic heart disease, smoker, diabetes mellitus (HbA1c > 8), age | Palliative | CSATa |
| 46 | B | Hematogenous | Pelvis | III | Smoking, local extent, pelvis | Curative | Marginal resection, PMMA beads |
| 38 | C | Contiguous post-operative | Humerus | IV | HIV infection (CD4<350cells/mm3), adjacent joint stiffness, local extent, diabetes mellitus | Palliative | CSATa |
| 48 | C | Contiguous post-traumatic | Proximal tibia | III | HIV infection (CD4 <350 cells/mm3), smoking, local extent, poor soft tissues | Palliative | CSATa |
Chronic suppressive antibiotic therapy.
4. Discussion
The complex nature of the disease necessitates an individualized approach to a patient with chronic osteomyelitis. Selecting low risk treatment options in high-risk patients reduces the risk of complications. Embarking on a curative protocol in a host who is unable to withstand the metabolic and immunological demands of complex limb reconstructive process may result in therapeutic failure and amputation. In such patients (C-hosts) unnecessary or unwanted limb ablation may be avoided by the institution of a palliative treatment strategy, that does not involve high-risk reconstructive surgical procedures. Existing classification systems however fails to provide discreet objective criteria that allow reproducible identification of C-hosts. This shortcoming prompted the implementation of a refined host stratification system, which incorporated a more pragmatic definition of C-hosts. By integrating the resulting host status with the appropriate curative, palliative or alternative treatment strategy we were able to achieve acceptable short-term outcomes in both low and high-risk cases while maintaining a low rate of amputation.
The reported success rates of the management of adult chronic osteomyelitis vary widely, with figures ranging from 40 to 95%.1,33,34 A recent Cochrane review, comparing the efficacy of oral and intravenous antibiotics following surgical debridement, found an overall remission rate of 78.8% at 12 months.35 In the original article by Cierny and Mader the success rate of limb-salvage procedures was reported to be 93.6%.6 Primary amputation was performed in 46 of the 189 (24%) of patients who received definitive treatment in their series. More recently, Cierny reported an 85% success rate of curative treatment, with 96% success in A-hosts and 74% in B-hosts.7 Ten percent of cases in this series were managed by primary amputation. The Bone Infection Unit in the United Kingdom reported an excellent cure rate of 90% at 5 years follow-up.34 Treatment strategy selection and host status were, however, not specifically discussed in this report of their outcomes. In comparison to these results we were able to achieve an overall success rate of 89.9% at a mean follow-up of 18 months, with 100% and 93% success in A- and B-hosts respectively. A success rate of 93.5% was achieved in patients treated curatively and through the judicious implementation of palliative treatment strategies we were able to achieve a primary amputation rate of only 5%. Lack of uniformity in the literature on chronic osteomyelitis, in terms of definition, classification and treatment protocols makes comparison of results problematic. Authors reviewing trials involving antibiotic therapy in chronic osteomyelitis came to a similar conclusion, citing the heterogeneous nature of the patients, classification systems and treatment strategies used as a stumbling block in making evidence based recommendations.30,33 Similarly our results cannot be directly compared to those of Cierny, who excluded C-hosts in whom a much higher failure rate can be expected when calculating outcome figures.6,7
The successful use of suppressive antibiotics in periprosthetic infections of hip or knee replacements has prompted implementation of similar strategies in patient with chronic osteomyelitis.5,36 To the best of our knowledge this is the first series to specifically look at the outcome of the use of chronic suppressive antibiotic therapy in chronic osteomyelitis. Success have however been reported in isolated cases involving infection associated with osteosynthesis through the use of long-term antibiotics without surgical removal of the implants.37,38 In our series we were able to achieve successful suppression in 87.2% of patients treated palliatively. Remission of disease was achieved in 62% of patient treated with chronic suppressive antibiotic therapy (CSAT). The efficacy of CSAT in chronic hematogenous osteomyelitis in adults has also not previously been reported.30 Successful suppression of disease was achieved in all of the five patients with hematogenous osteomyelitis treated palliatively. This finding suggests that chronic osteomyelitis without the presence of surgical implants can successfully be treated palliatively, in appropriately selected patients.
The overall success rate in this series is most likely related to host stratification and treatment selection, rather than therapeutic or surgical prowess. The selection of patient-matched treatment options may close the gap in successful outcomes between compromised and healthy patients. Our strategy involving C-host classification in accordance with certain predefined major and minor criteria, resulted in comparable success rate in both the palliative and curative treatment groups. The fact that there was no statistical difference in success rate between high and low risk patients (p-value = 0.201) suggests that the proposed decision tree may be relevant, at least in a developing world clinical environment. The majority of the suggested major criteria are modifiable. This implies that, in certain cases, palliative treatment can be utilized as a temporary measure while the patient is optimized for curative management.
