Abstract
Purpose
The study objective was to examine the role of physical activity (PA) and sedentary time (ST) on mortality risk among a population of low-income adults with diabetes.
Methods
Black (n = 11,137) and white (n = 4508) men and women with diabetes from the Southern Community Cohort Study self-reported total PA levels and total ST. Participants were categorized into quartiles of total PA and total ST. Hazard ratios (HRs) and 95% confidence intervals (CIs) for subsequent mortality risk were estimated from Cox proportional hazards analysis with adjustment for potential confounders.
Results
During follow-up, 2370 participants died. The multivariable risk of mortality was lower among participants in the highest quartile of PA compared with those in the lowest quartile (HR, 0.64; 95% CI: 0.57–0.73). Mortality risk was significantly increased among participants in the highest compared with the lowest quartile of ST after adjusting for PA (HR, 1.21; 95% CI: 1.08–1.37). Across sex and race groups, similar trends of decreasing mortality with rising PA and increasing mortality with rising ST were observed.
Conclusions
Although causality cannot be established from these observational data, the current findings suggest that increasing PA and decreasing ST may help extend survival among individuals with diabetes irrespective of race and sex.
Keywords: Physical activity, Diabetes, Low income, Sedentary behaviors, Cardiovascular disease, Mortality
Diabetes is the seventh leading cause of death in the United States [1]. When compared with those without diabetes, people with diabetes have a two-fold increase in relative risk of death [1,2]. Therefore, strategies to reduce premature death related to diabetes have important public health implications. Along with self-management behaviors such as dietary alterations and medication adherence, health care providers often recommend increased physical activity (PA) to those with diabetes [3,4]. Increased levels of PA have been found to increase insulin sensitivity and glucose tolerance, as well as positively impact serum lipid levels among individuals with type 2 diabetes [5].
Previous studies on the relationship between PA and all-cause mortality (hereafter referred to as mortality) among individuals with diabetes reported strong, inverse dose-response associations. However, these studies were conducted primarily among white males and populations with a low prevalence or absence of risk factors such as hypertension, obesity, hyperlipidemia, and poor glycemic control [6,7]. Moreover, most previous study populations comprised individuals from higher socioeconomic backgrounds, and thus, the results may not be generalizable to persons with diabetes of lower socioeconomic status.
Previous research conducted in the Southern Community Cohort Study (SCCS) examined the mortality experience among those with diabetes and reported that mortality risk was approximately 80% higher for those with versus without diabetes [8]. This study also observed that blacks with diabetes had a slightly lower mortality risk compared with whites with diabetes and similarly low socioeconomic status [8]. Herein, we examine the impact of PA and sedentary behavior on that mortality experience among a racially diverse population of low-income male and female adults with diabetes.
Methods
Subjects for this study were participants in the SCCS, an ongoing, prospective cohort study designed to examine health disparities in the incidence and mortality of chronic illnesses. Details of study methods are provided elsewhere [9–11]; in brief, study participants were 40–79 years of age at enrollment and recruited from community health centers (85%) and general population mailings (15%) across a 12-state area of the southeastern United States (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia, and West Virginia) between March 2002 and September 2009. On entry into the SCCS, personal computer-assisted interviews were conducted at the community health centers, while the general population recruits completed and mailed back an identical self-administered study questionnaire. The questionnaire ascertained information about demographic, socioeconomic, and anthropometric characteristics; personal and family medical history; PA; sedentary behaviors, tobacco and alcohol use; medication use; and other factors. The study population is unique, with black participants making up two-thirds of the study population and both black and white participants having similar, primarily low socioeconomic characteristics.
To be eligible for inclusion in the present analyses, subjects must have self-reported diabetes on the baseline questionnaire (responded yes to the question “Have you ever been told by a doctor that you had diabetes or high blood sugar or were treated for diabetes or high blood sugar?”). Participants were included in current analyses if they were diagnosed with diabetes after the age of 18 years and self-reported race as either “black” or “white.” If participants were missing information regarding the age of diabetes diagnosis (“What was your age at first diagnosis for diabetes or high blood sugar?”), diabetes medication use (“Are you currently taking prescription medication, including insulin, to lower your blood sugar?”), total PA (summary measure of all activity), total sitting time (summary measure of all sitting behavior times), or demographics (age, sex, or race) they were excluded from all analyses. Thus, after excluding participants due to missing data (n = 735), the final analytic cohort was comprised 15,645 SCCS participants with adult-onset diabetes.
Assessment of PA
PA was assessed at baseline using the SCCS physical activity questionnaire, which was specifically developed for the SCCS to assess PA at home, work, and for leisure [12]. Time spent conducting light, moderate, and strenuous activity at home and work were assessed for weekdays and weekends, both separately and combined using weighted averages. Participants were asked how much time they “typically” spent doing an activity involving light, moderate, or strenuous work, and about time spent doing moderate or vigorous sports. The physical activity questionnaire also assessed sedentary time (ST) by asking how much time per day was typically spent sitting for five separate activities: in a car or bus, at work, watching television or movies, using a computer, or other sitting activities (i.e., talking on the phone, reading, or sitting at meals).
Physically active times were converted from hours per day into a summary measure of metabolic equivalent tasks (METs)—hours per day. MET-hour was chosen as the measurement of PA frequency and intensity because it is independent of weight [13]. MET values were based on the values suggested by the Compendium of Physical Activities [14]. The exposure for the analysis was calculated as total PA (total of light, moderate, and strenuous household and/or occupational work and moderate and vigorous leisure-time PA) in MET-hours per day. All sitting times were summed into total hours per day spent in ST.
Participants were categorized by quartiles of total PA and ST as calculated from the distribution among all included participants, rather than according to standard cutoffs which were developed based on younger, predominately white populations. However, we have conducted sensitivity analyses using the cutoffs recommended by the 2008 Physical Activity Recommendations, and the results were not substantially different.
