Abstract
Microbial signals stimulate development and maintenance of the neonatal immune system. The process begins in utero, with limited exposure to microbes in the intrauterine environment, as well as maternal immune signals priming the developing immune system. After birth and initial colonization, the immune system must be able to activate against pathogens, but also achieve oral tolerance of food and resident gut microbes. Through microbial signals and appropriate nutrition, the immune system is able to achieve homeostasis. Major challenges to successful colonization and immune system regulation include abnormal microbial inoculi (cesarean section, hygiene) and antibiotics. When normal colonization is interrupted, dysbiosis occurs. This imbalance of microbes and subsequently of the immune system can result in allergic diseases, asthma or necrotizing enterocolitis. Probiotics and probiotic-derived therapies represent an exciting avenue to replete the population of commensal microbes and to prevent the immune-mediated sequelae of dysbiosis.
Keywords: microbiome, probiotics, allergies, necrotizing enterocolitis, neonatal colonization, nutrition, immune homeostasis
Introduction: Microbes, hygiene and immune dysreguation
Modern improvements in sanitation and public health have lead to an era of unprecedented cleanliness. Modern medicines have allowed for the near-eradication of previously common pathogens. However, these improvements have coincided with an explosion of autoimmune diseases and diseases of immune dysregulation1. Strachan2 and others proposed that a certain level of microbial stimulation is necessary to prevent disease. This concept of microbes as not only pathogens but also as protective commensals has led to investigation of the microbiome as a source of both health and disease. In this review, we will discuss infant colonization, factors affecting colonization and the role of microbes in shaping the immune system. When colonization does not develop normally, the infant is at risk for sequelae including necrotizing enterocolitis, allergies and asthma. Finally, we will discuss probiotics as potential preventative therapies against these immune-mediated conditions.
Prenatal and early postnatal microbial exposure
The colonization process begins in utero. Whereas it was previously believed that the intrauterine environment is sterile, evidence is mounting to the contrary. Not only is the uterine environment colonized under pathologic conditions, but it is now believed to be colonized during healthy pregnancies3,4. The colonizing microbes are believed to originate from the maternal gut. Jimenez et al. demonstrated the recovery of labeled bacterial DNA in meconium samples matching those bacteria administered to the mother5. These data underscore the importance of maternal microbial health in the peripartum period.
The development of a healthy microbiota depends on the initial inocula during birth and early colonization. This initial colonization is critical for shaping the immune system. Ideally, the infant is inoculated with microbes from his or her mother while passing through the birth canal. Vaginally-born infants inherit microbiota that resemble their mothers; cesarean-born infants are colonized with skin bacteria and other environmental microbes6. Several recent studies have demonstrated an association between birth mode (vaginal or cesarean delivery) and lifelong health outcomes including obesity7 and asthma8.
During pregnancy, the woman’s microbiome is also undergoing major shifts9. In early pregnancy, the women’s intestinal microbiota resemble those of nonpregnant women, whereas in later pregnancy these microbiota diverge. In later pregnancy, the microbiota of pregnant women become less diverse, while also becoming more different from one another and from nonpregnant individuals.
Immune programming in utero and during early life: Farm data
Exposure to farming has been demonstrated to protect against allergic disease. In a cohort of rural schoolchildren, those children whose parents are farmers are protected against allergic symptoms such as wheeze and hay fever when compared to their peers10. These same children were also less likely to have antibodies against both indoor and outdoor allergens. Finally, farm exposure seemed to have a dose-response relationship, with children of part-time farmers being less protected than those of full-time farmers.
To examine the earliest effects of farm exposure on the immune system, one study compared cord blood from infants born to farming mothers to that of infants born from non-farming rural mothers. Cord blood mononuclear cells from infants born to farming mothers contained an increased proportion of regulatory T cells (Treg)11. The Tregs (farm-exposed) were more efficient in suppressing proliferation of stimulated effector T cells, a change in activity mediated by epigenetic methylation. Finally, Th2 cytokine levels were decreased in the children of farming mothers. Th2 cytokines are responsible for humoral immunity and therefore IgE response.
