Abstract
Background
Apathy is a common neuropsychiatric symptom in Alzheimer’s disease (AD) dementia and mild cognitive impairment (MCI). Detecting apathy accurately may facilitate earlier diagnosis of AD. The Apathy Evaluation Scale (AES) is a promising tool for measurement of apathy in prodromal and possibly preclinical AD.
Objective
To compare the three AES sub-scales—subject-reported (AES-S), informant-reported (AES-I), and clinician-reported (AES-C)—over time in individuals at risk for AD due to MCI and advanced age (cognitively normal [CN] elderly).
Methods
Mixed effects longitudinal models were used to assess predictors of score for each AES sub-scale. Cox proportional hazards models were used to assess which AES sub-scales predict progression from MCI to AD dementia.
Results
Fifty seven MCI and 18 CN subjects (ages 53–86) were followed for 1.4±1.2 years and 0.7±0.7 years, respectively. Across the three mixed effects longitudinal models, the common findings were associations between greater apathy and greater years in study, a baseline diagnosis of MCI (compared to CN), and male sex. CN elderly self-reported greater apathy compared to that reported by informants and clinicians, while individuals with MCI under-reported their apathy compared to informants and clinicians. Of the three sub-scales, the clinician-reported AES (AES-C) best predicted transition from MCI to AD dementia.
Conclusion
In a sample of CN elderly and elderly with MCI, apathy increased over time, particularly in men and those with MCI. Self-reported AES scores may be more sensitive than informant and clinician-report when subjects are CN, but less reliable if subjects have MCI. Moreover, the clinician-reported AES sub-scale predicted progression from MCI to AD dementia.
Keywords: aged, Alzheimer’s disease, apathy, mild cognitive impairment, symptom assessment
INTRODUCTION
One of the earliest and most common neuropsychiatric symptoms in Alzheimer’s disease (AD) dementia and mild cognitive impairment (MCI) is apathy, characterized by a loss of interest, motivation and goal-oriented behavior, as well as social withdrawal [1–15]. Apathy has been found to predict progression from normal cognition to MCI [16] and from MCI to AD dementia, as well as global functional decline and cognitive decline in cognitively normal (CN) elderly [17–21]. These predictive properties suggest that measurement of apathy may be useful for earlier and more accurate diagnosis of preclinical AD and early prodromal AD [22].
A variety of scales have been used to measure apathy in the context of MCI and AD dementia. Scales designed to assess multiple neuropsychiatric symptoms, among those apathy, include the Frontal Systems Behavioral Inventory [20, 23], the Neuropsychiatric Inventory brief questionnaire [19, 24], and the full Neuropsychiatric Inventory questionnaire [9–14, 17, 25–29], which quantifies apathy with a score ranging from 0 to 12 points [30]. Scales designed to more thoroughly assess apathy with multiple items include the 14-item Apathy Scale [18], the Apathy Inventory [31], and the Apathy Evaluation Scale (AES). The AES, which uses 18 specific items to quantify apathy within a scoring range of 18 to 72, is an especially promising metric for apathy in AD. It has been shown to differentiate patients with probable AD dementia from CN elderly according to severity of apathy [32, 33], and has recently been employed as a measure of apathy in individuals at risk for AD due to MCI or advanced age (CN elderly) [22]. The AES can be scored by three separate reporters, resulting in three different sub-scales: the subject (self)-reported AES (AES-S), the informant-reported AES (AES-I), and the clinician-reported AES (AES-C). It is unclear if any one of the three AES sub-scales provides particular advantages as a predictor of MCI or AD dementia compared to the other sub-scales.
In order to further explore the utility of the three AES sub-scales in the context of CN elderly and MCI, we conducted a longitudinal study of CN and MCI subjects. We assessed the course of apathy as measured by each sub-scale over time, taking into consideration the initial diagnosis (CN or MCI). Finally, we tested each AES sub-scale’s ability to predict transition from a diagnosis of MCI to AD dementia. We hypothesized that a worse initial diagnosis (MCI as opposed to CN) would predict greater apathy over time. We further hypothesized that in MCI the informant and clinician-reported sub-scales as opposed to the subject sub-scale will best predict disease progression because these individuals already have cognitive impairment, which could impair the accuracy of their self-report of symptoms.