Mortality ascertainment
The primary outcome was defined as death from any cause. Vital status and date of death were ascertained through linkage of the SCCS cohort with the Social Security Administration vital status service for epidemiologic researchers and the National Death Index through December 31, 2011 [8].
Statistical analysis
Person-years of follow-up began on the date of enrollment into the SCCS cohort and concluded on the date of death, date of loss to follow-up, or the end of the study period (December 31, 2011), whichever came first. Descriptive statistics for the study population were calculated, including means and standard deviations for continuous variables as well as counts and percentages for categorical or dichotomous variables. Values for blacks and whites were compared using χ2 tests and one-way analysis of variance tests.
Cox proportional hazards models, using days of follow-up as the time scale, were constructed to estimate hazards ratios (HRs) and 95% confidence intervals (CIs) for mortality in relation to total PA and total ST, first separately and then mutually adjusted to determine whether the associations were independent of each other. The proportionality assumptions were tested using the goodness-of-fit testing and the log-log survival plots. The results did not indicate a violation of the proportional hazards assumption. Fully adjusted models included age at enrollment; race (black or white; not included in race-stratified models); sex (not included in sex-stratified models); body mass index (<25, 25–29.9, 30–39.9, and ≥40 kg/m2); educational attainment (less than high school, high school graduate, and beyond high school); annual household income (<$15, $15–$50K, and ≥$50K); insulin use (yes or no); smoking (current, former, and never); hypertension, high cholesterol, or cardiovascular disease (myocardial infarction and/or bypass and stroke) prevalent at baseline (all yes or no); and duration of diabetes (years). Covariates were selected for inclusion in the model based on a thorough review of the relevant literature. Certain covariates, such as marital status, were not included if they did not impact the associations between PA or ST and mortality. Information regarding activity limitations was not readily available, and we thus adjusted for comorbid conditions which may limit the frequency or type of activity conducted.
The dose-response trend for total PA or total ST was evaluated by entering the categorical form of the variable as a continuous variable into a proportional hazards model with death as the outcome. The P value for the likelihood ratio test was used to test the interactions of PA or ST with race or sex. To explore whether PA and ST jointly influence mortality, we examined the joint associations for these exposures using tertiles.
Sensitivity analyses were conducted to evaluate the potential effect of exposure outliers for PA and ST. To determine whether outlier values for PA and ST impacted estimates, a term (PA outlier: yes or no; ST outlier: yes or no) was added to the models in sensitivity analyses to determine whether adjusting for the distribution of those outliers would appreciably change the estimates. We also conducted secondary analyses excluding those who died in the first year of follow-up to mitigate the possibility of reverse causation related to increased risk of death due to pre-existing illness. In addition, we examined the association in those without prevalent cardiovascular disease (responded “no” to “Have you ever been told by a doctor that you had a heart attack or stroke?”). Finally, we conducted sensitivity analyses with the exclusion of those who died of external causes or causes believed unrelated to PA or diabetes.
All analyses were conducted using Stata software, version 11 (StataCorp LP, College Station, TX) and all tests were two sided.
Results
The 15,645 SCCS participants with diabetes (71.2% black, 28.8% white, and 65.0% female) had an average age at enrollment of 54.9 (8.9) years (Table 1). Approximately two-thirds of the population had a high school education or less, and more than 60% reported an annual household income of less than $15,000. Females were significantly more likely to report an annual household income of less than $15,000. Blacks were slightly younger than whites at both their time of enrollment and time of diabetes diagnosis. Males were younger at enrollment than females, but there were no significant differences in age at the time of diabetes diagnosis between the sexes. Whites were more likely than blacks to have obtained a high school education or beyond. A higher proportion of whites than blacks were also in the severely obese (body mass index ≥40) category and reported prevalent CVD at baseline. Females were more likely than males to be overweight or obese, whereas males were more likely to have prevalent CVD, high cholesterol, and hypertension. Blacks reported slightly but significantly higher total PA levels than whites and had higher proportions of individuals in both the highest and lowest categories of ST than whites. Blacks were more likely to never have smoked compared to whites. Of note, blacks were also significantly more likely to use insulin as a part of their diabetes medication regimen than were whites.
Table 1.