The exposure most associated with upregulation of the immunomodulatory Tregs was maternal exposure to farm milk11. The association between raw farm milk and protection against asthma and atopy was further elucidated in the GABRIELA study: milk consumption in rural families (both farming and non-farming) was assessed by survey and by direct sampling. Raw farm milk, but not boiled farm milk, was inversely associated with asthma, atopy and hay fever, even adjusting for other farm exposures12. In those that consumed both farm milk and shop milk, more frequent (daily) consumption of raw farm milk as well as earlier exposure to raw farm milk (<1 year old) showed stronger inverse associations with asthma, atopy and hay fever.
The PASTURE study, a prospective cohort study, also correlated maternal farming exposures during pregnancy with atopic disease and with immunologic components of cord blood. Maternal exposure to farm animals and to cats during pregnancy was protective in a dose-response relationship against the later diagnosis of atopic dermatitis in the child13. Of note, although children in farming families had an overall lower incidence of atopic dermatitis, this relationship was statistically significant only when comparing children whose mothers had worked on a farm during pregnancy to those whose mothers did not work on a farm while pregnant (Figure 1). This study revealed a potential role for innate immune regulation via Toll-like receptors (TLRs) as an intermediate between maternal farming exposure and childhood development of atopic dermatitis.
Figure 1.
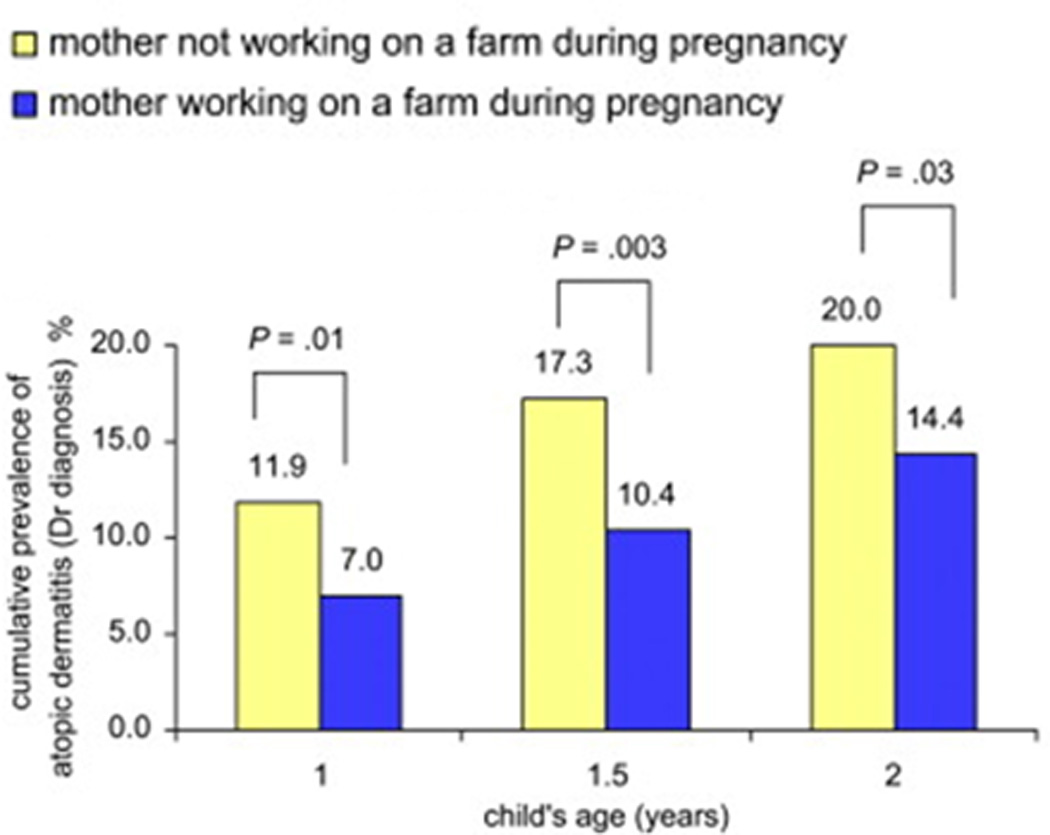
Maternal farm work during pregnancy is protective against child’s development of atopic dermatitis in first 2 years of life13.
Symbiosis shapes the developing immune system
Neonatal microbial colonization coincides with the rapidly developing infant’s immune system. Neonatal symbiosis has effects on the architecture of the developing gut and immune system, as well as on the stimulation and regulation of the innate and adaptive immune systems.
