Abstract
Curcumin provides various biological effects through its anti-inflammatory and antioxidant properties. Moreover, curcumin exerts a neuroprotective effect against ischemic condition-induced brain damage. Protein phosphatase 2A (PP2A) is a ubiquitous serine and threonine phosphatase with various cell functions and broad substrate specificity. Especially PP2A subunit B plays an important role in nervous system. This study investigated whether curcumin regulates PP2A subunit B expression in focal cerebral ischemia. Cerebral ischemia was induced surgically by middle cerebral artery occlusion (MCAO). Adult male rats were injected with either vehicle or curcumin (50 mg/kg) 1 h after MCAO and cerebral cortex tissues were isolated 24 h after MCAO. A proteomics study, reverse transverse-PCR and Western blot analyses were performed to examine PP2A subunit B expression levels. We identified a reduction in PP2A subunit B expression in MCAO-operated animals using a proteomic approach. However, curcumin treatment prevented injury-induced reductions in PP2A subunit B levels. Reverse transverse-PCR and Western blot analyses confirmed that curcumin treatment attenuated the injury-induced reduction in PP2A subunit B levels. These findings can suggest that the possibility that curcumin maintains levels of PP2A subunit B in response to cerebral ischemia, which likely contributes to the neuroprotective function of curcumin in cerebral ischemic injury.
Keywords: Curcumin, neuroprotection, protein phosphatase 2A subunit B
Stroke is a leading cause of death and disability in humans. Cerebral ischemia is the most general stroke type. Cerebral ischemia leads to the generation of reactive oxygen species and activates the inflammatory response [1,2]. Curcumin, a yellow phenolic agent present in the rhizomes of Curcuma longa Linn. (Zingiberaceae), has anti-oxidant, anti-inflammatory, and anti-cancer effects [3,4,5,6,7,8,9]. Moreover, curcumin has been shown to be protective in experimental models of cerebral ischemia [10,11,12]. Curcumin reduces infarct volume and neurological dysfunction after focal cerebral ischemia in rats [13,14].
Protein phosphatase 2A (PP2A) belongs to the family of serine and threonine protein phosphatases. PP2A modulates various cellular activities including cell metabolism, cell differentiation, development and apoptosis [15,16,17]. PP2A has three subunits: a structural subunit (subunit A), a regulatory subunit (subunit B), and a catalytic subunit (subunit C) [18]. Subunit A dimerizes with subunit C and binds to subunit B [19]. Subunits A and C of PP2A are expressed ubiquitously in various tissues, whereas subunit B is abundant in brain tissue. Subunit B modulates axonal outgrowth and neurogenesis in the nervous system [20,21]. We previously demonstrated that PP2A subunit B levels decrease in focal cerebral ischemic injury [22]. Although curcumin has a neuroprotective function in ischemic injury, little is known about the underlying mechanism. We hypothesized that the neuroprotective effects of curcumin in ischemic brain injury might be due in part to modulation of PP2A subunit B expression. To investigate this hypothesis, we assessed whether curcumin regulates the expression of PP2A subunit B in a middle cerebral artery occlusion (MCAO) animal model.
Adult male Sprague-Dawley rats (n=52, 210~230 g) were housed under controlled temperature (25℃), humidity (50%), and lighting (12/12 light/dark cycle) conditions. All experimental procedures for animal use were approved by the Institutional Animal Care and Use Committee at Gyeongsang National University (GNU-LA-021). Animals were divided randomly into four groups as follows: vehicle+sham, curcumin+sham, vehicle+MCAO, and curcumin+MCAO (n=13 per group). Curcumin (Sigma, St. Louis, MO, USA) was dissolved in normal saline containing 1% dimethyl sulfoxide as the vehicle. Curcumin (50 mg/kg) or vehicle was injected intraperitoneally at 1 h after the onset of MCAO as described previously [13,14].