Baseline characteristics of SCCS participants with diabetes by race and sex
| Characteristic | Total (n = 15,645) | Black (n = 11,137) | White (n = 4508) | P* | Male (n = 5483) | Female (n = 10,162) | P* |
|---|---|---|---|---|---|---|---|
| Age at enrollment (mean, SD) | 54.9 (8.9) | 54.4 (8.8) | 56.0 (8.9) | <.0001 | 54.6 (8.7) | 55.1 (8.9) | <.001 |
| Age at diabetes diagnosis (mean, SD) | 46.1 (10.5) | 45.4 (10.4) | 47.8 (10.8) | <.0001 | 46.3 (10.2) | 46.0 (10.7) | .13 |
| Time since diabetes diagnosis (n,%) | |||||||
| <5 y | 6284 (40.2) | 4368 (39.2) | 1916 (42.5) | <.0001 | 2228 (40.6) | 4056 (39.9) | <.0001 |
| 5–10 y | 4407 (28.2) | 3094 (27.8) | 1313 (29.1) | 1638 (29.9) | 2769 (27.3) | ||
| 11–19 y | 3019 (19.3) | 2214 (19.9) | 805 (17.9) | 1056 (19.3) | 1963 (19.3) | ||
| ≥20 y | 1935 (12.4) | 1461 (13.1) | 474 (10.5) | 561 (10.2) | 1374 (13.5) | ||
| Female (n, %) | 10,162 (65.0) | 7290 (65.5) | 2872 (63.7) | .04 | — | — | |
| Black race (n, %) | 11,137 (71.2) | — | — | 3847 (70.2) | 7290 (71.7) | ||
| Education (n,%) | |||||||
| Less than high school | 5374 (34.4) | 4067 (36.5) | 1307 (29.0) | <.0001 | 1861 (34.0) | 3513 (34.6) | .68 |
| High school | 5049 (32.3) | 3535 (31.8) | 1514 (33.6) | 1770 (32.3) | 3279 (32.3) | ||
| Beyond high school | 5216 (33.4) | 3530 (31.7) | 1686 (37.4) | 1849 (33.7) | 3367 (33.1) | ||
| Household income (n,%) | |||||||
| ≥$15K | 9391 (60.8) | 6908 (62.8) | 2483 (55.8) | <.0001 | 2961 (54.7) | 6430 (64.0) | <.0001 |
| $15K–$49K | 5124 (33.2) | 3604 (32.8) | 1520 (34.2) | 1950 (36.1) | 3174 (31.6) | ||
| ≥$50K | 940 (6.1) | 492 (4.5) | 448 (10.1) | 498 (9.2) | 442 (4.4) | ||
| Body mass index (n,%) | |||||||
| <25 | 1662 (10.8) | 1244 (11.3) | 418 (9.4) | <.0001 | 872 (16.0) | 790 (7.9) | <.0001 |
| 25–29.9 | 3655 (23.6) | 2663 (24.2) | 992 (22.2) | 1,669 (30.7) | 1986 (19.8) | ||
| 30–39.9 | 6991 (45.2) | 4959 (45.1) | 2032 (45.6) | 2301 (42.3) | 4690 (46.8) | ||
| ≥40 | 3155 (20.4) | 2137 (19.4) | 1018 (22.8) | 602 (11.1) | 2553 (25.5) | ||
| Prevalent conditions (n,%) | |||||||
| Hypertension | 12,432 (79.5) | 9009 (80.9) | 3423 (76.0) | <.0001 | 4287 (78.2) | 8145 (80.2) | .004 |
| High cholesterol | 8644 (55.4) | 5738 (51.6) | 2906 (64.7) | <.0001 | 2930 (53.6) | 5714 (56.3) | .001 |
| Heart attack/bypass | 2095 (13.4) | 1231 (11.1) | 864 (19.2) | <.0001 | 948 (17.3) | 1,147 (11.3) | <.0001 |
| Stroke/TIA | 1722 (11.0) | 1183 (10.6) | 539 (12.0) | .02 | 633 (11.6) | 1089 (10.7) | .12 |
| Smoking Status (n,%) | |||||||
| Current | 4553 (29.3) | 3267 (29.5) | 1286 (28.7) | <.0001 | 2013 (37.0) | 2540 (25.1) | <.0001 |
| Former | 4591 (29.5) | 3050 (27.5) | 1541 (34.4) | 1939 (35.6) | 2652 (26.2) | ||
| Never | 6418 (41.2) | 4768 (43.0) | 1650 (36.9) | 1490 (27.4) | 4928 (48.7) | ||
| Glucose-lowering medication | |||||||
| Oral Medication Only | 7838 (53.3) | 5502 (53.0) | 2336 (53.8) | <.0001 | 2505 (50.0) | 5333 (55.0) | <.0001 |
| Insulin Use | 4445 (30.2) | 3351 (32.3) | 1094 (25.2) | 1598 (31.9) | 2847 (29.3) | ||
| No Medication | 2431 (16.5) | 1521 (14.7) | 910 (21.0) | 906 (18.1) | 1525 (15.7) | ||
| PA (MET-h/d; n,%) | |||||||
| <6.9 | 3822 (24.4) | 2648 (23.8) | 1174 (26.0) | .004 | 1714 (31.3) | 2108 (20.7) | <.0001 |
| 6.9–14.1 | 4002 (25.6) | 2830 (25.4) | 1172 (26.0) | 1218 (22.2) | 2784 (27.4) | ||
| 14.2–24.8 | 3908 (25.0) | 2811 (25.2) | 1097 (24.3) | 1085 (19.8) | 2823 (27.8) | ||
| ≥24.9 | 3913 (25.0) | 2848 (25.6) | 1065 (23.6) | 1466 (26.7) | 2447 (24.1) | ||
| Sedentary time (h/d; n,%) | |||||||
| <6 | 3801 (24.3) | 2784 (25.0) | 1017 (22.6) | .002 | 1289 (23.5) | 2512 (24.7) | .002 |
| 6–8.4 | 3822 (24.4) | 2656 (23.9) | 1166 (25.9) | 1323 (24.1) | 2499 (24.6) | ||
| 8.5–11.9 | 3647 (23.3) | 2569 (23.1) | 1078 (23.9) | 1236 (22.5) | 2411 (23.7) | ||
| ≥12.0 | 4375 (28.0) | 3128 (28.1) | 1247 (27.7) | 1635 (29.8) | 2740 (27.0) |
n = sample size.
P-values are from χ2 tests or t tests comparing between blacks and whites, or males and females.
During follow-up (median follow-up time: 6.2 years, range = 0.01–9.8 years), 2370 deaths (15.2%, with similar percentages among blacks and whites; 12.3% among females; 20.4% among males) occurred among the study population for a crude annual death rate of 2.44%. The highest level of PA corresponds to doing moderate exercise for 1 hour, 5 d/wk. Table 2 summarizes the HRs (95% CI) for mortality across quartiles of PA in the SCCS cohort participants with diabetes, overall, and stratified by race and by sex. Increased PA was inversely associated with mortality in a dose-response manner after adjusting for ST (highest vs. lowest quartile: HR, 0.64; 95% CI: 0.57–0.73; P for trend <.0001). These associations persisted, but were not significantly different for blacks and whites separately (P for interaction by race = .29), or for women and men (P for interaction by sex = .89). The estimates did not appreciably change when participants who died within the first year of follow-up were excluded (results not shown; highest compared with lowest level of PA, HR, 0.66; 95% CI: 0.58–0.75; P for trend <.0001). Fewer than 10% of deaths could be attributed to causes that are not related to PA. When other and/or external causes of death were excluded, the associations remained consistent for the PA-mortality association (HR, 0.79; 95% CI: 0.68–0.86).