Architecture
Neonatal colonization is required for maturation of the functional gastrointestinal architecture. Although the intestinal epithelium serves as a barrier between the gut lumen and the immune system, there is constant surveillance of the gut luminal contents. The lumen is sampled by both specialized M cells and dendritic cells (DCs). M cells deliver samples from the apical lumen to the subepithelial lymphoid follicle via transcytosis, while DCs protrude between the tight junctions connecting epithelial cells to sample the contents of the apical surface14. It is through these interactions of the luminal commensal microbiota with the immune system that homeostasis is achieved.
While gut-associated lymphoid tissue (GALT) such as Peyer’s patches are present prior to delivery, colonization is required for the development of functional germinal centers. In germ-free (GF) mice, germinal centers do not appear until 10–14 days after colonization15.
Similarly, the microbiota are necessary for mature angiogenesis in the epithelium. Adult GF mice lack mature intestinal capillary networks16. With colonization by even a single species, B. thetaiotaomicron, these previously GF mice complete angiogenesis via a mechanism involving Paneth cells.
Innate immunity
Commensal microbiota constantly interact with the innate immune system to maintain homeostasis. This includes stimulation of the mucus layer and down-regulation of inflammatory cytokines and regulation of macrophages. Commensals stimulate the mucus layer, which in turn creates a physical barrier between microbiota and the immune system17,18. Although even commensals have many of the same microbe-associated molecular patterns (MAMPs) as pathogens, stimulation of the innate immune system by commensals decreases immune activation. This occurs primarily through the nuclear-factor kappa-B (NFκB) pathway, with commensals interfering with the pathway at several steps14. In interfering with NFκB activation, commensals inhibit the production of several pro-inflammatory cytokines.
Adaptive immunity
Commensals interact with the adaptive immune system to produce protective antibodies, balance T cell subsets and effect tolerance of oral antigens (resident microbes as well as food). These antibodies and T cells then complete the cycle of regulation by shaping the microbiota, with ongoing crosstalk allowing for immune homeostasis. Many of the genes involved in this regulation are susceptibility genes for autoimmune disease19.
Infants do not produce secretory IgA (sIgA) in the first weeks of life20. In the time before an infant is able to produce his or her own sIgA, it is provided as a component in breast milk. This breast milk sIgA shapes the initial microbiome and prevents excessive inflammation21. sIgA coat resident microbes of the gut, minimizing antigenic stimulation of the systemic immune system19. DCs, as part of the local immune system, sample luminal contents and phagocytose any penetrating microbes. Through this sampling, luminal bacteria stimulate the formulation of germinal centers and the production of sIgA19,22. In turn, these sIgA are produced along the length of the gut, and once again coat the microbes, minimizing antigenic stimulation23.
DCs also regulate the activation, proliferation and differentiation of B and T cells through antigen presentation and establishment of a cytokine milieu. While the DC body is in the subepithelium, the cell’s projections (dendrites) pass through the tight junctions of epithelial cells to have direct contact with the gut lumen. Although the question of, “How does a dendritic cell decide what is a commensal?” has not been fully answered, one possible mechanism includes recognition of repeating motifs in commensal DNA that are not commonly found in the DNA of pathogens24.
As part of the regulation of B and T cells, microbial signals are responsible for the balance of subsets of T helper cells, again acting through DCs. Each type of T helper cell is responsible for a different role (Th1 is for cellular immunity, Th2 for antibody secretion, Th17 for clearing extracellular pathogens); the proper balance of all of these is required for immune homeostasis. For example, asthma is associated with a Th2 predominance25 as are allergic reactions; both are caused by dysregulation of the Th2 (antibody, therefore IgE) response.
In another example, regulatory T cells are responsible for allowing for oral tolerance14. In this scenario, oral antigens present in the gut lumen are sampled by a DC. However, how this signal is handled depends on the presence of gut microbiota. In the balanced state, after a DC internalizes a food antigen, it presents it to an undifferentiated T helper cell as well as producing the regulatory cytokine interleukin-10 (IL-10). The T cell differentiates into a Treg, which then acts to suppress Th2 activation and differentiation of B cells into plasma cells.
This signaling is disrupted in germ free (GF) animals, in whom exposure to oral antigen ovalbumin (OVA) results in colitis rather than tolerance26,27. In specific-pathogen free mice, there was IgE production in response to the initial exposure to OVA, but this response diminished in later exposures. In contrast, in GF mice, IgE production took longer to appear and increased with further exposures27. In another study, mono-colonization with Bifidobacterium infantis was sufficient to restore oral tolerance26.