METHODS
Subjects
Seventy five subjects (57 MCI, 18 CN) ages 53 to 86 were recruited from the clinic and community to participate in an investigator-initiated study at the Brigham and Women’s Hospital and Massachusetts General Hospital Center for Alzheimer Research and Treatment where they underwent clinical assessments. At baseline, all subjects had generally good or stable health status, and did not have significant cerebrovascular disease or current primary psychiatric disorders. Specifically, subjects had a Modified Hachinski Ischemic Score ≤ 4 [34] and a Geriatric Depression Scale (long form) ≤ 10 [35]. Subjects had a study partner who provided collateral information about the subjects’ behavior, mood, and daily functioning.
Specific baseline inclusion criteria for CN subjects were as follows: cognitively normal with Clinical Dementia Rating (CDR) global score of 0 [36], Mini-Mental State Examination (MMSE) score of 28–30 (inclusive) [37], and performance above education adjusted cutoff scores on the Logical Memory IIa (story recall) of the Wechsler Memory Scale-Revised [38]. Of note, one subject received a diagnosis of CN despite a CDR global score of 0.5 after careful review by experienced clinicians (authors GAM and REA).
Specific baseline inclusion criteria for MCI subjects satisfied criteria for amnestic MCI, single or multiple domain, and were as follows: non-demented subjects with memory complaint, performance below education adjusted cutoff scores on Logical Memory IIa (story recall), CDR global score of 0.5 (with Memory Box score ≥ 0.5), MMSE score of 24–30 (inclusive), and essentially preserved activities of daily living (this was based on the clinical judgment of the investigator assessing the subject; this was not determined by using a cut-off on a specific test). Of note, based on consensus review, two subjects received a diagnosis of MCI despite an MMSE of 23.
Follow-up diagnoses were determined by consensus after careful review of current and previous neuropsychological test performance, CDR scores, and the Functional Activities Questionnaire scores (a measure of instrumental activities of daily living) [39]. In some instances the clinicians rating the AES-C also rated the CDR. However, the clinicians who determined diagnosis were blinded to AES scores. At follow-up subjects were assigned a diagnosis of CN, MCI, or AD dementia.
The study was approved by the Partners Human Research Committee, and informed consent was obtained from all subjects before any of the study procedures were carried out.
Clinical Assessments
The Apathy Evaluation Scale (AES) [32]: Apathy was quantified using the AES, consisting of 18 items relating to apathy, each scored on a 4-point Likert-type scale (total AES score range 18–72 with lower score indicating greater apathy). For each subject, three AES sub-scale scores were collected, with each sub-scale scored based on the impressions of a single reporter: (1) the subject or self-report (AES-S), (2) an informant, usually a family member or close friend (AES-I) or (3) the clinician (AES-C). For the AES-C, the clinician takes into consideration both the subject and informant report and uses his or her clinical judgment to determine the score for each item.
The Rey Auditory Verbal Learning Test (RAVLT) total learning score was used to assess memory [40]; the Wechsler Adult Intelligence Scale-Revised Digit Symbol test was used to assess aspects of executive function, including processing speed, visual scanning, and working memory [41]; and the American National Adult Reading Test (AMNART) intelligence quotient (IQ) was used as an estimate of premorbid verbal IQ, a proxy of cognitive reserve [42].
Statistical Analysis
We employed SAS version 9.4 (the Mixed and Phreg procedures) (SAS Software) for primary analyses and SPSS version 22 (IBM) for analyses of baseline characteristics. In supplementary analyses, we applied factor analysis to the AES to identify symptom clusters driving AES scores (online only).