Table 2.
HRs (95% CIs) for the association between quartiles of total PA and all-cause mortality risk among SCCS participants with diabetes
| Activity level | Person-years | No. of events | HR (95% CI)* | HR (95% CI)† | HR (95% CI)‡ |
|---|---|---|---|---|---|
| Total (MET-h/d, n = 15,645) | |||||
| <6.9 | 22,004 | 887 | Reference | Reference | Reference |
| 6.9–14.1 | 24,050 | 591 | 0.70 (0.62–0.79) | 0.78 (0.69–0.87) | 0.77 (0.69–0.86) |
| 14.2–24.8 | 24,275 | 466 | 0.64 (0.56–0.72) | 0.66 (0.59–0.75) | 0.66 (0.58–0.74) |
| ≥24.9 | 24,245 | 426 | 0.72 (0.63–0.82) | 0.65 (0.57–0.74) | 0.64 (0.57–0.73) |
| P for trend | <.0001 | <.0001 | |||
| Black (MET-h/d, n = 11,137) | |||||
| <6.9 | 15,780 | 601 | Reference | Reference | Reference |
| 6.9–14.1 | 17,578 | 428 | 0.77 (0.67–0.88) | 0.84 (0.73–0.96) | 0.83 (0.73–0.95) |
| 14.2–24.8 | 18,067 | 332 | 0.66 (0.57–0.76) | 0.69 (0.59–0.80) | 0.68 (0.59–0.79) |
| ≥24.9 | 18,368 | 324 | 0.79 (0.68–0.93) | 0.70 (0.61–0.82) | 0.69 (0.60–0.80) |
| P for trend | .005 | <.0001 | <.0001 | ||
| White (MET-h/d, n = 4508) | |||||
| <6.9 | 6224 | 286 | Reference | Reference | Reference |
| 6.9–14.1 | 6472 | 163 | 0.56 (0.45–0.70) | 0.66 (0.54–0.81) | 0.66 (0.54–0.82) |
| 14.2–24.8 | 6208 | 134 | 0.59 (0.47–0.74) | 0.63 (0.51–0.79) | 0.64 (0.51–0.79) |
| ≥24.9 | 5877 | 102 | 0.56 (0.43–0.72) | 0.55 (0.43–0.70) | 0.55 (0.43–0.70) |
| P for trend | .002 | <.0001 | <.0001 | ||
| Males (MET-h/d, n = 5483) | |||||
| <6.9 | 9473 | 497 | Reference | Reference | Reference |
| 6.9–14.1 | 7045 | 240 | 0.71 (0.60–0.84) | 0.79 (0.67–0.93) | 0.79 (0.67–0.93) |
| 14.2–24.8 | 6573 | 182 | 0.69 (0.57–0.84) | 0.66 (0.55–0.80) | 0.66 (0.55–0.79) |
| ≥24.9 | 8772 | 199 | 0.66 (0.55–0.81) | 0.63 (0.52–0.75) | 0.62 (0.52–0.75) |
| P for trend | <.001 | <.0001 | <.0001 | ||
| Females (MET-h/d, n = 10,162) | |||||
| <6.9 | 12,531 | 390 | Reference | Reference | Reference |
| 6.9–14.1 | 17,005 | 351 | 0.81 (0.69–0.95) | 0.77 (0.66–0.90) | 0.77 (0.66–0.90) |
| 14.2–24.8 | 17,702 | 284 | 0.73 (0.62–0.86) | 0.66 (0.56–0.78) | 0.66 (0.56–0.77) |
| ≥24.9 | 15,473 | 227 | 0.85 (0.71–1.02) | 0.68 (0.57–0.81) | 0.67 (0.56–0.79) |
| P for trend | .11 | <.0001 | <.0001 | ||
Age-adjusted model.
Model adjusted for age, sex (except for in sex-stratified models), race (except for in race-stratified models), BMI, socioeconomic status (income and education), history of comorbidities (hypertension, high cholesterol, myocardial infarction, and stroke), smoking, diabetes characteristics (insulin use and time since diabetes diagnosis).
Model adjusted for previously mentioned covariates and total ST (h/d).
Table 3 summarizes HRs (95% CIs) for the association between quartiles of ST and mortality, overall, and stratified by race and by sex. Sedentary time in the highest quartile corresponds to spending more than half of the 24-hour day or three-fourth of usual waking hours (16 hours) in sedentary behaviors. After adjusting for PA, participants with diabetes in the highest quartile of ST had mortality risk approximately 21% higher than those in the lowest quartile (HR, 1.21; 95% CI: 1.08–1.37), with a significant dose-response trend (P for trend = .001). When other and/or external causes of death were excluded, the estimates remained similar for the ST-mortality association (HR, 1.22; 95% CI: 1.06–1.36). Race-specific analyses showed that high levels of ST were associated with similarly increased mortality risk among blacks and whites (P for interaction by race = .46). Similarly, there was a positive trend for mortality among males and females separately, and sex did not modify the association between ST and mortality (P for interaction by sex = .19).
Table 3.