There are several shortcomings to this study. Due to the short follow-up our results are likely to deteriorate over time due to the recurrence of infection. A minimum follow-up of 12 months, as applied by Simpson et al and Conterno et al, may however be reasonable as 95% of recurrences can be expected within the first year.6,30,35,39 In the palliative treatment group in particular recurrence can be expected. The lack of a control group and randomization are further shortcomings. The lack of randomization could however be difficult to overcome. Exposing all patients, including the most compromised hosts, to the rigors of limb salvage surgery in order to see which risk factors is associated with treatment failure (which frequently would involve amputation) represents an ethical dilemma. The fact that we defined success differently in the curative and palliative also resulted in an apparent improvement in our results. However, it would be unrealistic to expect cure (clinical, biochemical and radiological absence of infection) in patients treated palliatively and thus the definition of success or failure of treatment is intimately bound to the management strategy selected. The final shortcoming of this study its retrospective nature and we have embarked on a prospective study in order to validate these results.
Many questions remain and while this approach may prove to be useful in the developing world, it may not be applicable in all clinical scenarios. Our hope is that the introduction of the concept of a pragmatically defined C-host will spark further research, which could lead to uniformity in host classification and ultimately treatment strategy selection. This may in turn facilitate comparison of different treatment protocols or interventions.
Conflicts of interest
All authors have none to declare.
Acknowledgements
The corresponding author has received a research grant from the South African Orthopaedic Association for research in the field of chronic osteomyelitis.
References
- 1.Walter G., Kemmerer M., Kappler C., Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int. 2012;109:257–264. doi: 10.3238/arztebl.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall B.B., Fitzgerald R.H., Rosenblatt J.E. Anaerobic osteomyelitis. J Bone Joint Surg Am. 1984;65-A:30–35. [PubMed] [Google Scholar]
- 3.Haas D.W., McAndrew M.P. Bacterial osteomyelitis in adults: evolving considerations in diagnosis and treatment. Am J Med. 1996;101:550–561. doi: 10.1016/s0002-9343(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 4.Rodner C.M., Browner B.D., Pestani E. Chronic osteomyelits. In: Browner B.D., editor. Skeletal Trauma. 4th ed. Saunders Elsevier; Philadelphia: 2003. pp. 483–506. [Google Scholar]
- 5.Calhoun J.H., Manring M.M. Adult osteomyelitis. Infect Dis Clin N Am. 2005;19:765–786. doi: 10.1016/j.idc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Simpson A.H., Deakin M., Latham J.M. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br. 2001;83:403–407. doi: 10.1302/0301-620x.83b3.10727. [DOI] [PubMed] [Google Scholar]
- 7.Cierny G., Mader J.T., Penninck J.J. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 8.Cierny G. Surgical treatment of osteomyelitis. Plast Reconstr Surg. 2011;127(suppl 1):S190–S204. doi: 10.1097/PRS.0b013e3182025070. [DOI] [PubMed] [Google Scholar]
- 9.Roa N., Ziran B.H., Lipsky B.A. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg. 2011;127(suppl 1):177S–187S. doi: 10.1097/PRS.0b013e3182001f0f. [DOI] [PubMed] [Google Scholar]
- 10.Cierny G., DiPasquale D. Treatment of chronic infection. J Am Acad Orthop Surg. 2006;14:S105–S110. doi: 10.5435/00124635-200600001-00025. [DOI] [PubMed] [Google Scholar]
- 11.Marais L.C., Ferreira N., Aldous C., Le Roux T.L.B. The classification of chronic osteomyelitis. S Afr J Orthop. 2014;13:22–28. [Google Scholar]
- 12.Govender S., Harrison W.J., Lukhele M. Impact of HIV on bone and joint surgery. Best Prac Res Clin Rheum. 2008;22:605–619. doi: 10.1016/j.berh.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Liu B., Guo C., Liu L. Management and prognosis of HIV infected patients with postoperative sepsis. Sci Res Essays. 2011;6:2389–2394. [Google Scholar]
- 14.Aird J., Noor S., Lavy C., Rollinson P. The effect of HIV on early wound healing in open fractures treated with internal and external fixation. J Bone Joint Surg Br. 2011;93-B:678–683. doi: 10.1302/0301-620X.93B5.26081. [DOI] [PubMed] [Google Scholar]
- 15.Su J., Tsun A., Zhang L. Preoperative risk factors influencing the incidence of postoperative sepsis in human immunodeficiency virus-infected patients: a retrospective cohort study. World J Surg. 2013;37:774–779. doi: 10.1007/s00268-013-1915-y. [DOI] [PubMed] [Google Scholar]
