Defective MBL2 diplotypes in Danish children younger than 5 years are not associated with meningococcal meningitis or bacteremia. Nor did we find any association between diplotypes and mortality.
Keywords: invasive meningococcal disease, MBL deficiency, MBL2 genotypes
Abstract
Background. Neisseria meningitidis is the cause of meningococcal bacteremia and meningitis, and nasopharyngeal colonization with this pathogen is common. The incidence of invasive disease is highest in infants, whereas adolescents more often are carriers. Altered regulation or dysfunction of the innate immune system may predispose to invasive meningococcal disease (IMD). In this study, we investigated the effect of genetic variation in the mannose-binding lectin gene, MBL2, and its promoter on susceptibility to IMD and IMD-associated mortality among children.
Methods. Children (<5 years) diagnosed during 1982–2007 with IMD and controls were identified through Danish national registries. DNA was obtained from the Danish Neonatal Screening Biobank. The associations between MBL2 diplotypes and IMD susceptibility and 30- and 90-day mortality were investigated using logistic regression analysis.
Results. We included 1351 children: 406 with meningitis, 272 with bacteremia, and 673 age- and sex-matched controls. Of the children studied, 1292 (96%) were successfully genotyped and assigned MBL2 diplotypes. The median age in IMD cases was 19.1 months (interquartile range [IQR], 8.8–32.2 months). Children with defective MBL2 diplotypes were not at higher risk for meningococcal meningitis than children with intermediate and normal diplotypes (odds ratio [OR] = 0.69; 95% confidence interval [CI], .47–1.02). Similar results were found for children with bacteremia and defective diplotypes (OR = 0.84; 95% CI, .53–1.32) as well as for all cases (OR = 0.75; 95% CI, .56–1.01). There was no association between MBL2 diplotypes and mortality.
Conclusions. Defective MBL2 diplotypes did not predict either an increased IMD susceptibility or mortality in a Danish population of children.
Invasive meningococcal disease (IMD) is of major public health importance due to its global distribution, epidemic potential, disease burden in children and young adults, and potential for severe clinical manifestations, such as meningitis and sepsis [1]. Carriage prevalence increases with age, from 0.71% in infants and toddlers under the age of 4 years [1] to 25% in children aged 5–19 years [2] and up to 33% in young adults aged 18–26 years [3]. Thereafter, the prevalence declines steadily with age [2].
In a small proportion of carriers, colonization leads to invasive disease. However, the mechanisms remain incompletely understood but are probably a combination of bacterial virulence factors, environmental conditions, and host susceptibility [1]. The severity of a meningococcal infection ranges from bacteremia with mild symptoms to meningococcal meningitis associated with 5%–10% mortality and to fulminant meningococcal sepsis, characterized by septic shock and disseminated intravascular coagulation with thrombotic lesions in multiple organ systems and up to 40% mortality [4].
Differences in host susceptibility to IMD are at least partly determined by genetic factors in the host [4], but the exact role of each component remains to be elucidated [5].
Mannose-binding lectin (MBL) is the central molecule of the lectin pathway of complement activation [6]. Mannose-binding lectin binds the meningococci and targets membrane proteins such as opA and porB [7]. Mannose-binding lectin stimulates phagocytosis of the bacteria by neutrophils, macrophages, and monocytes, increases the effectiveness of the host's neutralization of the pathogen, and regulates the immune response by reducing production of proinflammatory cytokines [8].
Mannose-binding lectin deficiency is a relatively common phenomenon, and in a Caucasian population the expected prevalence of MBL deficiency is approximately 5% [9, 10]. Although it has been linked to increased susceptibility to certain infectious diseases [8], the vast majority of individuals with MBL deficiency seems to be unaffected [10]. Its precise role and importance is under discussion in the context of meningococcal disease [6, 11, 12]. Previous studies have described that children under the age of 2 years with MBL deficiency may be at an age-dependent increased risk for infections [12, 13]. We studied the associations between (1) MBL deficiency and meningococcal meningitis or bacteremia and (2) mortality in a large pediatric case-control study.
