Abstract
In order to assess inhibitory potentials of white rose petal extracts (WRPE) on the activities of enzymes related to dermal aging according to the extraction conditions, three extraction methods were adopted. WRPE was prepared by extracting dried white rose (Rosa hybrida) petals with 50% ethanol (WRPE-EtOH), Pectinex® SMASH XXL enzyme (WRPE-enzyme) or high temperature-high pressure (WRPE-HTHP). In the inhibition of matrix metalloproteinase-1, although the enzyme activity was fully inhibited by all 3 extracts at 100 µg/mL in 60 min, partial inhibition (50-70%) was achieved only by WRPE-EtOH and WRPE-enzyme at 50 µg/mL. High concentrations (≥250 µg/mL) of all 3 extracts markedly inhibited the elastase activity. However, at low concentrations (15.6-125 µg/mL), only WRPE-EtOH inhibited the enzyme activity. Notably, WRPE-EtOH was superior to WRPE-enzyme and WRPE-HTHP in the inhibition of tyrosinase. WRPE-EtOH significantly inhibited the enzyme activity from 31.2 µM, reaching 80% inhibition at 125 µM. In addition to its strong antioxidative activity, the ethanol extract of white rose petals was confirmed to be effective in inhibiting skin aging-related enzymes. Therefore, it is suggested that WRPE-EtOH could be a good candidate for the improvement of skin aging such as wrinkle formation and pigmentation.
Keywords: Rosa hybrida, white rose petal extract, matrix metalloproteinase-1, elastase, tyrosinase
With the improvement of economic level and the desire for beauty, the interest in health and skin care is increasing. For these demands, a variety of cosmetics and foods with novel bioactive ingredients for skin beauty are under research and development, attracting consumers' attention [1].
Skin is influenced by various factors such as UV, stresses, hormones, and so on. During normal aging and photo-aging, the major features of the skin are wrinkle formation, decreased elasticity, and pigmentation. As a mechanism, increase in collagenase activity that causes skin wrinkles is one of the major factors that bring about the degeneration of collagen fiber and breakdown [1,2]. Reactive oxygen species (ROS) generated in the body also promote the activation of collagenase such as matrix metalloproteinases (MMPs) and restrain synthesis of collagen, which results in the decrease of skin elasticity and the acceleration of wrinkle formation [2,3]. Notably, skin elastase plays a pivotal role in wrinkling and/or sagging of the skin via the impairment of elastic fiber configuration and the subsequent loss of skin elasticity [4,5]. On the other hand, tyrosinase is a rate-limiting enzyme for the biosynthesis of melanin that causes the skin pigmentation such as melasma, freckle, and age spot [4,6,7,8]. Tyrosinase converts L-tyrosine into 3,4-dihydroxyphenylalanine (L-DOPA) as an initial step of melanin synthesis. In the pigmentation process, it is also known that ROS play an important role of mediator [2].
The rose (genus Rosa) might be one of the flowers most-widely developed to species and subspecies for decoration and as a source of aromatic oils for the perfume industry [9]. The pharmacological activities of rose flower extracts have been demonstrated in our recent series of reports. We have reported that extracts of flowers from Rosa hybrida and Rosa rugosa exerted antiallergic effects in vitro cell culture system as well as in vivo systemic and local hypersensitivity models including atopic dermatitis [10,11,12]. Especially, n-hexane and butanol fractions of white rose petal extract (WRPE) were found to have strong antioxidative activity [13,14] so that substantially prevented brain infarction in an ischemia-reperfusion model of stroke [15].
Particularly, it was suggested that rose petals and roots also contain organic molecules for natural resistance to microbial attack in the wild environment [16,17]. We demonstrated that n-hexane and butanol fractions of WRPE exerted antimicrobial activity [14] as well as anti-inflammatory potential [15,18]. Hexane fraction of WRPE was confirmed to contain high amounts of gallic acid and volatile compounds for the broad range of antioxidative, antimicrobial, antiallergic, and anti-inflammatory effects [13,14,18], however, it is still not clear whether such ingredients are responsible for the inhibition of enzymes related to skin aging.
It has been known that antiaging potentials are in part derived from antioxidative activity. Therefore, in addition to the antioxidative and anti-inflammatory capacities, the inhibitory activity of WRPE on the enzymes important for dermal aging was demonstrated in the present study, although active ingredients in the extracts are under analysis. We extracted white rose petals via three different methods, and measured for their relative inhibitory effects on MMP-1, elastase, and tyrosinase activities to assess their potential as a candidate for skin beauty.