Mixed Effects Models
We used mixed fixed and random effects models to test for clinical predictors of AES sub-scale scores over time. Three mixed random and fixed coefficient regression models with backward elimination (p<0.05 cutoff) were used, one model for each of the three AES sub-scales (AES-S, AES-I, AES-C) as dependent variables. In order to account for relationships between apathy and diagnosis (CN vs. MCI) over time, each model included as fixed predictors diagnosis (CN vs. MCI), time (measured as year in study) and the interaction between the two (data were sufficient to reliably estimate only linear effects of time). To examine the relationship between apathy and sex over time, we also included as fixed predictors sex and its interaction with time. Given the possibility that sex may modify relationships of apathy with diagnosis and vice versa, we also included as fixed predictors the interaction between sex and diagnosis as well as the three-way interaction between sex, diagnosis, and time. Finally, each model also included as fixed covariates age at baseline and AMNART IQ. Random terms were correlated intercepts and linear slope of change across time. Partial β regression coefficients with 95% confidence intervals (CI) were reported. The percent of variance of the dependent variable linearly accounted for by each model was defined as the square of the correlation between actual values and values predicted by the model. Residuals from fixed and random components of models were checked graphically for model fit and conformance to assumptions of normality and homogeneity of variance.
Cox Model
We employed a Cox proportional hazards multiple regression model to assess whether the individual AES sub-scales were significant predictors of time to change from a baseline diagnosis of MCI to an endpoint diagnosis of AD dementia. Of the 18 CN subjects in the study, none progressed to MCI over a mean of 0.7±0.7 years. Therefore no hazard ratios (HR) were calculated for progression from CN to MCI, and only subjects diagnosed as MCI at baseline were included in these analyses. Subjects who remained stable at MCI were censored at time of last follow-up. Predictors were tested in a backward elimination algorithm (cutoff p<0.05). The initial pool of predictors in the Cox regression included scores of the three AES sub-scales (AES-S, AES-I, and AES-C), as well as the following covariates: sex, AMNART IQ, and baseline values of age, Digit Symbol score, and RAVLT total learning score. After backward elimination produced an optimal subset of predictors, the validity of the proportional hazard model assumption was tested for each of the retained predictors using a Kolmogorov simulation test (see SAS Phreg Procedure).
RESULTS
Baseline Characteristics
Demographics and characteristics for all subjects and each diagnostic group are displayed in Table 1. Compared to CN subjects, MCI subjects demonstrated significantly lower MMSE scores, Digit Symbol scores, RAVLT total learning scores, AES-C scores, and AES-I scores (lower scores indicating greater apathy), and significantly higher CDR sum of boxes scores. The difference in AES-S scores between CN and MCI subjects was not statistically significant. The MCI group also had a higher percentage of males than the CN group.
Table 1.
Baseline demographics and characteristics of all subjects and individual diagnostic groups.
| Characteristic | All subjects | MCI | CN | Significant Differences between MCI vs CN* |
|---|---|---|---|---|
| N | 75 | 57 | 18 | |
| Age (years) | 74.7±8.0 (53–86) | 74.5±8.6 (53–86) | 75.4±6.0 (63–84) | — |
| Sex (% male) | 58.7 | 70.2 | 22.2 | Chi-square test=13.0, p=0.0003 |
| Education (years) | 16.8±2.6 (12–20) | 16.8±2.6 (12–20) | 16.8±2.9 (12–20) | — |
| AMNART IQ | 122.4±6.6 (100–131) | 122.1±7.0 (100–131) | 123.4±5.3 (111–130) | — |
| MMSE | 27.8±1.9 (23–30) | 27.3±1.9 (23–30) | 29.4±0.8 (28–30) | Mann-Whitney U test, p<0.0001 |
| CDR-SB | 1.3±1.1 (0.0–3.5) | 1.7±1.0 (0.5–3.5) | 0.0±0.1 (0–0.5) | Mann-Whitney U test, p<0.0001 |
| Digit Symbol | 46.0±12.0 (22–71) | 43.8±11.4 (22–69) | 52.4±11.5 (34–71) | t=2.8, p=0.008 |
| RAVLT Total Learning | 37.9±12.1 (18–71) | 33.2±9.0 (18–55) | 50.4±10.3 (34–71) | t=6.6, p<0.0001 |
| AES-C | 62.7±7.7 (39–72) | 60.9±7.7 (39–72) | 68.4±4.3 (55–72) | Mann-Whitney U test, p<0.0001 |
| AES-I | 62.8±7.9 (42–72) | 61.1±8.0 (42–72) | 68.3±4.5 (58–72) | Mann-Whitney U test, p=0.0002 |
| AES-S | 64.3±7.4 (40–72) | 63.3±8.0 (40–72) | 67.2±4.2 (56–72) | — |
AES-C Total (Apathy Evaluation Scale, clinician-reported score), AES-I Total (Apathy Evaluation Scale, informant-reported score), AES-S Total (Apathy Evaluation Scale, subject-reported score), AMNART IQ (American National Adult Reading Test intelligence quotient), CDR-SB (Clinical Dementia Rating sum of boxes), CN (cognitively normal), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), RAVLT (Rey Auditory Verbal Learning Test).