- 16.Guild G.N., Moore T.J., Barnes W., Hermann C. CD4 count is associated with postoperative infection in patients with orthopaedic trauma who are HIV positive. Clin Orthop Relat Res. 2012;470:1507–1512. doi: 10.1007/s11999-011-2223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvizi J., Sullivan T.A., Pagnano M.W., Trousdale R.T., Bolander M.E. Total joint arthroplasty in human immunodeficiency virus-positive patients: an alarming rate of early failure. J Arthroplasty. 2003;18:259–264. doi: 10.1054/arth.2003.50094. [DOI] [PubMed] [Google Scholar]
- 18.Lubega N., Harrison W.J. Orthopaedic and trauma surgery in HIV positive patients. Orthop Trauma. 2010;24:298–302. [Google Scholar]
- 19.Foster M.R., Heppenstall R.B., Friedenberg Z.B., Hozack W.J. A prospective assessment of nutritional status and complications with fractures of the hip. J Orthop Trauma. 1990;4:49–57. doi: 10.1097/00005131-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Masatu D.M., Mcharo C.N. Predictive values of serum nutritional indices for early portoperative wound infections in surgically treated closed femoral fractures. SA Orthop J. 2010;9:63–67. [Google Scholar]
- 21.Jaberi F.M., Parvizi J., Haytmanek C.T., Joshi A., Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466:1368–1371. doi: 10.1007/s11999-008-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iorio R., Williams K.M., Marcantonio A.J., Specht L.M., Tilzey J.F., Healy W.L. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplast. 2012;27:726–729. doi: 10.1016/j.arth.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Richards J.E., Kauffmann R.M., Zuckerman S.L., Obremskey W.T., May A.K. Relationship of hyperglycemia and surgical-site infection in orthopaedic. J Bone Joint Surg Am. 2012;94-A:1181–1186. doi: 10.2106/JBJS.K.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R., The Hospital Infection Control Practices Advisory Committee Guideline for prevention of surgical site infection. Am J Infect Control. 1999;27:97–134. [PubMed] [Google Scholar]
- 25.Aggarwal V.K., Tischler E.H. Mitigation and education. J Arthroplasty. 2014;29(suppl 1):19–25. doi: 10.1016/j.arth.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Lautenbach E. Chronic osteomyelitis: irrigation and suction after surgery. Bone Joint Surg Br. 1975;57-B:245–262. [PubMed] [Google Scholar]
- 27.Hashmi M.A., Norman P., Saleh M. The management of chronic osteomyelitis using the Lautenbach method. J Bone Joint Surg Br. 2004;86-B:269–275. doi: 10.1302/0301-620x.86b2.14011. [DOI] [PubMed] [Google Scholar]
- 28.Masquelet A.C., Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Lazzarini L., Mader J.T., Calhoun J.H. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004;86-A:2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Lazzarini L., Lipsky B.A., Mader J.T. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Inf Dis. 2005;9:127–138. doi: 10.1016/j.ijid.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 31.The South African Antiretroviral Treatment Guidelines 2013. http://www.kznhealth.gov.za/medicine/2013_art_guidelines.pdf. Date last accessed: 15 January 2015.
- 32.World Health Organization . 2007. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-related Disease in Adults and Children.http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf (date last accessed 30 August 2012) [Google Scholar]
- 33.Spellberg B., Lipsky B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNally M., Nagarajah K. Osteomyelitis. Orthop Trauma. 2010;24:416–429. [Google Scholar]
- 35.Conterno L., Turchi M.D. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD004439.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segreti J., Nelson J.A., Trenholme G.M. Prolonged suppressive antibiotic therapy for infected orthopedic prostheses. Clin Infect Dis. 1998;27:711–713. doi: 10.1086/514951. [DOI] [PubMed] [Google Scholar]
- 37.Stein A., Bataille J.F., Drancourt M. Ambulatory treatment of multidrug-resistant Staphylococcus-infected orthopedic implants with high-dose oral co-trimoxazole (trimethoprim-sulfamethoxazole) Antimicrob Agents Chemother. 1998;42:3086–3091. doi: 10.1128/aac.42.12.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javaloyas de Morlius M., Monreal Portella M. Oral antibiotic therapy in the adult bacterial osteomyelitis: results after two years of follow-up. Med Clin (Barc) 1999;113:488–489. [PubMed] [Google Scholar]
- 39.Tice A.D., Hoaglund P.A., Shoultz D.A. Risk factors and treatment outcomes in osteomyelitis. J Antimicr Chemother. 2003;51:1261–1268. doi: 10.1093/jac/dkg186. [DOI] [PubMed] [Google Scholar]