For the extraction of white rose flowers, three kinds of extraction methods using ethanol (WRPE-EtOH), enzyme (WRPE-enzyme), and HTHP (WRPE-HTHP) were adopted. For ethanol extraction, 10 g milled dry petals were suspended in 250 mL 50% ethyl alcohol in a 500 mL glass bottle. The bottle was heated at 50℃ for 3 hours in a shaking water bath (Jeio Tech, Seoul, Korea). In the case of enzyme extraction, 10 g milled dry petals and 0.5 g Pectinex® SMASH XXL (Novozymes, Bagsvaerd, Denmark) were suspended in 250 mL of distilled water (pH 4.5) in a 500 mL glass bottle. The bottle was heated at 40℃ for 25 hours in the shaking water bath. For high temperature-high pressure (HTHP) extraction, 10 g milled dry petals were suspended in 250 mL distilled water in a 500 mL glass bottle. The bottle was heated at 105℃ for 1 hour using autoclave (AC-01) (Jeio Tech). After the different extraction processes, the extracts were filtered through a Whatman filter paper (No. 4; Whatman, Clifton, NJ, USA) using a vacuum pump (BOC International, London, UK).
Male C57BL/6 mice to obtain blood neutrophils were purchased from the Daehan Biolink (Eumseong, Korea), and housed in a room with constant environmental conditions (23±2℃; 45-65% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). Pellet feed and purified water were available ad libitum. All the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University (CBNU), Korea, and conducted according to the Standard Operation Procedures (SOP) of the Laboratory Animal Research Center, CBNU.
MMP-1 activity was assayed using a SensoLyte® Generic MMP assay kit (AnaSpec, Fremont, CA, USA). In brief, Pro-MMP was incubated with 10 µg/mL trypsin for 20 min at room temperature in a 96-well microplate. Trypsin inhibitor (final concentration of 100 µg/mL) and serially-diluted WRPE (final concentrations of 20-100 µg/mL) were added to each well. After adding diluted enzyme and MMP substrate (50 µL each), mixed completely by shaking the plate. The absorbance change was recorded for 60 min at 412 nm.
Elastase activity was measured using a drug discovery kit following a protocol as in Enzo Life Science (Farmingdale, NY, USA). WRPE was serially diluted (final concentrations of 20-100 µg/mL) in 150 mM Tris-HCl buffer (pH 8.0), in a 96-well plate. Membrane homogenate of mouse blood neutrophils, as a source of elastase [19] and its substrate (100 µM N-succinyl-Ala-Ala-Ala-p-nitroanilide) were added into the wells. After incubation for 30 min at 25℃, the absorbance was measured at 410 nm using a microplate reader. The inhibition ratio of elastase was calculated as follows: Inhibition (%)=[(A-B)/A]×100, where A is the absorbance of control group, and B is the absorbance after reacting with each extract.
Tyrosinase activity was measured using a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Serially-diluted WRPE (final concentrations of 15.6-1,000 µg/mL) was pre-incubated with mushroom tyrosinase (2,500 U/mL; Sigma-Aldrich, St. Louis, MO, USA) in 100 mM sodium phosphate buffer (pH 6.5), and followed by adding substrate (1.5 mM tyrosine). After incubation for 30 min at 37℃, the absorbance was measured at 490 nm using a microplate reader. The inhibition ratio of tyrosinase was calculated as follows: Inhibition (%)=[(A-B)/A]×100, where A is the absorbance of control group, and B is the absorbance after reacting with each extract.
The statistical significance was determined by one-way analysis of variance, followed by post-hoc Tukey's multiple-comparison test. P-values (<0.05, <0.01 or <0.001) were considered to be statistically significant.
In our previous studies, the pharmacological activities of WRPE were different according to the extraction solvents. For example, the antioxidative potential was strongest in hexane fraction, followed by butanol fraction and water extract. It was confirmed that the antioxidant activity of hexane fraction was comparable to well-known antioxidants butylated hydroxyanisole (BHA) and L-ascorbic acid (vitamin C) [13,14]. In the present study, we adopted ethanol, enzyme, and HTHP as solvents feasible for oral administration and dermal application. As results, all 3 kinds of extract exerted strong antioxidative activities in DPPH-scavenging assay as well as in lipid peroxidation test (data not shown), implying that the 3 types of WRPE might have antiaging potential [2].