Rats were anesthetized with sodium pentobarbital (Sigma, 30 mg/kg), and MCAO was carried out according to a previously described method [23]. Briefly, the two main branches of the right common carotid artery, external carotid, and internal carotid were exposed through a midline incision. A 4/0 nylon filament with a heated rounded tip was implanted into the external carotid artery and advanced into the internal carotid artery further about 2.5 cm until the origin of the middle cerebral artery. Sham-operated animals were subjected to the same procedures except for insertion of the filament. At 24 h after the onset of permanent occlusion, animals were decapitated and the brain was collected.
Right cerebral cortices were homogenized in buffer solution (8 M urea, 4% CHAPS, ampholytes, and 40 mM Tris-HCl) followed by centrifugation at 16,000 g for 20 min at 4℃. The supernatant was collected and protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol. Immobilized pH gradient (IPG, range pH 4-7 and pH 6-9, 17 cm, Bio-Rad) gel strips were incubated in rehydration buffer (8 M urea, 2% CHAPS, 20mM DTT, 0.5% IPG buffer, and bromophenol blue) for 13 h at room temperature. Assayed protein samples were loaded on IPG strips via a sample cup and subjected to first-dimension isoelectric focusing using an Ettan IPGphor 3 (GE Healthcare, Uppsala, Sweden) instrument with the following protocol: 250 V (15 min), 10,000 V (3 h), and then 10,000-50,000 V at 20℃. After first-dimension separation, the strips were incubated in equilibration buffer (6 M urea, 30% glycerol, 2% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl, bromophenol blue) containing DTT and iodoacetamide. Strips were then loaded onto gradient gels (7.5-17.5%) and second-dimension electrophoresis was performed using Protein-II XI electrophoresis equipment (Bio-Rad) at 5 mA for 2 h followed by 10 mA at 10℃ until the bromophenol blue dye migrated off the bottom of the gel.
The following steps were used to stain gels. Gels were fixed in a fixation solution (12% acetic acid, 50% methanol) for 2 h and stained with silver stain solution containing 0.2% silver nitrate and 0.75mL/L formaldehyde. Gels were then washed with deionized water and developed in 0.2% sodium carbonate solution. Stained gels were scanned using an Agfar ARCUS 1200™ (Agfar-Gevaert, Mortsel, BEL) and gel images were collected. PDQuest 2-D analysis software (Bio-Rad) was used to analyze protein spots according to a standard protocol. Protein spots were excised and destained. Gel particles were digested in trypsin-containing buffer and the extracted peptides were analyzed by a Voyager-DETM STR biospectrometry workstation (Applied Biosystem, Foster City, CA, USA) for peptide mass fingerprinting. MS-Fit and ProFound programs were used to detect proteins and SWISS-PROT and NCBI were used as the protein sequences. For proteomic study, right cerebral cortices were separately performed and were repeated three times. These data was presented as average for each experimental data.
Right cerebral cortices were homogenized in lysis buffer (1 M Tris-HCI, 5M sodium chloride, 0.5% sodium deoxycholate, 10% sodium dodecyl sulfate, 1% sodium azide, 10% NP-40) containing 200 µM phenylmethylsulfonyl fluoride and centrifuged at 15,000 g for 20 min at 4℃. Supernatants were collected and protein concentration was determined using a bicinchoninic acid kit (Pierce, Rockford, IL, USA) according to the manufacturer's protocol. Equal volumes of protein (30 µg per sample) were electrophoresed on 10% SDS-polyacrylamide gels. Proteins were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). To minimize non-specific antibody binding, membranes were blocked with skim milk for 1 h at room temperature. Membranes were washed in Tris-buffered saline containing 0.1% Tween-20 (TBST) and then incubated with anti-PP2A subunit B antibody (diluted 1:1,000, Cell Signaling Technology, Beverly, MA, USA) and anti-actin (diluted 1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as primary antibodies. After washing with TBST, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000; Pierce), and Amersham ECL western blotting detection reagents (GE Healthcare, Uppsala, Sweden) were used to detect signals according to the manufacturer's manual. Intensity analysis was carried out using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, USA) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, USA).