PATIENTS AND METHODS
Study Population
We conducted the research as a nationwide study in Denmark within a population of approximately 5.5 million inhabitants. The unique 10-digit identification number provided for all residents in Denmark through the Danish Civil Registration System (CRS) allowed linkage of data between the different registries. Through the registries at the Danish National Neisseria and Streptococcus Reference Center, Statens Serum Institute (SSI), we identified all children born in the period 1982–2006 who had IMD below age of 5 years. From the CRS [14], we obtained information on country of birth of the children and their parents as well as data on mortality. To minimize effects of population stratification, we excluded children if they or 1 of their parents were born outside Scandinavia or Germany.
For cases with meningitis, we identified controls through CRS, and for cases with bacteremia, controls were obtained from the Danish Neonatal Screening Biobank (DNSB) [15] by selecting 1 of the dried blood spot (DBS) card stored adjacent to the case. Because the DBS cards are stored according to sample date, controls could thereby be matched on sex and age. We also required that controls and their parents were born in Scandinavia or Germany. We used the risk set sampling technique (ie, the control children had to be alive and without a hospitalization with IMD at the date the corresponding case had IMD diagnosed) [16].
Information on all hospitalizations in cases and controls was obtained from the Danish National Patient Registry [17]. Cases and controls with a hospitalization for any cause before the IMD episode were excluded, due to their possible severe comorbidity. Data were obtained in February 2007, and all children were born in the period 1982 to 2006. Meningococcal vaccines are not included in Danish Immunization schedules.
DNA Extraction and Genotyping
We obtained access to DNA from IMD patients and the healthy controls by using archived residual blood samples from the DNSB, which contains dried blood samples collected from almost every Dane born after 1981 as a part of the neonatal screening program [18]. Procured samples are stored in the DNSB [15, 19]. For the current study, DNA extraction and genome amplification were performed by the Department of Clinical Biochemistry at SSI, as previously described [18]. In brief, two 3.2 mm discs were punched from each DBS sample. Genomic DNA was extracted using the Extract-N-Amp kit (Sigma-Aldrich). To attenuate possible unequal amplification of alleles, whole-genome amplification (WGA) was carried out in triplicate using the REPLI-g mini kit (QIAGEN) and pooled after completed WGA.
Three single-nucleotide polymorphisms (SNPs) in exon 1 (variant B, rs1800450; variant C, rs1800451; and variant D, rs5030737) and 1 promoter SNP (rs7096206) in the MBL2 gene were genotyped.
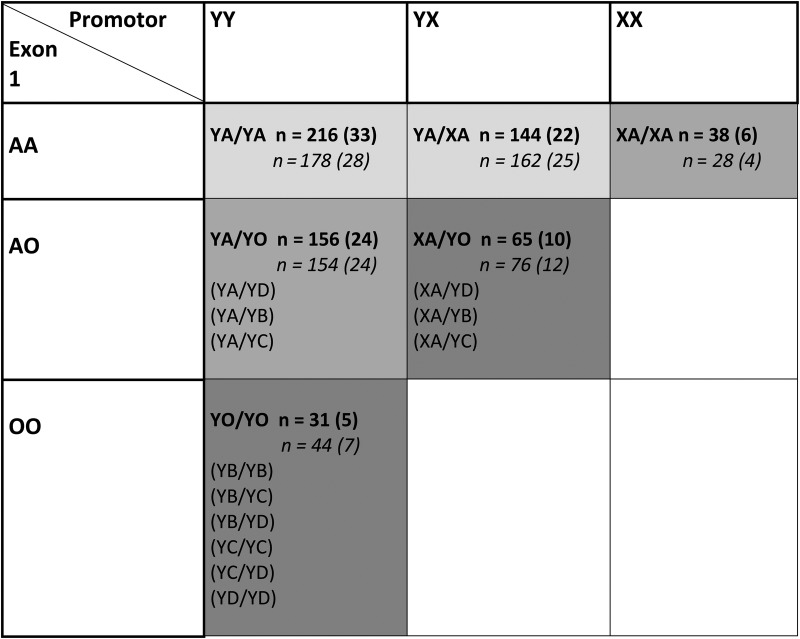
Individuals with MBL deficiency are homozygous (or compound heterozygous, ie, having 2 heterogeneous polymorphisms) for any of the 3 structural polymorphisms in exon 1 of the MBL2 gene, and these genotypes are strongly correlated with undetectable levels of MBL in plasma; individuals that are homozygous for the variant promotor SNP or with 1 polymorphism in exon 1 have reduced plasma MBL concentrations, as previously described [20]. For exon 1 SNPs, the wild-type allele is denoted A. The B, C, and D variants are pooled and given the designation “O,” ie, 1 wild-type haplotype combined with 1 SNP in B, C, or D is denoted A/O. Homozygosity and compound heterozygosity are denoted O/O (YO/YO). The promoter SNP is expressed as X/Y, where Y is the wild-type allele [20].