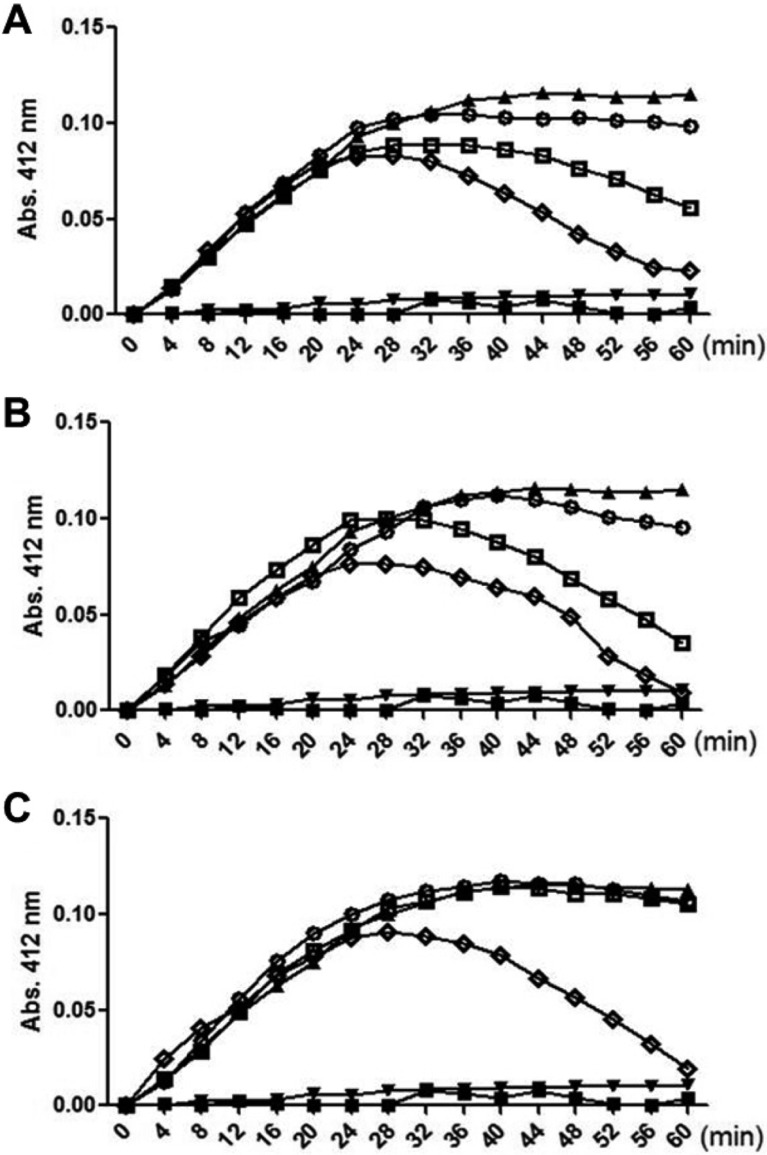
It was well known that degradation of a major portion (higher than 90%) of collagens affects directly on the decline of skin elasticity and wrinkle formation. There are many types of collagen-breakdown enzyme, wherein MMP-1 (collagenase) is one of the enzymes playing a central role [20,21,22]. The MMP-1 activity was inhibited by WRPE after 30min-lag time (Figure 1). By comparison, it was found that WRPE-EtOH and WRPE-enzyme were more effective than WRPE-HTHP in the inhibition of MMP-1. Although the enzyme activity was fully inhibited by all 3 extracts at 100 µg/mL in 60 min, a partial inhibition (50-70%) was achieved only by WRPE-EtOH and WRPE-enzyme at 50 µg/mL.
Figure 1. Inhibition of matrix metalloproteinase-1 (MMP-1) by white rose petal extracts (WRPE). White rose petals were extracted with ethanol (WRPE-EtOH, A), enzyme (WRP-enzyme, B) or high temperature-high pressure (WRPE-HTHP, C). ■, blank (buffer alone); ▲, vehicle (MMP-1 alone); ▼, trypsin inhibitor; ○, 25 µg/mL WRPE; □, 50 µg/mL WRPE; ◇, 100 µg/mL WRPE.