Total RNA from right cerebral cortices was extracted using Trizol reagent according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA, USA). RNA (1 µg) was reverse-transcribed using the Superscript III first-strand system for reverse transcription-PCR (Invitrogen) according to the manufacturer's protocol. The primers sequences used to amplify the PP2A subunit B were 5'-CCTGGTATGCCAAACTCGAT-3' (forward primer), 5'-ACAATAGCCACCTGGTCGTC-3' (reverse primer). The primers sequences used to amplify actin were 5'-GGGTCAGAAGGACTCCTACG-3' (forward primer), and 5'-GGTCTCAAACATGATCTGGG-3' (reverse primer). PCR reactions were performed as follows: 5 min at 94℃, 30 sec at 94℃, 30 sec at 54℃, 1 min at 72℃ and 10 min at 72℃. Samples were amplified for 30 cycles. PCR products were loaded on 1% agarose gels and visualized under UV light.
All data are expressed as means±S.E.M. Results in each group were compared by two-way analysis of variance (ANOVA) followed by post-hoc Scheffe's test. P<0.05 was considered to represent statistical significance.
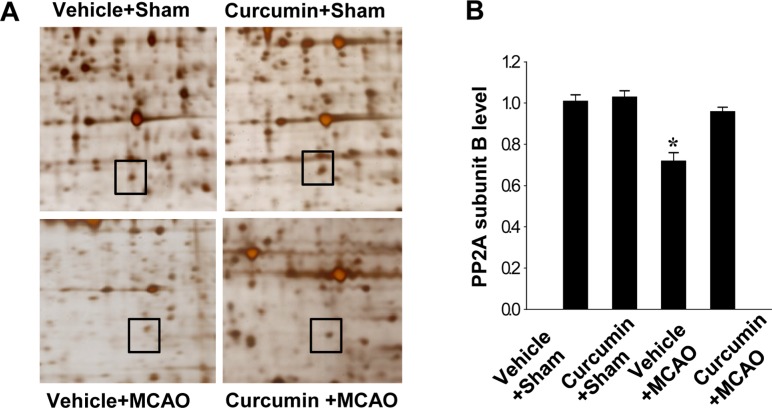
We found a change in PP2A subunit B protein spots in response to curcumin treatment after surgical induction of focal cerebral ischemia using a proteomic approach. Figure 1 shows that PP2A subunit B level decreased in the cerebral cortices of the vehicle+MCAO group. However, this decrease in phosphatase 2A (PP2A) subunit B expression was attenuated in the curcumin+MCAO group. Phosphatase 2A (PP2A) subunit B levels were similar between the vehicle+sham and curcumin+sham groups.
Figure 1. Protein phosphatase 2A (PP2A) subunit B protein spots identified by MALDI-TOF in the cerebral cortices from vehicle+sham, curcumin+sham, vehicle+middle cerebral artery occlusion (MCAO), and curcumin+MCAO animals. Squares indicate the protein spots. The intensity of spots was measured using PDQuest software. The ratio of intensity is described as spots intensity of these animals to spots intensity of sham+vehicle animals. Data (n=3) are shown as mean±S.E.M. *P<0.05.