An overview of diplotypes (haplotype pairs) is provided in Figure 1. The functional diplotypes are known to correspond to MBL serum concentrations and can be divided into 3 groups: normal (YA/YA, YA/XA), intermediate (XA/XA, YA/YO), and deficient (XA/YO, YO/YO) [9, 21–23].
Figure 1.
Overview of construction of diplotypes and diplotype frequencies in the combined group of children with meningitis and bacteremia. Construction of diplotypes: The three structural variant alleles (B, C and D) in exon 1 are pooled and denoted “O”. Inferred consequence for MBL protein expression: Light grey, normal; Grey, intermediate; Dark grey, defective. For diplotype frequencies (%): Bold, meningitis + bacteremia cases; Italics, meningitis + bacteremia controls.
Single-nucleotide polymorphism genotyping was performed by LGC Genomics (LGC Ltd, Teddington, Middlesex, United Kingdom) using a competitive allele-specific polymerase chain reaction [24].
Statistical Analysis
We calculated medians and interquartile ranges (IQR) of quantitative variables, and we compared SNP frequencies in each group using χ2 statistics. To examine a possible association between MBL2 diplotypes and IMD susceptibility and 30- and 90-day mortality, logistic regression was performed. The analyses were adjusted for sex, and results are presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Data were stratified for source of infection (bacteremia/meningitis), and in some analyses we made age strata and hereby tested IMD susceptibility in children younger than 24 months, younger than 12 months, and 6–18 months. Genotype equilibrium was tested applying the Hardy and Weinberg method to controls [25]. All statistical analyses were performed using Statistical Analysis Systems (SAS version 9.4, SAS institute, Cary, NC).
The study was approved by the Danish Data Protection Agency (record nos. 2005-41-6012 and 2015-41-3716). Ethical permission was obtained from the Ethical Committee of Central Denmark Region (record no. 20060008). According to Danish Legislation, the Research Ethics Committee can grant an exemption from obtaining informed consent for research projects based on biological material under certain circumstances, and such an exemption was granted for this study.
RESULTS
Children With Invasive Meningococcal Disease
We included 406 cases who presented with meningitis and 272 cases who presented with bacteremia. In addition, we included 673 age- and sex-matched population controls. Table 1 provides an overview of MBL2 diplotypes in each subgroup. Genotype call rates were >95%. Diplotypes were constructed for a total of 1292 children. An overview of haplotype frequencies is presented in Table 2. Among study participants, there was a predominance of boys (Table 2). Age at time of diagnosis did not differ substantially between those who presented with meningitis (median, 18.5 months; IQR, 8.2–32.4 months) and the subgroup with bacteremia (median, 19.6 months; IQR, 9.2–31.8 months). The genotype frequencies of controls were in Hardy-Weinberg equilibrium (HWE) for rs1800450 (B) (χ2 = 0.43 and P > .2); rs1800451 (C) (χ2 = 0.21 and P > .2); and rs5030737 (D) (χ2 = 1.46 and P > .2). For rs7096206 (X/Y) (χ2 = 5.24 and P = .02), HWE departed [25].