Furthermore, this tolerance is dependent on the timing of gut colonization. When comparing animals colonized as neonates versus those colonized as adults (GF until adulthood), those colonized as neonates showed greater tolerance of OVA28 This improved tolerance in neonates was due to a greater ability to suppress the immune response. The role of intestinal colonization in infancy as necessary for immune programming and tolerance has been replicated: Animals raised GF until 5 weeks of age demonstrated epigenetic changes of cytokine genes and increased invariant natural killer T cell activity resulting in a phenotype of severe induced colitis and OVA-induced asthma when compared to SPF animals26.
Nutrition
Neonatal enteral nutrition follows the initial inoculation of the infant intestine as the next major determinant of the infant microbiome. Breast-fed infants have dramatically higher proportions of Bifidobacteria than do infants fed formula29. Improved molecular techniques have demonstrated high proportions of Bacteroidetes in the early infant gut, demonstrating that an anaerobic environment is established within the first week of life in healthy breastfed infants30. The identities and diversity of these bacteria first colonizing the infant intestine may also be protective against later disease. For example, infants colonized with a greater number of species of Bifidobacteria at one month of age have greater levels of salivary IgA, which also correlates with protection from allergic symptoms31. In the same cohort, those infants colonized with a higher relative percentage of Bacteroidetes fragilis at one month demonstrated decreased production of inflammatory cytokine interleukin (IL) 6 in response to bacterial endotoxin lipopolysaccharide (LPS)31.
DYSBIOSIS
Dysbiosis occurs when there is abnormal colonization of the body, often thought of as an imbalance between commensal and pathogenic microbes. The factors causing dysbiosis include altered microbial exposures (hygiene1,2, cesarean delivery6,32), genetics33–35, diet36 and antibiotic exposure37. Dysbiosis causes immune disequilibrium in animal models33–35 and is thought to be responsible for disease states such as allergies and necrotizing enterocolitis (NEC).
Allergic disease and asthma
As mentioned previously, the Th2 subset of T helper cells stimulate IgE production and are therefore associated with allergy. An observational study of atopic and non-atopic infants measured the immune responses of blood samples to allergens, and demonstrated how failed immune regulation leads to allergy38. Non-atopic infants initially (in the newborn period) exhibited a greater Th2 response to allergens than did atopic infants. In non-atopic infants this Th2 response was progressively suppressed over the first 2 years of life. In atopic infants this response progressively increased over this same time period. More recent data have demonstrated that cesarean delivery causes delayed colonization and prolonged Th2 predominance32, potentially explaining one mechanism by which allergen sensitization results in an increasing (rather than decreasing) immune response.
Some of the strongest data linking dysbiosis and allergies are epidemiologic associations with conditions causing dysbiosis- particularly cesarean section. Cesarean delivery has repeatedly been linked to allergic rhinitis and asthma39. One particularly striking feature of these data is that repeat cesarean deliveries are associated even more strongly with allergic disease39. Infants born by repeat cesarean delivery likely have the least exposure to maternal vaginal flora: these deliveries are scheduled and typically occur before rupture of membranes. In contrast, infants born by emergent cesarean are likely to have had ruptured membranes for a significant amount of time, and were likely exposed to vaginal secretions during labor.
Dysbiosis may interact with other risk factors, such as genetic predisposition, in order to cause disease. The relationship between cesarean delivery and childhood allergies strengthens when looking only at children whose mothers have a history of allergic disease. Among these children whose mothers also had a history of allergies, the effect size of cesarean delivery on the relative risk of the child also having allergies was dramatically increased.40 (Figure 2).
Figure 2.