Hazard ratios (95% confidence intervals) for the association between quartiles of total sedentary time and all-cause mortality risk among SCCS participants with diabetes
| Sedentary time level | Person-years | No. of events | HR (95% CI)* | HR (95% CI)† | HR (95% CI)‡ |
|---|---|---|---|---|---|
| Total (h/d, n = 15,645) | |||||
| <6 | 23,162 | 562 | Reference | Reference | Reference |
| 6–8.4 | 23,263 | 589 | 1.00 (0.88–1.14) | 1.02 (0.90–1.15) | 1.03 (0.91–1.16) |
| 8.5–11.9 | 22,025 | 542 | 1.13 (0.99–1.28) | 1.09 (0.96–1.24) | 1.10 (0.97–1.25) |
| ≥12 | 26,124 | 677 | 1.38 (1.22–1.57) | 1.20 (1.06–1.35) | 1.21 (1.08–1.37) |
| P for trend | .001 | .001 | .001 | ||
| Black§ (h/d, n = 11,137) | |||||
| <6 | 17,608 | 422 | Reference | Reference | Reference |
| 6–8.4 | 16,731 | 413 | 1.02 (0.86–1.17) | 0.99 (0.86–1.14) | 1.00 (0.87–1.16) |
| 8.5–11.9 | 16,080 | 386 | 1.08 (0.92–1.26) | 1.07 (0.83–1.24) | 1.09 (0.94–1.26) |
| ≥12 | 19,374 | 464 | 1.32 (1.14–1.53) | 1.13 (0.98–1.30) | 1.15 (1.00–1.33) |
| P for trend | <.0001 | .05 | .03 | ||
| White§ (h/d, n = 4508) | |||||
| <6 | 5554 | 140 | Reference | Reference | Reference |
| 6–8.4 | 6532 | 176 | 1.02 (0.80–1.31) | 1.08 (0.86–1.37) | 1.07 (0.85–1.35) |
| 8.5–11.9 | 5945 | 156 | 1.28 (0.99–1.64) | 1.14 (0.89–1.44) | 1.13 (0.89–1.44) |
| ≥12 | 6750 | 213 | 1.57 (1.24–2.00) | 1.38 (1.09–1.74) | 1.36 (1.08–1.72) |
| P for trend | <.0001 | .005 | .007 | ||
| Males§ (h/d, n = 5483) | |||||
| <6 | 7491 | 262 | Reference | Reference | Reference |
| 6–8.4 | 7923 | 265 | 0.89 (0.73–1.08) | 0.94 (0.79–1.13) | 0.95 (0.79–1.14) |
| 8.5–11.9 | 7095 | 257 | 1.10 (0.90–1.33) | 1.16 (0.96–1.39) | 1.18 (0.98–1.42) |
| ≥12 | 9354 | 334 | 1.18 (1.00–1.42) | 1.14 (0.96–1.36) | 1.16 (0.97–1.38) |
| P for trend | .01 | .04 | .03 | ||
| Females§ (h/d, n = 10,162) | |||||
| <6 | 15,671 | 300 | Reference | Reference | Reference |
| 6–8.4 | 15,340 | 324 | 1.09 (0.92–1.30) | 1.09 (0.92–1.29) | 1.09 (0.92–1.29) |
| 8.5–11.9 | 14,930 | 285 | 1.15 (0.96–1.37) | 1.05 (0.88–1.24) | 1.05 (0.88–1.24) |
| ≥12 | 16,770 | 343 | 1.48 (1.25–1.76) | 1.25 (1.06–1.48) | 1.27 (1.07–1.50) |
| P for trend | <.0001 | .02 | .01 |
Age-adjusted model.
Model adjusted for age, sex (except for in sex-stratified models), race (except for in race-stratified models), BMI, socioeconomic status (income and education), history of comorbidities (hypertension, high cholesterol, myocardial infarction, and stroke), smoking, and diabetes characteristics (insulin use and time since diabetes diagnosis).
Model adjusted for previously mentioned covariates and total PA (MET-h/d).
Race- and sex-specific analyses were presented for illustrative purposes.
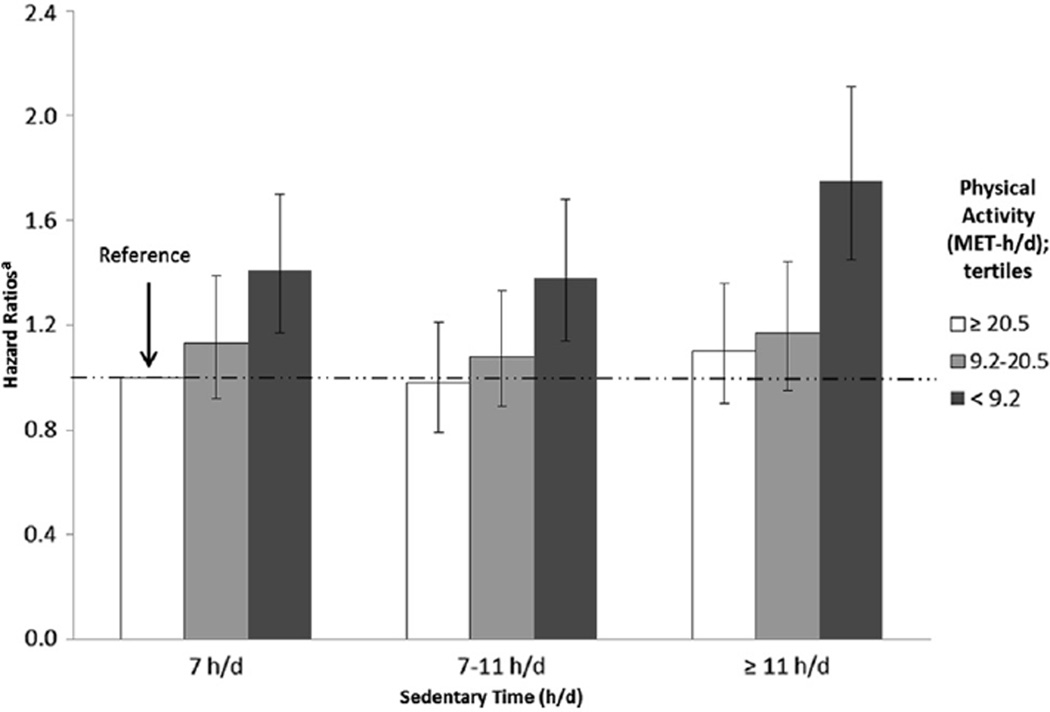
Analysis of the joint associations of PA and ST on mortality (Fig.1 and Appendix 1) showed that individuals who were the most sedentary (≥11 h/d) and the least active (<9.2 MET-h/d) were at the greatest risk of death (HR, 1.75; 95% CI: 1.45–2.11) compared with other PA-ST categories. We observed the highest risk of death among the lowest levels of PA, regardless of ST.