Table 1.
MBL2 Diplotypes in Children With Invasive Meningococcal Disease and Controls
| Group | Status | Normal Diplotypes: YA/YA, XA/YA (%) | Intermediate Diplotypes: XA/XA, YA/YO (%) | Defective Diplotypes: XA/YO, YO/YO (%) | Total, (%) | P Valuea | Odds Ratio (95% Confidence Interval)b |
|---|---|---|---|---|---|---|---|
| Meningitis | Control | 211 (55) | 100 (26) | 71 (19) | 382 | .15 | 0.69 (.47–1.02) |
| Case | 219 (57) | 115 (30) | 53 (14) | 387 | |||
| Bacteremia | Control | 129 (50) | 82 (32) | 49 (19) | 260 | .62 | 0.84 (.53–1.32) |
| Case | 141 (54) | 79 (30) | 43 (16) | 263 | |||
| Combined | Control | 340 (53) | 182 (28) | 120 (19) | 642 | .17 | 0.75 (.56–1.01) |
| Case | 360 (55) | 194 (30) | 96 (15) | 650 |
a 2 × 3 χ2 test, degrees of freedom = 2.
b Comparison of defective diplotypes vs intermediate combined with normal diplotypes and adjusted for sex.
Table 2.
Characteristics of Cases and Controls and Overview of Haplotypesa
| YA (%) | XA (%) | YB (%) | YC (%) | YD (%) | Total | |
|---|---|---|---|---|---|---|
| Bacteremia | ||||||
| Cases | 289 (55) | 116 (22) | 86 (16) | 5 (1) | 30 (6) | 526 |
| Male: 145 (53%) | ||||||
| Median age at infection, months: 19.6 (IQR = 9.2–31.8) | ||||||
| Controls | 274 (53) | 109 (21) | 82 (16) | 8 (2) | 45 (9) | 518 |
| Male: 148 (54%) | ||||||
| Total | 563 | 225 | 168 | 13 | 75 | 1044 |
| Male: 293 (54%) | ||||||
| Meningitis | ||||||
| Cases | 443 (57) | 169 (22) | 106 (14) | 14 (2) | 42 (5) | 774 |
| Male: 237 (58%) | ||||||
| Median age at infection, months: 18.5 (IQR = 8.2–32.4) | ||||||
| Controls | 398 (52) | 185 (24) | 106 (14) | 15 (2) | 60 (8) | 764 |
| Male: 234 (59%) | ||||||
| Total | 841 | 354 | 212 | 29 | 102 | 1538 |
| Male: 471 (59%) | ||||||
Abbreviations: IQR, interquartile range.
a One bacteremia control was left out of the table, because it had genotype T:C in rs1800450 (B) and T:T in rs5030737 (D), because this combination should not exist. This was likely due to a genotyping error.
MBL2 Diplotypes and Associations With Invasive Meningococcal Disease
MBL2 diplotype associations were tested in the meningitis and bacteremia subgroups and in the combined group (Table 1). Children with defective MBL2 diplotypes were not at higher risk for meningococcal meningitis than the group of children with intermediate or normal diplotypes (OR = 0.69; 95% CI, .47–1.02). Similar results were found for children with bacteremia and defective diplotypes (OR = 0.84; 95% CI, .53–1.32) as well as for all cases (OR = 0.75; 95% CI, .56–1.01). No significant associations with IMD were found when we repeated the analyses testing the defective YO/YO diplotype versus all remaining diplotypes (OR = 0.70 and 95% CI, .73–1.30 for meningitis and OR = 0.65 and 95% CI, .31–1.35 for bacteremia) and compared normal diplotypes to defective diplotypes (OR = 0.72 and 95% CI, .48–1.08 for meningitis and OR = 0.79 and 95% CI, .49–1.28 for bacteremia). In these analyses, genotypes were grouped as in previous studies [26–28]. In age-stratified analyses, we found no significant associations between susceptibility to IMD and MBL deficiency in children aged 6–18 months and under the age of 1 and 2 years.