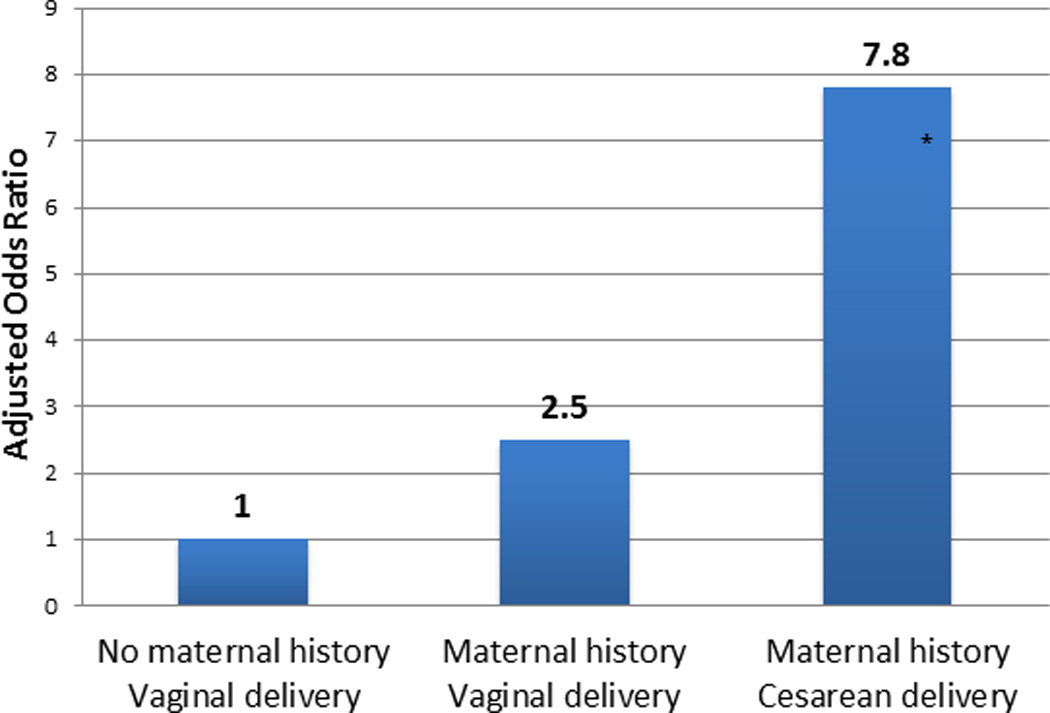
Maternal history of allergic disease and cesarean delivery have additive effects as risk factors for child’s development of allergy. Adapted from (40).
* p< 0.01; adjusted for covariates.
Food Allergy to egg confirmed by testing at age 1 – 2.
Antibiotics also dramatically influence the microbiome during childhood, with lasting effects. A recent paper demonstrated that even short courses of low doses of penicillin result in durable phenotypes of altered gene expression mediated through dysbiosis37. The hazard ratio of developing asthma is directly proportional to the number of antibiotic prescriptions in the first two years of life. Macrolides and cephalosporins demonstrated particularly significant associations with development of asthma41. Finally, infants with atopic eczema have less diverse microbiota, particularly within phylum Bacteroidetes42.
Finally, genetic mutations alter the microbiome to put the host at risk for disease. In mice genetically predisposed to develop food allergy, the intestinal microbiota are significantly different from those of control mice33. Furthermore, when the genetically at-risk mice were exposed to allergen OVA, they developed allergic symptoms in conjunction with a shift of the microbiota that was even farther from that of control mice.
These epidemiologic, cohort and animal model data all provide evidence that altered microbiota are the intermediate between risk factors (cesarean delivery, antibiotics, genetics) and allergy.
Necrotizing Enterocolitis (NEC)
NEC is characterized by unchecked inflammation and necrosis in the small and large intestine of premature infants. NEC is believed to be caused by altered colonization and an excessive immune response to even non-pathologic bacteria. Although research has failed to identify a single causal pathogen or specific microbial signature, numerous studies have found that the microbiota of infants with NEC are distinct from those of unaffected premature infants. Studies have found that the microbiota of infants with NEC are less diverse than those of controls43 and have a predominance of proteobacteria43–45. Furthermore, infants with NEC have been exposed to more days of antibiotics than controls (Figure 3)43. In extremely low birthweight infants, the duration of initial empiric antibiotic therapy is associated with increased rates of NEC and death, even after adjusting for sicker infants receiving more antibiotics46.
Figure 3.