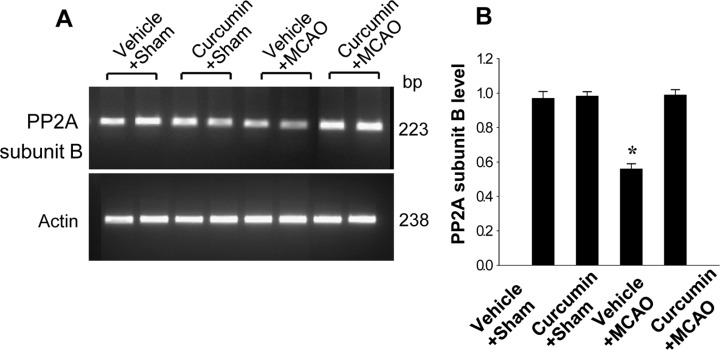
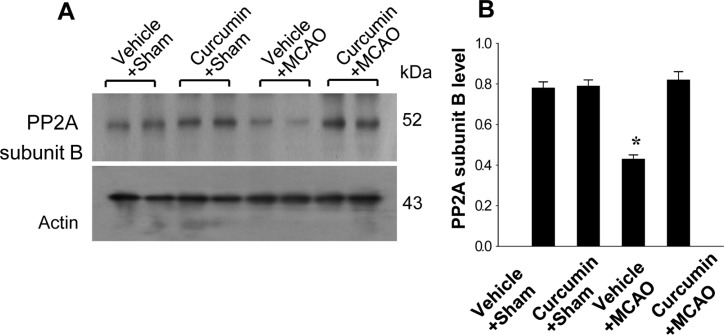
Reverse transcription-PCR and Western blot analyses also showed changes in PP2A subunit B levels in response to curcumin treatment. PP2A subunit B transcript level was lower in the vehicle+MCAO group than the sham-operated group, while curcumin treatment attenuated the injury-induced decrease in PP2A subunit B transcript levels (Figure 2). Transcript levels of PP2A subunit B were similar in the vehicle+sham and curcumin+sham groups. Western blot analysis revealed that PP2A subunit B protein level was decreased in the vehicle+MCAO group compared to the sham-operated group. Curcumin prevented the injury-induced decrease in PP2A subunit B protein expression (Figure 3).
Figure 2. Reverse transverse-PCR analysis of protein phosphatase 2A (PP2A) subunit B in the cerebral cortices from vehicle+sham, curcumin+sham, vehicle+middle cerebral artery occlusion (MCAO), and curcumin+MCAO animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as intensity of PP2A subunit B to intensity of actin. Data (n=5) are represented as mean±S.E.M. *P<0.05.
Figure 3. Western blot analysis of protein phosphatase 2A (PP2A) subunit B in the cerebral cortices from vehicle+sham, curcumin+sham, vehicle+middle cerebral artery occlusion (MCAO), and curcumin+MCAO animals. Each lane represents an individual experimental animal. Densitometric analysis is represented as intensity of PP2A subunit B to intensity of actin. Data (n=5) are represented mean±S.E.M. *P<0.05.
Curcumin exerts a neuroprotective effect following focal cerebral ischemia through its anti-inflammatory and anti-oxidant effects [3,4,5,6,7,8]. Moreover, curcumin decreases infarct volume and prevents neuronal cell death by modulating apoptosis signaling pathways in cerebral ischemia [14]. In this study, we clearly showed that MCAO injury reduces expression of PP2A subunit B, whereas curcumin treatment prevents this decrease.