All values (except for n and sex) represent mean ± standard deviation (range).
Results are displayed for statistically significant differences only.
Lower AES-C score (indicating greater apathy) was associated with lower AES-S score (r = 0.58, p<0.0001), AES-I score (r = 0.79, p = <0.0001), MMSE (r = 0.30, p=0.01), Digit Symbol score (r = 0.35, p=0.003), and RAVLT total learning score (r = 0.47, p=0.0001), and with higher CDR sum of boxes score (r = −0.60, p<0.0001). Lower AES-I score was associated with lower AES-C score (as above), lower AES-S score (r = 0.35, p=0.002), MMSE (r = 0.26, p=0.03), Digit Symbol score (r = 0.30, p=0.01), RAVLT total learning score (r = 0.44, p=0.0002), and higher CDR sum of boxes score (r = −0.56, p<0.0001). Lower AES-S score was associated with lower AES-C and AES-I scores (as above), lower Digit Symbol score (r = 0.24, p=0.05) and higher CDR sum of boxes score (r = −0.28, p=0.02).
Mixed Effects Models
Tables 2 displays results for the mixed effects longitudinal analyses, including predictors retained as significant following backward elimination. All significant fixed effects were in the expected direction. Random slopes and intercepts for time were included in all final models and occasional significant negative and positive correlations between them. For the reduced model for each analysis, residuals from values predicted by the combined fixed and random coefficients conformed reasonably to assumptions of normality and homoscedascity.
Table 2.
Mixed Effect Longitudinal Models with AES Sub-Scale Scores as Dependent Variables
| Dependent Variable | Retained Predictors | Effect (β) | 95% CI | p | % Var Acct F | % Var Acct F+R |
|---|---|---|---|---|---|---|
| AES-S | Male Sex | −3.09‡ | (−5.74, −0.44) | 0.02 | 7.6 | 85.8 |
| Year in Study | −0.85 | (−1.7347, 0.03623) | 0.06 | |||
| AES-I | Baseline Diagnosis MCI | −7.60‡ | (−11.61, −3.60) | 0.0003 | 17.6 | 83.6 |
| Year in Study | −2.61 | (−3.73, −1.49) | <0.0001 | |||
| AES-C | Baseline Diagnosis MCI | −7.28‡ | (−11.27, −3.30) | 0.0005 | 23.3 | 85.6 |
| Male Sex | −0.84‡ | (−4.39, 2.72) | 0.64 | |||
| Year in Study | −3.05 | (−4.18, −1.92) | 0.002 | |||
| Year in Study X Male Sex | −2.89 | (−4.87, −0.92) | 0.005 |
X denotes an interaction.