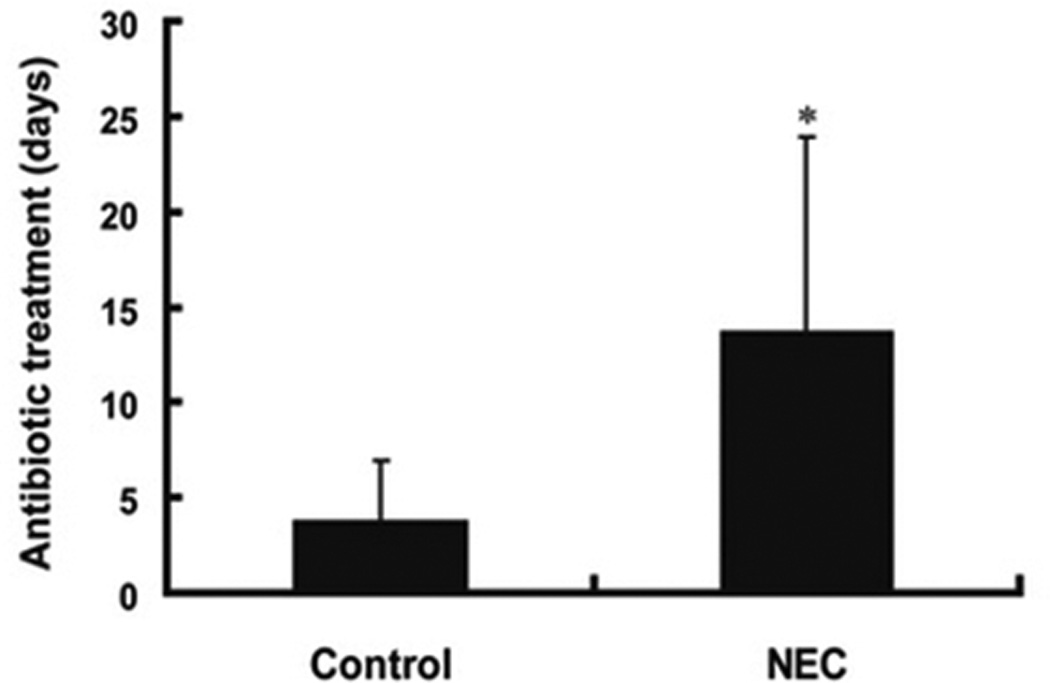
Infants who develop NEC have been exposed to more days of antibiotics than those infants who do not develop NEC43.
PROBIOTICS
The relationship between the microbiota and conditions such as allergies and NEC make probiotics an appealing option for prevention. Probiotics are live bacteria administered to the host, which confer some benefit to the host, presumably by restoring the balance between potentially helpful and potentially harmful bacteria (commensals and pathogens)47. As this review has discussed the role of microbe-host interactions in homeostasis and disease in the prenatal period, infancy and childhood relative to allergic diseases and NEC, here we will discuss the potential probiotic therapies for these same situations.
Asthma and allergic disease
There may be a role for probiotics in the prenatal period for prevention of asthma, in a convergence of the fetal programming hypothesis48 and the hygiene hypothesis2. Probiotics administered during late pregnancy affect innate immune receptor (TLRs) expression in the placenta and infant gut4. In a mouse model of allergic airway inflammation, maternal exposure to cowshed-derived, nonpathogenic Acinetobacter lwoffii reduced asthma symptoms in the offspring49. This effect was mediated by the maternal innate immune system via TLRs. Pregnant mice developed an inflammatory response to A. lwoffii exposure; however, those mice exhibited a down-regulation of TLR expression and therefore a decrease in inflammatory cytokine production in the placenta. In the A. lwoffii exposed offspring, immune responsiveness to OVA exposure was reduced when compared with controls.
Similarly, in children genetically at-risk for atopic disease, administration of probiotic Lactobacillus GG significantly reduced clinical atopic dermatitis at both 2 and 4 years of follow-up50,51. The probiotic was given to pregnant mothers and to the children during the first 6 months of life.
Yet, the data are not uniform. In one trial, administration of Lactobacillus reuteri to pregnant women and their infants was found to decrease atopic sensitization and eczema at 2 years of age52, but had no effect on respiratory allergies at 7 years of age53. In another trial, a probiotic cocktail was administered to pregnant mothers and their children at high risk for allergy. The cocktail reduced the proportion of children who developed IgE-associated allergic disease only among cesarean-delivered children, but failed to alter the proportion of children who developed allergic disease in the entire cohort51.
Both animal models and a short list of human trials support the concept of using probiotics during late pregnancy and infancy to modify the risk of developing allergic disease or asthma in in high-risk families. Evidence are lacking to recommend a specific strain, dosing or duration of therapy. Finally, there are no data as yet to recommend the routine usage of probiotics in families without high-risk family histories.