Fig. 1.
Joint effects of total PA and total ST on all-cause mortality risk among SCCS participants with diabetes. aAdjusted for age, sex, race, BMI, socioeconomic status (education and income), smoking, hypertension, high cholesterol, myocardial infarction, stroke, insulin use, and duration of diabetes
Conclusions
In this prospective analysis of a racially diverse, low-income population with diabetes, we found that higher levels of total PA were associated with a 36% reduced risk of mortality after adjusting for ST. Increased total time spent in sedentary behaviors was linked to an approximate 21% increase in mortality risk in this population after adjusting for PA. Furthermore, the analysis of the joint associations of both PA and ST on mortality risk revealed that across all levels of ST, low levels of PA were associated with an increased risk of death. To our knowledge, the present study contributes the first assessment of the independent relationship between total PA and ST and mortality in both black and white men and women with diabetes, as well as a comparison of these associations by race and sex and the presentation of joint effects models for PA and ST. Our study also examined this association in a population from a low socioeconomic status background, populations among whom diabetes prevalence is elevated.
Our findings are generally consistent with prior studies of mortality among those with diabetes, most of which were conducted among white men and reported a reduced risk of mortality as activity levels increased [15–29]. The strengths of the previously reported associations varied, even among relatively similar populations. Studies of males reported risk reductions ranging from 52 to 78% [15,18,21,23,27,29] among those with (variously defined) highest versus lowest PA. The highest quartile in the present study is lower than some studies that were conducted among those with diabetes who were without any other comorbid condition, but higher than studies of general populations that tended to adjust for comorbidities.
Studies that included both males and females had results ranging from 24 to 60% reductions in risk associated with high PA levels [16,17,19,20,22,24,26,28]. It is unclear whether the weaker associations observed in mixed-gender populations was a result of differences in study methodologies or an actual sex difference in the impact of PA on mortality among those with diabetes. The overall reduction in mortality risk (36%) we observed was within the range of other mixed-gender studies, and our sex-specific results demonstrated, for the first time, similar mortality associations in both males and females for both PA and ST.
The effect estimates may range between studies in part because of differences in how PA was measured for each study, as well as how PA levels were categorized and compared for each study. Sluik et al. [24] used quartiles for the assessment of leisure-time PA, and their top quartile was more than 113 MET-h/wk (corresponds with approximately >16.1 MET-h/d). Mortality risk among their top quartile was reduced by almost 40% compared with the lowest quartile (HR, 0.62; 95% CI: 0.46–0.85). Trichopoulou et al. [28] assessed PA using quintiles, and the highest quintile was 37 MET-h/d or more. Those in the highest quintile had a decreased mortality risk of more than 20% compared with the lowest quintile (HR, 0.76; 95% CI: 0.63–0.92). Quintiles for PA were also the exposure variable used in the study conducted by Tanasecu et al. [27], and the highest quintile (≥37.2 MET-h/wk) also had a decreased risk of mortality compared with those in the lowest quintile (HR, 0.65; 95% CI: 0.45–0.93). Our study showed comparable results with the reduction in risk. Most studies, like ours, assessed self-reported total or leisure-time PA. However, studies that measured cardiorespiratory fitness as the index of PA likewise consistently reported inverse associations with mortality in those with diabetes, with risk reductions of 57%–87% when comparing highest level of activity to lowest [15,21,23,29].
ST and mortality risk have been explored previously but not specifically among those with diabetes. Most studies have treated ST as if it were the inverse of PA and have not evaluated its independent association with mortality. Our study demonstrated an increased mortality risk of 21% for those who were the most sedentary compared with those who were the least sedentary. These results were after adjustment for PA, further highlighting the independence of the relationship. We did however, also explored how PA and ST interact and effect mortality risk. Across all levels of ST, those in the lowest category of PA with diabetes (<9.2 MET-h/d) were at an increased risk of mortality compared to the most active and least sedentary individuals with diabetes. Those who were the least active and most sedentary had the highest risk. These results highlight the synergistic relationship between PA and ST. This relationship suggests that PA may have a greater impact on mortality risk than does ST. Future studies should examine this effect for cause-specific mortality and among more active populations with fewer comorbid conditions.
Previous investigations also differed regarding covariates used in multivariate models. A number of studies did not adjust for diabetes duration, insulin use, or diabetes medication, which have been previously implicated as significant risk factors for mortality among individuals with diabetes [8,30–34]. Our analysis included adjustment for demographic and socioeconomic variables, as well as diabetes duration and treatment variables, which were often not included in multivariate models of other studies.
Currently, the available evidence supporting PA as a protective factor against mortality for people with diabetes is derived almost exclusively from white populations. The only study that published race-specific estimates reported a weaker and less graded association among black male veterans than white male veterans [21], similar to our findings of qualitatively weaker trends among blacks than whites. However, our overall findings do not support a differential effect of PA or ST on mortality by race, and there would be limited biologic plausibility for such an interaction.
The present study population was racially diverse and by design, black and white SCCS participants were of generally similar socioeconomic status, enabling evaluation of the risk of mortality with minimal confounding by differences in socioeconomic status (with residual confounding adjusted statistically). The findings of the present study suggest that increased PA and decreased ST is associated with decreased mortality risk in a mainly low-income population with systematic and standardized follow-up to determine death status.
The analyses were limited by several factors. All questionnaire data were collected at baseline, and we did not have follow-up data for any of the covariates included in multivariate analyses. However, concerns about lack of repeated measures are mitigated by the rather short follow-up period. Also, the questionnaire data were self-reported and subject to potential misclassification. For our main exposure variables, total PA and total ST, some participants in our analyses reported total hours spent in PA or ST in excess of 24 hours (n = 1547). Because participants were asked about individual activity or sitting times and their responses were not confined to the 24-hour day, we could not reliably determine which individual times were over-reported. Because of our categorization of total PA and total ST into quartiles, the participants who over-reported would largely be contained in the highest quartiles of activity or sitting, where they would arguably belong even in the absence of over-reporting.