Apathy Evaluation Scale, subject-reported sub-scale (AES-S); Apathy Evaluation Scale, informant-reported sub-scale (AES-I); Apathy Evaluation Scale, clinician-reported sub-scale (AES-C); confidence interval (CI); percent variance in dependent variable accounted for by fixed effects of model (% Var Acct F); percent variance in dependent variable accounted for by fixed and random effects of model (% Var Acct F+R); mild cognitive impairment (MCI)
β for a categorical predictor is the estimated difference in adjusted means for the categories.
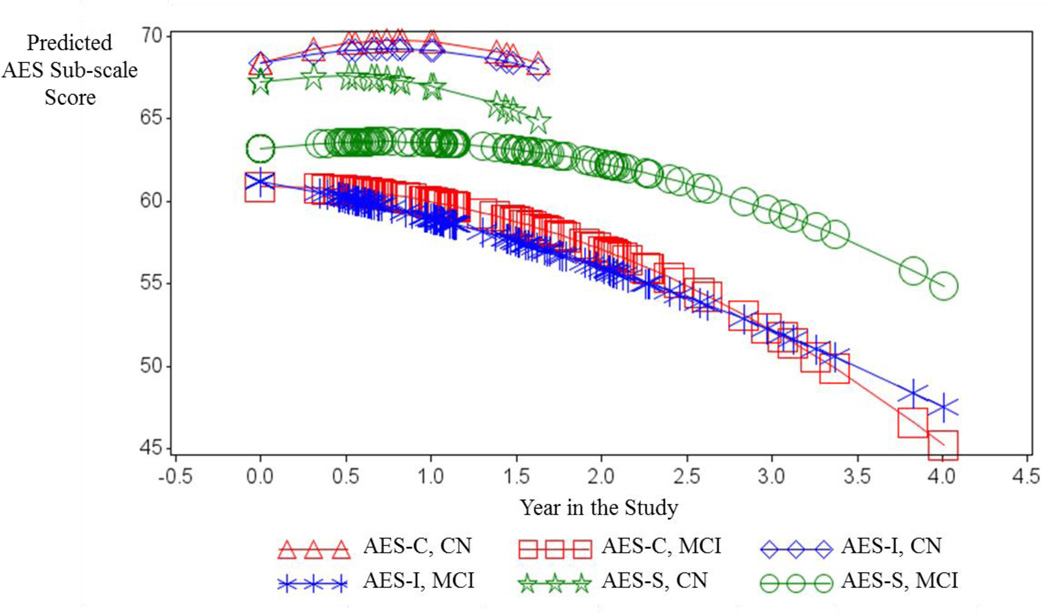
Across the three models, we found associations between greater apathy and greater years in study, a diagnosis of MCI (compared to CN), and male sex. A visual comparison of the effects of baseline diagnosis and time on the scores of the three AES sub-scales is provided in Figure 1, which displays predicted scores from simplified models for each sub-scale. In all three mixed effects models there was a significant increase in apathy (i.e. declining AES sub-scale score) over time at a rate independent of baseline diagnosis (CN vs. MCI). However, as illustrated in Figure 1, a baseline diagnosis of MCI (compared to CN) was a significant predictor of greater informant and clinician-reported apathy (i.e. lower AES-I score and AES-C score) additive to time effects (p=0.0003 and p=0.0005, respectively) (see Table 2). Moreover, Figure 1 illustrates that CN individuals self-report (AES-S) greater apathy over time when compared to informant or clinician-report (AES-I and AES-C), while this pattern reverses in MCI individuals where the informant or clinician-report greater apathy over time when compared to self-report. Male sex was a significant predictor of lower AES-S score additive to time (p=0.02) and more rapid decline in AES-C score over time (p=0.005), as illustrated in Figure 2.
Figure 1.
Predicted values for AES-C, -I and -S sub-scale scores from the fixed portions of respective mixed effects models including the fixed predictors: Baseline Diagnosis (CN vs. MCI), Time in the Study (linear and quadratic), and the interaction of Baseline Diagnosis X Time in Study (linear and quadratic). (Intercepts and the linear term for time were random terms). Each symbol corresponds to a person-visit record. Forced best fit models were used to graphically illustrate the different trajectories of the AES-C vs AES-I vs AES-S scores, irrespective of whether individual predictors in models were statistically significant or not.