Necrotizing enterocolitis (NEC)
Due to the relatively low incidence of NEC, most trials of probiotics for prevention of NEC have only a few confirmed cases. There have been several meta-analyses published: In 2010, a meta-analysis of 11 studies (N=2176 patients) identified at 30% reduction in the incidence of NEC (p<0.00001) without a change in rate of sepsis or increase of adverse events54. Another meta-analysis in 2012 aimed to evaluate the level of evidence for use of probiotics in premature infants; the overall level of evidence was evaluated to be 2b (lesser quality randomized controlled trial)55. This analysis also pointed out the heterogeneity of study criteria and methods, and thus divided analysis by organism. Although these data suggested a benefit of probiotics in preventing NEC, the trends were not significant when looking at individual organisms such as Bifidobacterium lactis and Lactobacillus rhamnosus GG (LGG)55. A Cochrane Review in 2014 included a broad range of trials (N=24 trials), and calculated a relative risk of 0.43 (95% confidence interval 0.33–0.56) of developing NEC, as well as a reduction in overall mortality, with probiotic supplementation compared to controls56. In none of these meta-analyses were there any cases of sepsis with a probiotic organism, nor any major reported adverse events54–56. Finally, a recent cohort study (before and after implementation of a protocol administering probiotics to all premature infants <32 weeks in a neonatal intensive care unit) identified a substantial reduction in the rate of NEC, from 9.8% to 5.4%, p<0.0257.
Probiotic secretions
As an alternative to administering live organisms, the concept of using microbial secretions, known as probiotic conditioned media (PCM), to stimulate the neonatal immune system is gaining traction. A paper from this lab in 2013 administered PCM (but no live bacteria) from Bifidobacterium infantis and Lactobacillus acidophilus, to several models of NEC58. A striking decrease in expression of inflammatory markers in response to stimulation, as well as an increase in inflammatory regulators was found, particularly when using PCM from B. infantis. In another study, use of LGG-derived supernatants increased immunomodulatory cytokine expression in cell culture and decreased asthma-related pathology in a mouse model59. These results suggest that PCM holds promise as low-risk, effective interventions to address asthma, allergy and NEC.
SUMMARY AND CONCLUSIONS
The microbial-host relationship is central to immune system stimulation and homeostasis. In addition to the inoculum of initial colonizing microbes, maternal microbial and immune status, infant nutrition and antibiotic exposure all affect the developing gut microbiome, and therefore the developing immune system. Dysbiosis can result in immune-mediated diseases including allergies and NEC. Probiotics and probiotic secretions represent appealing options to replace microbes and their signals in an effort to return to microbial and immune homeostasis.
Supplementary Material
Acknowledgements
Supported by NIH grants (P30 DK040561; P01 DK033506; R01 HD012437; R01 HD059126; 3T32DK007191-39S1)
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest.
References
- 1.Bach J. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funkhouser LJ, Bordenstein SR. Mom knows best: The universality of maternal microbial transmission. PLoS biology. 2013;11(8):e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rautava S, Collado MC, Salminen S, Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: A randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102(3):178–184. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez E, Marín ML, Martín R, et al. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159(3):187–193. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesquita DN, Barbieri MA, Goldani HA, et al. Cesarean section is associated with increased peripheral and central adiposity in young adulthood: Cohort study. PloS one. 2013;8(6):e66827. doi: 10.1371/journal.pone.0066827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thavagnanam S, Fleming J, Bromley A, Shields M, Cardwell C. A meta-analysis of the association between caesarean section and childhood asthma. Clinical & Experimental Allergy. 2008;38(4):629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 9.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun-Fahrländer C, Gassner M, Grize L, et al. Prevalence of hay fever and allergic sensitization in farmer's children and their peers living in the same rural community. Clinical and experimental allergy. 1999;29(1):28–34. doi: 10.1046/j.1365-2222.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 11.Schaub B, Liu J, Höppler S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123(4):774–782. e5. doi: 10.1016/j.jaci.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 12.Loss G, Apprich S, Waser M, et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J Allergy Clin Immunol. 2011;128(4):766–773. e4. doi: 10.1016/j.jaci.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Roduit C, Wohlgensinger J, Frei R, et al. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol. 2011;127(1):179–185. e1. doi: 10.1016/j.jaci.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Reviews Immunology. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 15.Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): The roles of enteric bacteria and viruses. Journal of Immunology Research. 1998;6(1–2):13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via paneth cells. Proc Natl Acad Sci U S A. 2002;99(24):15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to infants' and children's health? J Pediatr Gastroenterol Nutr. 2015;60(3):294–307. doi: 10.1097/MPG.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deplancke B, Gaskins HR. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(6):1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 19.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selner J, Merrill D, Claman H. Salivary immunoglobulinand albumin: Development during the newborn period. J Pediatr. 1968;72(5):685–689. doi: 10.1016/s0022-3476(68)80014-9. [DOI] [PubMed] [Google Scholar]