It is well known that PP2A can modulate physiological neuronal function and neuronal cell activity, including cell development and apoptosis [15,16,17]. A reduction in PP2A mediates the neurodegenerative disease processes and a decrease in PP2A activity contributes to Alzheimer's disease [24,25]. Moreover, PP2A plays a critical role in regulating the phosphorylation of the microtubuleassociated protein tau [26]. Tau binds to tubulin to stabilize microtubules, and assembles tubulin into microtubules. Tau exists mostly in neurons and modulates the stability of axonal microtubules. PP2A has the ability to dephosphorylate tau. A reduction in PP2A activity leads to hyperphosphorylation of tau [24,25,26]. The phosphorylation of tau leads to aggregation of microtubules and formation of neurofibrillary tangles. Moreover, neurofibrillary tangles cause neuronal degeneration, cognitive impairment, and synaptic dysfunction [28,29,30]. Hyperphosphorylated tau results in axonal degeneration and ultimately induces a neuronal disorder [25]. The phosphorylation of tau protein can be modulated by PP2A [26]. Thus, consistent maintenance of PP2A levels is considered to be an essential event in the modulation of physiological neuronal cell function. Among PP2A subunits, the regulatory B subunit is mostly found in nerve tissue and regulates various roles of PP2A in the nervous system. Thus, it is accepted that in the brain, subunit B can regulate the substrate specificity of PP2A [19]. In this study, our proteomic approach clearly showed that MCAO-induced injury diminished the expression of PP2A subunit B, while curcumin treatment prevented the injury-induced decline in expression of PP2A subunit B. Moreover, we clearly demonstrated regulation of PP2A subunit B expression by curcumin treatment during MCAO injury using reverse transcription-PCR and western blot analyses. Curcumin restored the injury-induced decrease in PP2A subunit B expression. The maintenance of PP2A subunit B by curcumin in focal cerebral ischemia could ultimately lead to prevention of neuronal cell death. Although further studies are needed to elucidate the relation between curcumin and PP2A subunit B, our results suggest that curcumin maintains the levels of PP2A subunit B expression in an MCAO animal model, and that the maintenance of PP2A subunit B expression contributes to the neuroprotective function of curcumin. In conclusion, we demonstrated that curcumin modulates PP2A subunit B expression in focal cerebral ischemia and that regulation of the level of PP2A subunit B by curcumin mediates the protection of neuronal cells.
Acknowledgments
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MEST)(NRF-2013R1A1A2007300).
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60(2):208–212. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr Med Chem. 2015;22(10):1258–1277. doi: 10.2174/0929867322666150209154036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta S, Padhye S, Priyadarsini KI, Newton C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett. 2005;15(11):2738–2744. doi: 10.1016/j.bmcl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit. 2007;13(12):BR286–BR292. [PubMed] [Google Scholar]
- 5.Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, Ha I, Han JS. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005;82(6):831–838. doi: 10.1002/jnr.20692. [DOI] [PubMed] [Google Scholar]
- 7.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7(1-2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6(3):259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 9.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25(2):278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 10.Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46(3):273–279. doi: 10.1016/s1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 11.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74(8):969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82(1):138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35(3):374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 15.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353(Pt 3):417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13(1):7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 17.Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60(8):1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 18.Stone SR, Hofsteenge J, Hemmings BA. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987;26(23):7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- 19.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24(5):186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 20.Sim AT. The regulation and function of protein phosphatases in the brain. Mol Neurobiol. 1991;5(2-4):229–246. doi: 10.1007/BF02935548. [DOI] [PubMed] [Google Scholar]
- 21.Strack S, Zaucha JA, Ebner FF, Colbran RJ, Wadzinski BE. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392(4):515–527. [PubMed] [Google Scholar]
- 22.Koh PO. Focal Cerebral Ischemia Reduces Protein Phosphatase 2A Subunit B Expression in Brain Tissue and HT22 Cells. Lab Anim Res. 2011;27(1):73–76. doi: 10.5625/lar.2011.27.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000;275(8):5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Wang JZ. Protein phosphatase 2A in Alzheimer's disease. Pathophysiology. 2009;16(4):273–277. doi: 10.1016/j.pathophys.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17(6):1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 27.Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, White CL., 3rd Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol. 2004;63(10):1080–1091. doi: 10.1093/jnen/63.10.1080. [DOI] [PubMed] [Google Scholar]
- 28.Cancino GI, Toledo EM, Leal NR, Hernandez DE, Yévenes LF, Inestrosa NC, Alvarez AR. STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer's beta-amyloid deposits. Brain. 2008;131:2425–2442. doi: 10.1093/brain/awn125. [DOI] [PubMed] [Google Scholar]
- 29.Kanemaru K. [Immunotherapy targeting misfolded proteins in neurodegenerative disease] Brain Nerve. 2013;65(4):469–474. [PubMed] [Google Scholar]
- 30.Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27(45):12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]