AES (Apathy Evaluation Scale); AES-C (Apathy Evaluation Scale, clinician-reported sub-scale); AES-I (Apathy Evaluation Scale, informant-reported sub-scale); AES-S (Apathy Evaluation Scale, subject-reported sub-scale); CN (clinically normal); MCI (mild cognitive impairment)
Figure 2.
Predicted values for AES-C scores from the fixed portion of the mixed effects model including the fixed predictors: Baseline Diagnosis (CN vs MCI), Sex, Time in the Study (linear), and the interaction of Sex X Time in Study. (Intercepts and the linear term for time were random terms). Each symbol corresponds to a person-visit record.
AES-C (Apathy evaluation scale, clinician-reported sub-scale); CN (clinically normal); MCI (mild cognitive impairment)
Cox Model
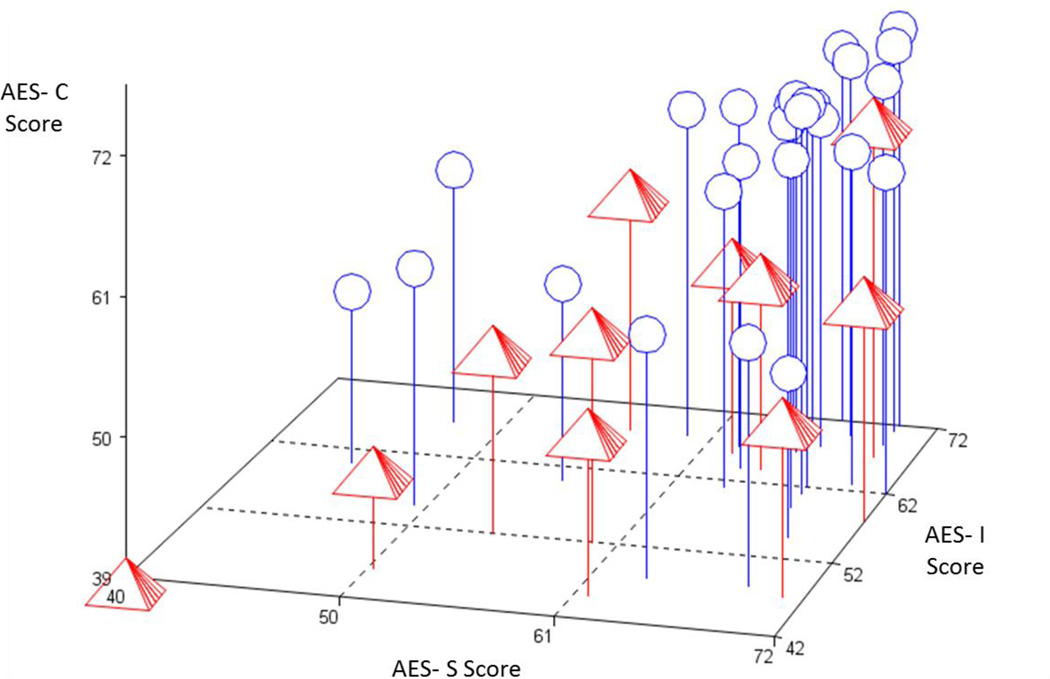
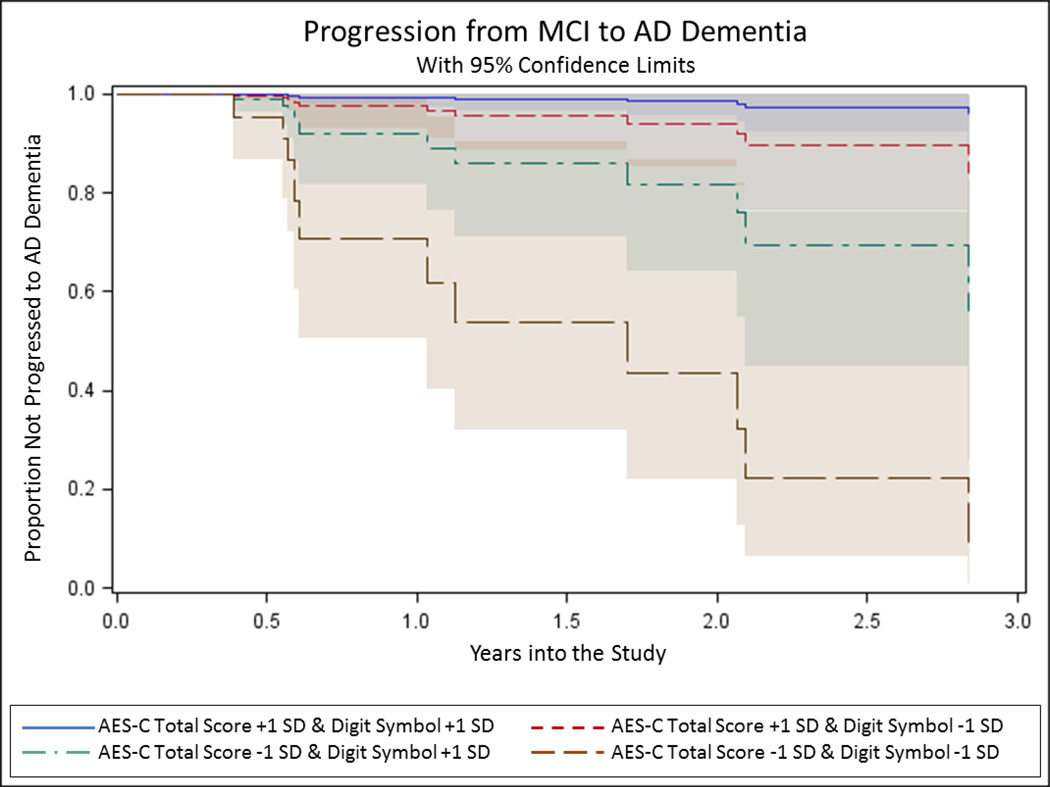
Thirty-nine subjects with MCI were included in the Cox Regression analysis, of which 11 transitioned to AD dementia over a mean of 1.4±1.2 years. The remaining 28 MCI subjects were censored. Figure 3 displays a 3-dimensional plot of baseline AES-C vs. AES-I vs. AES-S scores for subjects with a baseline diagnosis of MCI, with labels denoting subjects who progressed to AD dementia and those who did not as of last follow-up and were censored. Note that the subjects with stable MCI diagnosis tend to cluster in the region where