- 21.Rogier EW, Frantz AL, Bruno ME, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111(8):3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell host & microbe. 2007;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci. 2004;1029(1):36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 24.Kant R, de Vos WM, Palva A, Satokari R. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. J Med Microbiol. 2014;63(Pt 2):293–308. doi: 10.1099/jmm.0.064220-0. [DOI] [PubMed] [Google Scholar]
- 25.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 26.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. The Journal of Immunology. 1997;159(4):1739–1745. [PubMed] [Google Scholar]
- 28.Karlsson MR, Kahu H, Hanson LÅ, Telemo E, Dahlgren UI. Neonatal colonization of rats induces immunological tolerance to bacterial antigens. Eur J Immunol. 1999;29(1):109–118. doi: 10.1002/(SICI)1521-4141(199901)29:01<109::AID-IMMU109>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72(3):317–321. [PubMed] [Google Scholar]
- 30.Jost T, Lacroix C, Braegger CP, Chassard C. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7(8):e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sjögren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clinical & Experimental Allergy. 2009;39(12):1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 32.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 33.Noval Rivas M, Burton OT, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131(1):201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couturier-Maillard A, Secher T, Rehman A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123(2):700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell host & microbe. 2010;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from europe and rural africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. The Lancet. 1999;353(9148):196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 39.Neu J, Rushing J. Cesarean versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38(2):321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggesbø M, Botten G, Stigum H, Nafstad P, Magnus P. Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol. 2003;112(2):420–426. doi: 10.1067/mai.2003.1610. [DOI] [PubMed] [Google Scholar]
- 41.Marra F, Marra CA, Richardson K, Lynd LD, Fitzgerald MJ. Antibiotic consumption in children prior to diagnosis of asthma. BMC Pulm Med. 2011;11 doi: 10.1186/1471-2466-11-32. 32-2466-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–440. e2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. The ISME journal. 2009;3(8):944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torrazza RM, Ukhanova M, Wang X, et al. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PloS one. 2013;8(12):e83304. doi: 10.1371/journal.pone.0083304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mai V, Young CM, Ukhanova M, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PloS one. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 48.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatrica. 2004;93(s446):26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 49.Conrad ML, Ferstl R, Teich R, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe acinetobacter lwoffii F78. The Journal of Experimental Medicine. 2009;206(13):2869–2877. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. The Lancet. 2001;357(9262):1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 51.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. The Lancet. 2003;361(9372):1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 52.Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(5):1174–1180. doi: 10.1016/j.jaci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Abrahamsson TR, Jakobsson T, Björkstén B, Oldaeus G, Jenmalm MC. No effect of probiotics on respiratory allergies: A seven-year follow-up of a randomized controlled trial in infancy. Pediatric Allergy and Immunology. 2013;24(6):556–561. doi: 10.1111/pai.12104. [DOI] [PubMed] [Google Scholar]
- 54.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125(5):921–930. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 55.Mihatsch WA, Braegger CP, Decsi T, et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clinical Nutrition. 2012;31(1):6–15. doi: 10.1016/j.clnu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 56.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews. 2014;(4) doi: 10.1002/14651858.CD005496.pub4. [DOI] [PubMed] [Google Scholar]
- 57.Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a north american neonatal intensive care unit. J Pediatr. 2014;164(5):980–985. doi: 10.1016/j.jpeds.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 58.Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013;304(2):G132–G141. doi: 10.1152/ajpgi.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harb H, van Tol EAF, Heine H, et al. Neonatal supplementation of processed supernatant from lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in life. Clinical & Experimental Allergy. 2013;43(3):353–364. doi: 10.1111/cea.12047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