MBL2 Diplotypes and Mortality
Forty-four children (6.5%) died within the first 30 days after their IMD diagnosis: 26 (6.4%) with meningitis and 18 (6.6%) with bacteremia. Forty-six (6.8%) died within the first 90 days after receiving their diagnosis. MBL2-defective diplotypes were not associated with increased 30-day mortality in meningitis cases (OR = 1.17; 95% CI, .39–3.58) or in bacteremia cases (OR = 0.62; 95% CI, .14–2.80) compared with cases with normal or intermediate diplotype. For 90-day mortality, the corresponding results were OR = 1.13 and 95% CI, .37–3.41 for meningitis and OR = 0.96 and 95% CI, .27–3.44 for bacteremia, compared with cases with normal or intermediate diplotype.
DISCUSSION
We found no evidence that genetic variation in the MBL2 gene affected susceptibility to IMD in previously healthy children. Furthermore, there was no association between MBL2 gene variation and mortality. Our results are in line with other recent studies of MBL2 deficiency and meningococcal infection [11, 29]. However, the importance of MBL-deficiency in susceptibility to infection is still discussed, and previous studies have yielded conflicting results [11, 12, 29, 30].
A large population-based Danish study found no evidence for differences in susceptibility to infectious disease or to mortality in MBL-deficient individuals compared with population controls [10]. Darton et al [11] showed that individuals with MBL2 deficiency had higher genomic bacterial load in plasma, and particularly in children under the age of 2. However, they found no association between MBL deficiency and IMD susceptibility. In a study of 296 Caucasian cases and 5196 controls, there was no increased risk of IMD in MBL-deficient individuals [29]. In certain South American ethnic groups, more than 60% of the population is homozygous for the B variant [9]. This lends support to the hypothesis that MBL is without major importance in contemporary infections and that MBL deficiency is overcome by other immune defense systems [9].
In a study of respiratory tract infections of different etiologies in children from West Greenland, Koch et al [13] found that MBL deficiency among children 6 to 17 months old put them at increased risk of acute respiratory infections. Likewise, Faber et al [12] reported that MBL deficiency was significantly associated with susceptibility to childhood meningococcal disease in an age-dependent manner. We were unable to confirm these findings in our population.
Earlier studies showing an association between MBL2 and susceptibility to infection differ from our study in several important aspects, including sample size, inclusion of mixed ethnic groups, of various age groups, and of children with comorbidity, as well as culture unconfirmed IMD diagnosis based on clinical symptoms [12, 30].
An increased incidence of infectious disease may be seen in immunocompromised individuals who are also MBL deficient [31, 32], eg, in individuals with common variable immunodeficiency [33]. We only included children without previous hospitalizations to avoid inclusion of participants with severe comorbidities. Yet, the assumption that children without previous hospitalizations are healthy may not be correct. This could be a potential limitation in our study, and this assumption may have resulted in a potential underestimation of MBL deficiency's impact on susceptibility to IMD.
However, the frequency of MBL2 diplotype variants in cases and controls in our study corresponded well with previously described distributions in the Danish population [10, 31].
We assume that members of our study population consisted of individuals of North European origin, based on the birthplace of the children and their parents. Although in a Danish setting, the vast majority of the children are likely Caucasian, and different ancestry could have induced a systematic difference in allele frequencies between cases and controls, but this is unlikely due to the results in this study. All but 1 of the SNPs were in HWE, and this does not suggest ethnic heterogeneity either. We chose to include the single SNP that departed from HWE to enable construction of MBL diplotypes.
We found no significant associations between MBL2 diplotypes and mortality; however, these estimates were limited by a low statistical power expressed as wide CIs.