The current analyses were not conducted separately for occupational activity and leisure-time activity. Occupational activity has been associated with increased work-related stress and low socioeconomic status [35–37]. Thus, occupational activity may be less strongly associated with decreased risk of mortality than leisure-time activity, and combining occupational activity and leisure-time activity may have biased the effect toward the null. Sluik et al. [24] conducted a prospective study which stratified the association between activity and total mortality among individuals with diabetes by total PA and leisure-time activity. The estimates for the effect of total PA were weaker than that for leisure-time activity alone (active vs. inactive; total PA–HR: 0.81, 95% CI: 0.61–1.08; leisure-time-HR: 0.62, 95% CI: 0.45–0.85). However, another study conducted among those with diabetes reported similar estimates between occupational activity and leisure-time activity (active vs. light; occupational-HR: 0.67, 95% CI: 0.57–0.78; leisure-time-HR: 0.72, 95% CI: 0.55–0.95) [20]. The SCCS questionnaire contains questions regarding work-related activity, but the definition of work-related activity is not restricted to occupational activities. Our study was designed to assess total PA to understand how all avenues of PA can impact mortality risk.
Our study relied on self-reported diabetes diagnosis; however, previous validation studies conducted within the SCCS using medical records for 124 participants reported that 96% of the self-reported diabetes diagnoses were validated using International Classification of Diseases, Ninth Revision codes, HbA1c levels, treatment, or physicians’ notes [38]. Therefore, we do not believe this is a significant concern in our study. Our study did not have a method to definitively distinguish between type 1 and type 2 diabetes. In an effort to remove those likely to have type 1 diabetes, we only included those who reported diagnosis after 18 years of age.
Finally, to control for the possibility of reverse causation, we conducted secondary analyses excluding participants who died within the first year of follow-up (n = 241), and the results were similar to the main analyses. In an additional analysis, we removed those who died within the first year of the study, who were in the lowest and highest categories of PA and ST, respectively. Again, the results of these analyses were similar to those of the main analyses. Analyses were also conducted excluding those with prevalent CVD, an independent risk factor for death. Those results were similar for PA, but the association for ST was slightly attenuated (highest vs. lowest quartile; HR, 1.15; 95% CI: 1.02–1.24). In studies of PA and mortality, reverse causation could impart bias because of the inability to be physically active due to a pre-existing illness or condition that may result in death. Our results do not suggest that this bias is operating but we were not able to assess physical inability to be more physically active, and further studies would be needed to determine the impact of this factor in the association between PA and mortality. The evidence for the occurrence of this phenomenon in cohort studies has been weak and not well studied.
In summary, the findings suggest that increased PA and decreased ST may be viable prevention efforts to reduce mortality burden within a racially diverse, low-income population with diabetes. The study extends to blacks and to women findings that have previously been reported primarily among white men with diabetes and signals that intervention efforts could be similarly effective across sex and racial groups. This study also revealed a synergistic relationship between PA and ST in mortality risk. The risk of mortality was increased for the lowest levels of PA within each level of ST, emphasizing the importance of PA for those with diabetes and mortality risk. Further research exploring objective, repeated measures of PA and ST, and examining different types and frequencies of activity suitable for populations with diabetes may help to clarify intervention strategies aimed at reducing the burden of this increasingly common illness.
Appendix
Appendix 1.
HRs (95% CIs) for the joint effect of PA and ST on mortality among SCCS participants with diabetes
| PA/ST level | Least sedentary | Sedentary | Most sedentary |
|---|---|---|---|
| <7 h/d | 7–11 h/d | ≥11 h/d | |
| Most active, PA ≥20.5 MET-h/d | Reference | 0.98 (0.79–1.21) | 1.10 (0.90–1.36) |
| Active, PA 9.2–20.5 MET-h/d | 1.13 (0.92–1.39) | 1.08 (0.89–1.33) | 1.17 (0.95–1.44) |
| Least active, PA <9.2 MET-h/d | 1.41 (1.17–1.70) | 1.38 (1.14–1.75) | 1.75 (1.45–2.11) |
References
- 1.CDC. U.S. Department of Health and Human Services. Atlanta, GA: Centers for Disease Control and Prevention; 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. [Google Scholar]
- 2.Preis SR, Hwang S-J, Coady S, Pencina MJ, D’Agostino RB, Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119(13):1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treatment of type 2 diabetes mellitus. Prescriber’s Letter. 2006;22(11):221103. [Google Scholar]
- 4.Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah BR, Mamdani M, Jaakkimainen L, Hux JE. Risk modification for diabetic patients. Are other risk factors treated as diligently as glycemia? Can J Clin Pharmacol. 2004;11(2):e239–e244. [PubMed] [Google Scholar]
- 6.Kodama S, Tanaka S, Heianza Y, Fujihara K, Horikawa C, Shimano H, et al. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: a meta-analysis. Diabetes Care. 2013;36(2):471–479. doi: 10.2337/dc12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koivula RW, Tornberg AB, Franks PW. Exercise and diabetes-related cardiovascular disease: systematic review of published evidence from observational studies and clinical trials. Curr Diab Rep. 2013;13(3):372–380. doi: 10.1007/s11892-013-0373-0. [DOI] [PubMed] [Google Scholar]
- 8.Conway BN, May ME, Blot WJ. Mortality among low-income African Americans and whites with diabetes. Diabetes Care. 2012;35(11):2293–2299. doi: 10.2337/dc11-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21(1):26–37. doi: 10.1353/hpu.0.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, et al. Southern Community Cohort Study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97(7):972–979. [PMC free article] [PubMed] [Google Scholar]