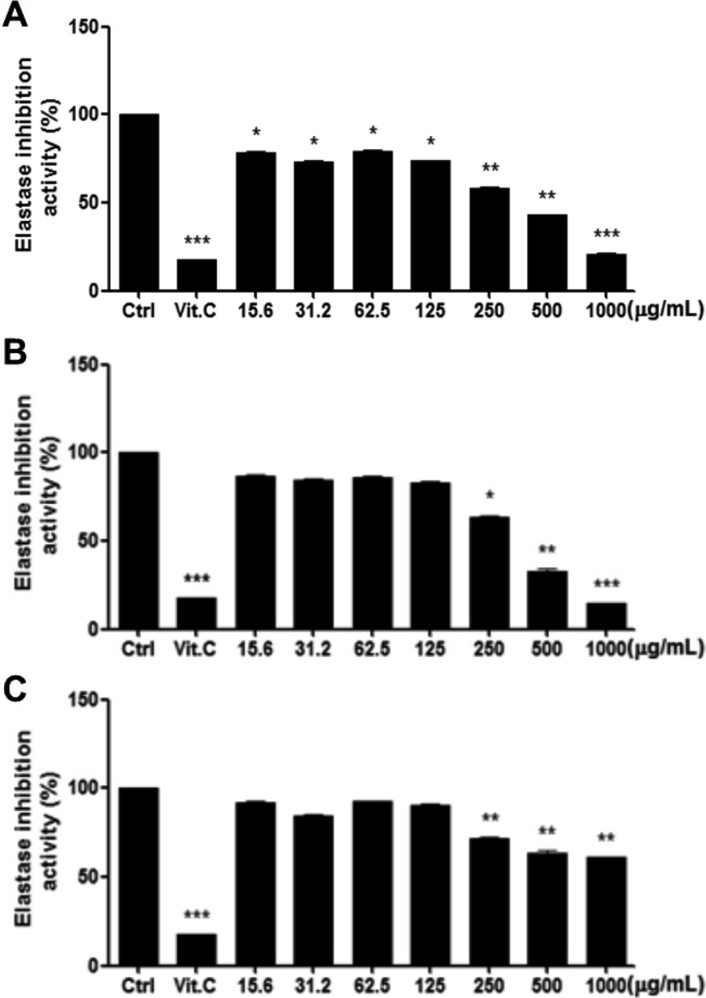
It was known that up-regulated activity of skin fibroblast-derived elastase causes impairment of elastic fiber configuration and the subsequent loss of skin elasticity, in which UVA-exposed fibroblasts play important and additional roles in UVA-induced sagging and wrinkling by up-regulating neprilysin and MMP-1, respectively [5]. In the present study, high concentrations (≥250 µg/mL) of WRPE markedly inhibited the elastase activity (Figure 2). In addition, low concentrations (15.6-125 µg/mL) of WRPE-EtOH significantly inhibited the enzyme activity.
Figure 2. Inhibition of elastase by white rose petal extracts (WRPE). White rose petals were extracted with ethanol (WRPE-EtOH, A), enzyme (WRP-enzyme, B) or high temperature-high pressure (WRPE-HTHP, C). *, **, ***Significantly different from vehicle control (Ctrl) (*<0.05, **<0.01, ***<0.001).
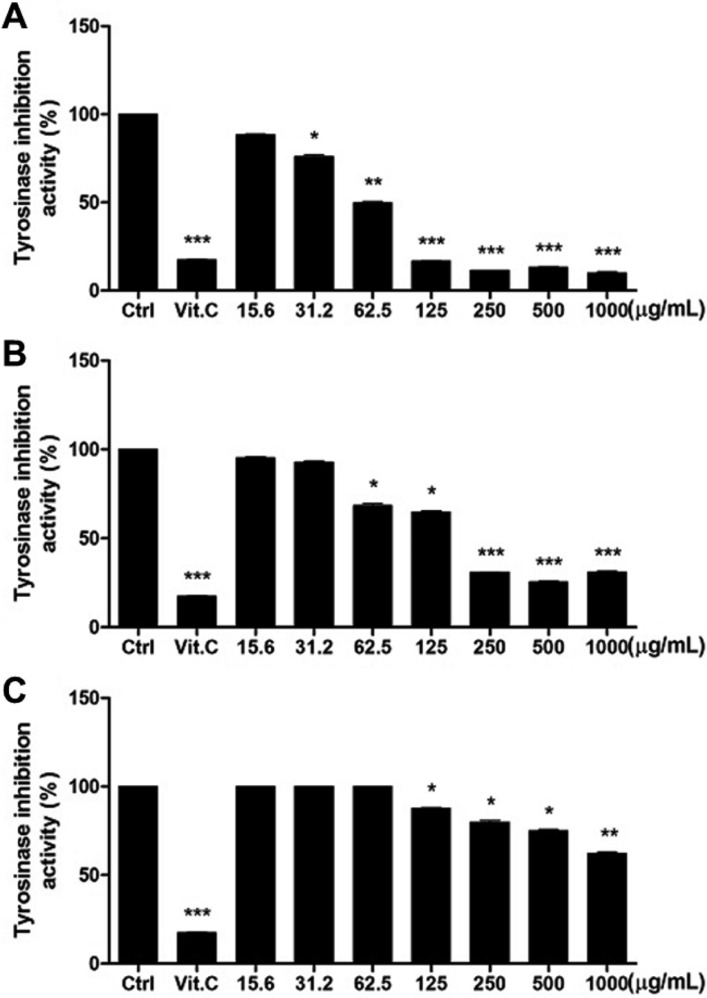
Melanocytes, activated by α-melanocyte-stimulating hormone (α-MSH) or UVB, are major cells responsible for synthesis of melanin pigment in the skin. Melanin is synthesized by tyrosinase which is supported by tyrosinase related protein-1 (TRP-1) and TRP-2 [23,24]. Being substrates of tyrosinase for melanin biosynthesis, L-tyrosine and L-DOPA are components in melanosomes of melanocytes located in dermal stratum basale. Thus, screening bioactive ingredients for skin whitening is focused on the inhibition of tyrosinase activity [25]. In the present study, it was confirmed that tyrosinase was remarkably inhibited by WRPE (Figure 3). It is of interest to note that WRPE-EtOH was superior to WRPE-enzyme and WRPE-HTHP in the inhibition of tyrosinase. WRPE-EtOH significantly inhibited the enzyme activity from 31.2 µM, reaching 80% inhibition at 125 µM, a similar degree to that obtained with 500 µM vitamin C. WRPE-enzyme significantly inhibited tyrosinase activity from 62.5 µM.