CONCLUSIONS
In conclusion, we conducted one of the larger studies on MBL2 deficiency in previously healthy children and their matched controls, and we did not find any association with susceptibility to IMD.
Acknowledgments
Author contributions. T. B. provided the study concept. M. N., Z. B. H., M. V. H., H. T. S., and T. B. designed the study. M. V. H., D. M. H., L. F. L. and T. B. performed the experiments. L. F. L., L. N. C., and T. B. analyzed the data. H. B. K. coordinated the national surveillance system. Z. B. H., M. V. H., and H. B. K. identified and collected samples. L. F. L. and T. B. wrote the first draft of the manuscript. All authors participated in writing the final manuscript.
Financial support. This work was supported by the Lundbeck Foundation; the Novo Nordisk Foundation; King Christian the 10th Foundation; Jacob Madsen's Foundation; TrygVesta, Ebba Celinder′s Foundation; the Danish Medical Association Foundation; the Foundation for Advancement of Medical Science; the Augustinus Foundation; Peder Laurits Pedersen′s Foundation; A.P. Møller Foundation for the Advancement of Medical Science; the Danish Medical Research Council; Preben and Anna Simonsen′s Foundation; Ferdinand and Ellen Hindsgaul′s Foundation; Hartmann′s Foundation; and the Dagmar Marshalls Fond. L. F. L. was supported by a PhD scholarship from the University of Copenhagen. H. T. S. was supported by the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation.
Potential conflicts of interest. T. B. reports grants from Lundbeck Foundation, grants from Novo Nordisk Foundation, grants from Hindsgaul Fund, grants from Preben and Anna Simonsen Foundation, during the conduct of the study; personal fees from GSK, personal fees from Bristol Myers Squibb, personal fees from Gilead, grants from Pfizer, personal fees from Bristol Myers Squibb, personal fees from GSK, personal fees from Bristol Myers Squibb, personal fees from GSK, nonfinancial support from Bristol Myers Squibb, nonfinancial support from Gilead, nonfinancial support from Janssen, nonfinancial support from MSD, and personal fees from Abbvie, outside the submitted work.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012; 30(suppl 2):B3–9. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright KA, Stuart JM, Jones DM, Noah ND. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect 1987; 99:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus H, Maiden MC, Wilson DJ et al. . Genetic analysis of meningococci carried by children and young adults. J Infect Dis 2005; 191:1263–71. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer MC, de Gans J, Heckenberg SG et al. . Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis 2009; 9:31–44. [DOI] [PubMed] [Google Scholar]
- 5.Chapman SJ, Khor CC, Vannberg FO et al. . IkappaB genetic polymorphisms and invasive pneumococcal disease. Am J Respir Crit Care Med 2007; 176:181–7. [DOI] [PubMed] [Google Scholar]
- 6.Sprong T, Mollnes TE, Neeleman C et al. . Mannose-binding lectin is a critical factor in systemic complement activation during meningococcal septic shock. Clin Infect Dis 2009; 49:1380–6. [DOI] [PubMed] [Google Scholar]
- 7.Estabrook MM, Jack DL, Klein NJ, Jarvis GA. Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J Immunol 2004; 172:3784–92. [DOI] [PubMed] [Google Scholar]
- 8.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis 2003; 37:1496–505. [DOI] [PubMed] [Google Scholar]
- 9.Garred P, Larsen F, Seyfarth J et al. . Mannose-binding lectin and its genetic variants. Genes Immun 2006; 7:85–94. [DOI] [PubMed] [Google Scholar]
- 10.Dahl M, Tybjærg-Hansen A, Schnohr P, Nordestgaard BG. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J Exp Med 2004; 199:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darton TC, Jack DL, Johnson M et al. . MBL2 deficiency is associated with higher genomic bacterial loads during meningococcemia in young children. Clin Microbiol Infect 2014; 20:1337–42. [DOI] [PubMed] [Google Scholar]