- 11.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing diabetes prevalence between African Americans and whites of similar socioeconomic status. Am J Public Health. 2007;97(12):2260–2267. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchowski MS, Matthews CE, Cohen SS, Signorello LB, Fowke JH, Hargreaves MK, et al. Evaluation of a questionnaire to assess sedentary and active behaviors in the Southern Community Cohort Study. J Phys Act Health. 2012;9(6):765–775. doi: 10.1123/jpah.9.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinstein AR, Sesso HD, Lee IM, Rexrode KM, Cook NR, Manson JE, et al. The joint effects of physical activity and body mass index on coronary heart disease risk in women. Arch Intern Med. 2008;168(8):884–890. doi: 10.1001/archinte.168.8.884. [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 15.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27(1):83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 16.de Fine Olivarius N, Siersma V, Nielsen A, Hansen L, Rosenvinge L, Mogensen C. Predictors of mortality of patients newly diagnosed with clinical type 2 diabetes: a 5-year follow up study. BMC Endocr Disord. 2010;10(1):14. doi: 10.1186/1472-6823-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford ES, DeStefano F. Risk factors for mortality from all causes and from coronary heart disease among persons with diabetes: findings from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Am J Epidemiol. 1991;133(12):1220–1230. doi: 10.1093/oxfordjournals.aje.a115834. [DOI] [PubMed] [Google Scholar]
- 18.Gaziano T, Bubes V, Gaziano J. Exercise and mortality among diabetics in the physicians’ health study enrolment cohort. Cardiovasc J S Afr. 2005;16(Suppl 2):12. [Google Scholar]
- 19.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan K. Relationship of walking to mortality among us adults with diabetes. Arch Intern Med. 2003;163(12):1440–1447. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 20.Hu G, Eriksson J, Barengo NC, Lakka TA, Valle TT, Nissinen A, et al. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation. 2004;110(6):666–673. doi: 10.1161/01.CIR.0000138102.23783.94. [DOI] [PubMed] [Google Scholar]
- 21.Kokkinos P, Myers J, Nylen E, Panagiotakos DB, Manolis A, Pittaras A, et al. Exercise capacity and all-cause mortality in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2009;32(4):623–628. doi: 10.2337/dc08-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nothlings U, Ford ES, KrÖGer J, Boeing H. Lifestyle factors and mortality among adults with diabetes: findings from the European Prospective Investigation into Cancer and Nutrition–Potsdam study*. J Diabetes. 2010;2(2):112–117. doi: 10.1111/j.1753-0407.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 23.Nylen ES, Kokkinos P, Myers J, Faselis C. Prognostic effect of exercise capacity on mortality in older adults with diabetes mellitus. J Am Geriatr Soc. 2010;58(10):1850–1854. doi: 10.1111/j.1532-5415.2010.03068.x. [DOI] [PubMed] [Google Scholar]
- 24.Sluik D, Buijsse B, Muckelbauer R, Kaaks R, Teucher B, Johnsen NF, et al. Physical activity and mortality in individuals with diabetes mellitus: a prospective study and meta-analysis. Arch Intern Med. 2012;172(17):1285–1295. doi: 10.1001/archinternmed.2012.3130. [DOI] [PubMed] [Google Scholar]
- 25.Smith TC, Wingard DL, Smith B, Kritz-Silverstein D, Barrett-Connor E. Walking decreased risk of cardiovascular disease mortality in older adults with diabetes. J Clin Epidemiol. 2007;60(3):309–317. doi: 10.1016/j.jclinepi.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sone H, Tanaka S, Suzuki S, Seino H, Hanyu O, Sato A, et al. Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with type 2 diabetes: analysis from the Japan Diabetes Complications Study (JDCS) Diabetologia. 2013;56(5):1021–1030. doi: 10.1007/s00125-012-2810-z. [DOI] [PubMed] [Google Scholar]
- 27.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107(19):2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 28.Trichopoulou A, Psaltopoulou T, Orfanos P, Trichopoulos D. Diet and physical activity in relation to overall mortality amongst adult diabetics in a general population cohort. J Intern Med. 2006;259(6):583–591. doi: 10.1111/j.1365-2796.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 29.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 30.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 31.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 32.Bangstad H-J, Danne T, Deeb LC, Jarosz-Chobot P, Urakami T, Hanas R. Insulin treatment. Pediatr Diabetes. 2007;8(2):88–102. doi: 10.1111/j.1399-5448.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 33.Boyle PJ. Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am J Med. 2007;120(9) Suppl. 2:S12–S17. doi: 10.1016/j.amjmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 34.De Feyter HM, Praet SF, van den Broek NM, Kuipers H, Stehouwer CD, Nicolay K, et al. Exercise training improves glycemic control in longstanding insulin-treated type 2 diabetic patients. Diabetes Care. 2007;30(10):2511–2513. doi: 10.2337/dc07-0183. [DOI] [PubMed] [Google Scholar]
- 35.Holtermann A, Hansen JV, Burr H, Søgaard K, Sjøgaard G. The health paradox of occupational and leisure-time physical activity. Br J Sports Med. 2012;46(4):291–295. doi: 10.1136/bjsm.2010.079582. [DOI] [PubMed] [Google Scholar]
- 36.Martins L, Lopes C. Rank, job stress, psychological distress and physical activity among military personnel. BMC Public Health. 2013;13(1):716. doi: 10.1186/1471-2458-13-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beenackers M, Kamphuis C, Giskes K, Brug J, Kunst A, Burdorf A, et al. Socioeconomic inequalities in occupational, leisure-time, and transport related physical activity among European adults: a systematic review. Int J Behav Nutr Phys Act. 2012;9(1):116. doi: 10.1186/1479-5868-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huizinga M, Elasy TA, Villegas R, Signorello LB, Blot W, Cavanaugh K. Validation of diabetes self-report and characteristics of undiagnosed diabetes in the Southern Community Cohort Study (abstract). Proceedings of the 69th Annual Meeting of the American Diabetes Association; American Diabetes Association; New Orleans, LA. 2009. p. A279. [Google Scholar]