Figure 3. Inhibition of tyrosinase by white rose petal extracts (WRPE). White rose petals were extracted with ethanol (WRPE-EtOH, A), enzyme (WRP-enzyme, B) or high temperature-high pressure (WRPE-HTHP, C). *, **, ***Significantly different from vehicle control (Ctrl) (*<0.05, **<0.01, ***<0.001).
In the present study, the inhibitory effects on MMP-1, elastase, and tyrosinase activities were assessed for 3 different extracts of white rose petals. By comparison, WRPE-EtOH and WRPE-enzyme were similar in MMP-1- and elastase-inhibitory capacities, exhibiting higher efficacy than WRPE-HTHP. Notably, there was a marked different in tyrosinase inhibition among WRPE-EtOH, WRPE-enzyme, and WRPE-HTHP: i.e., WRPE-EtOH was the most effective. Although 36 peaks were obtained from GC/MS analysis of WRPE, and gartanin derivatives were identified as possible active ingredients during fractionation in our ongoing study (data not shown) [26], additional ingredients remain to be clarified. In addition to its strong antioxidative activity [27], the ethanol extract of white rose petals was confirmed to be effective in inhibiting skin aging-related enzymes. Therefore, it is suggested that WRPE-EtOH could be a good candidate for the improvement of skin aging such as wrinkle formation and pigmentation.
Acknowledgment
This research was supported by High Value-Added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA; grant number 113034-3).