- 12.Faber J, Schuessler T, Finn A et al. . Age-dependent association of human mannose-binding lectin mutations with susceptibility to invasive meningococcal disease in childhood. Pediatr Infect Dis J 2007; 26:243–6. [DOI] [PubMed] [Google Scholar]
- 13.Koch A, Melbye M, Sørensen P et al. . Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA 2001; 285:1316–21. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29:541–9. [DOI] [PubMed] [Google Scholar]
- 15.Nørgaard-Pedersen B, Hougaard DM. Storage policies and use of the Danish Newborn Screening Biobank. J Inherit Metab Dis 2007; 30:530–6. [DOI] [PubMed] [Google Scholar]
- 16.Navidi W, Weinhandl E. Risk set sampling for case-crossover designs. Epidemiology 2002; 13:100–5. [DOI] [PubMed] [Google Scholar]
- 17.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011; 39:30–3. [DOI] [PubMed] [Google Scholar]
- 18.Hollegaard MV, Grove J, Grauholm J et al. . Robustness of genome-wide scanning using archived dried blood spot samples as a DNA source. BMC Genet 2011; 12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollegaard MV, Thorsen P, Norgaard-Pedersen B, Hougaard DM. Genotyping whole-genome-amplified DNA from 3- to 25-year-old neonatal dried blood spot samples with reference to fresh genomic DNA. Electrophoresis 2009; 30:2532–5. [DOI] [PubMed] [Google Scholar]
- 20.Lundbo LF, Harboe ZB, Clausen LN et al. . Mannose-binding lectin gene, MBL2, polymorphisms are not associated with susceptibility to invasive pneumococcal disease in children. Clin Infect Dis 2014; 59:e66–71. [DOI] [PubMed] [Google Scholar]
- 21.Vengen IT, Madsen HO, Garred P et al. . Mannose-binding lectin deficiency is associated with myocardial infarction: the HUNT2 study in Norway. PLoS One 2012; 7:e42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency—revisited. Mol Immunol 2003; 40:73–84. [DOI] [PubMed] [Google Scholar]
- 23.Steffensen R, Thiel S, Varming K et al. . Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods 2000; 241:33–42. [DOI] [PubMed] [Google Scholar]
- 24.Abhishek A, Doherty S, Maciewicz R et al. . The association between ANKH promoter polymorphism and chondrocalcinosis is independent of age and osteoarthritis: results of a case–control study. Arthritis Res Ther 2014; 16:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009; 169:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S, Knox K, Segal S et al. . MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 2002; 359:1569–73. [DOI] [PubMed] [Google Scholar]
- 27.García-Laorden MI, de Castro F, Solé-Violán J et al. . The role of mannose-binding lectin on pneumococcal infection. Eur Respir J 2013; 41:131–9. [DOI] [PubMed] [Google Scholar]
- 28.Lausen B, Schmiegelow K, Andreassen B et al. . Infections during induction therapy of childhood acute lymphoblastic leukemia–no association to mannose-binding lectin deficiency. Eur J Haematol 2006; 76:481–7. [DOI] [PubMed] [Google Scholar]
- 29.Bradley DT, Bourke TW, Fairley DJ et al. . Genetic susceptibility to invasive meningococcal disease: MBL2 structural polymorphisms revisited in a large case-control study and a systematic review. Int J Immunogenet 2012; 39:328–37. [DOI] [PubMed] [Google Scholar]
- 30.Hibberd ML, Sumiya M, Summerfield JA et al. . Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet 1999; 353:1049–53. [DOI] [PubMed] [Google Scholar]
- 31.Kronborg G, Weis N, Madsen HO et al. . Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J Infect Dis 2002; 185:1517–20. [DOI] [PubMed] [Google Scholar]
- 32.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet 1995; 346:941–3. [DOI] [PubMed] [Google Scholar]
- 33.Andersen P, Permin H, Andersen V et al. . Deficiency of somatic hypermutation of the antibody light chain is associated with increased frequency of severe respiratory tract infection in common variable immunodeficiency. Blood 2005; 105:511–7. [DOI] [PubMed] [Google Scholar]