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Lee HB, Lee HB, Lee CY, Kim EK. Trend of depigmenting research based on patent analysis. J Soc Cosmet Sci Korea. 2007;33(4):209–217. [Google Scholar]
- 2.Karim AA, Azlan A, Ismail A, Hashim P, Abd Gani SS, Zainudin BH, Abdullah NA. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement Altern Med. 2014;14:381. doi: 10.1186/1472-6882-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L, Meewes C, Wlaschek M. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35(3):307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63(1):1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 5.Imokawa G, Nakajima H2, Ishida K3. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: over-expression of neprilysin plays an essential role. Int J Mol Sci. 2015;16(4):7776–7795. doi: 10.3390/ijms16047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alaluf S, Heath A, Carter N, Atkins D, Mahalingam H, Barrett K, Kolb R, Smit N. Variation in melanin content and composition in type V and VI photoexposed and photoprotected human skin: the dominant role of DHI. Pigment Cell Res. 2001;14(5):337–347. doi: 10.1034/j.1600-0749.2001.140505.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58(2):85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Ohloff G, Demole E. Importance of the odoriferous principle of Bulgarian rose oil in flavour and fragrance chemistry. J Chromatogr. 1987;406:181–183. [Google Scholar]
- 10.Kwon SC, Shin S, Jeon JH, Park D, Jang MJ, Kim JJ, Kim CH, Jeong JH, Kim YB. Anti-allergic effects of rose petal extract and Ganoderma luciderm culture on mast cell-mediated allergy model. Lab Anim Res. 2008;24(1):93–97. [Google Scholar]
- 11.Jeon JH, Kwon SC, Park D, Shin S, Jang MJ, Joo SS, Kang H, Kim SH, Oh JY, Jeong JH, Kim YB. Effects of red and white rose petal extracts and Ganoderma luciderm culture on ovalbumin-induced atopic dermatitis. Lab Anim Res. 2008;24(3):347–354. [Google Scholar]
- 12.Jeon JH, Kwon SC, Park D, Shin S, Jeong JH, Park SY, Hwang SY, Kim YB, Joo SS. Anti-allergic effects of white rose petal extract and anti-atopic properties of its hexane fraction. Arch Pharm Res. 2009;32(6):823–830. doi: 10.1007/s12272-009-1602-6. [DOI] [PubMed] [Google Scholar]
- 13.Park D, Jeon JH, Kwon SC, Shin S, Jang JY, Jeong HS, Lee DI, Kim YB, Joo SS. Antioxidative activities of white rose flower extract and pharmaceutical advantages of its hexane fraction via free radical scavenging effects. Biochem Cell Biol. 2009;87(6):943–952. doi: 10.1139/o09-065. [DOI] [PubMed] [Google Scholar]
- 14.Joo SS, Kim YB, Lee DI. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. Plant Pathol J. 2010;26(1):57–62. [Google Scholar]
- 15.Yang G, Park D, Lee SH, Bae DK, Yang YH, Kyung J, Kim D, Choi EK, Hong JT, Jeong HS, Kim HJ, Jang SK, Joo SS, Kim YB. Neuroprotective Effects of a Butanol Fraction of Rosa hybrida Petals in a Middle Cerebral Artery Occlusion Model. Biomol Ther (Seoul) 2013;21(6):454–461. doi: 10.4062/biomolther.2013.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olech M, Nowak R, Nowacka N, Pecio L, Oleszek W, Los R, Malm A, Rzymowska J. Evaluation of rose roots, a post-harvest plantation residues as a source of phytochemicals with radical scavenging, cytotoxic, and antimicrobial activity. Ind Crop Prod. 2015;69:129–136. [Google Scholar]
- 17.Zhang W, Abdel-Rahman FH, Saleh MA. Natural resistance of rose petals to microbial attack. J Environ Sci Health B. 2011;46(5):381–393. doi: 10.1080/03601234.2011.572502. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Kim HS, Kim ST, Park D, Hong JT, Kim YB, Joo SS. Anti-inflammatory effects of hexane fraction from white rose flower extracts via inhibition of inflammatory repertoires. Biomol Ther. 2011;19(3):331–335. [Google Scholar]
- 19.Oh H, Siano B, Diamond S. Neutrophil isolation protocol. J Vis Exp. 2008;(17):745. doi: 10.3791/745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo S. The roles of cytokines in photoaging. J Dermatol Sci. 2000;23(Suppl 1):S30–S36. doi: 10.1016/s0923-1811(99)00076-6. [DOI] [PubMed] [Google Scholar]
- 21.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu BM, Qian ZJ, Kim MM, Nam KW, Kim SK. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat Phys Chem Oxf Engl 1993. 2009;78(2):98–105. [Google Scholar]
- 23.Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt G, Todd C, Cresswell JE, Thody AJ. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107(Pt 1):205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 25.Yoon JH, Shim JS, Cho Y, Baek NI, Lee CW, Kim HS, Hwang JK. Depigmentation of melanocytes by isopanduratin A and 4-hydroxypanduratin A isolated from Kaempferia pandurata ROXB. Biol Pharm Bull. 2007;30(11):2141–2145. doi: 10.1248/bpb.30.2141. [DOI] [PubMed] [Google Scholar]
- 26.Kim YB, Joo SS. Korean patent submission No. 10-2013-0065333 Cosmetic composition comprising white rose flower extract for skin whitening and improving skin wrinkle.
- 27.Choi JK, Lee YB, Lee KH, Im HC, Kim YB, Choi EK, Joo SS, Jang SK, Han NS, Kim CH. Extraction conditions for phenolic compounds with antioxidant activities from white rose petals. J Appl Biol Chem. 2015;58(2):117–124. [Google Scholar]