all 3 sub-scale scores are high (consistent with lesser apathy). Table 3 displays significant results from the Cox Regression Models. All predictors retained as significant in the final reduced models passed the test of the proportional hazard assumption. Of the three AES sub-scales, only the AES-C survived the backward elimination along with Digit Symbol score, whereby for both, lower scores consistent with greater apathy and greater executive dysfunction predicted greater hazard of transition from MCI to AD dementia (see Figure 4).
Figure 3.
AES-S vs. AES-I vs. AES-C scores of subjects with MCI at baseline, who remained stable or progressed to AD dementia over time. Pyramid = MCI progressed to AD dementia; Circle = Stable MCI. AES Total scores are values at baseline. Due to overlap of points, 2 stable persons are hidden in upper right. Mild cognitive impairment (MCI); AES-S (Apathy Evaluation Scale, subject-reported sub-scale); AES-I (Apathy Evaluation Scale, informant-reported sub-scale); AES-C (Apathy Evaluation Scale, clinician-reported sub-scale).
Table 3.
Cox Proportional Hazards Regression Analysis Showing Significant Predictors of Time to Progression from MCI (n = 39) to AD dementia (n = 11) Retained in the Final Models
| Full Model χ2‡ | df | P | Predictors Retained | HR† | 95% CI | Predictor χ2* (df = 1) | p |
|---|---|---|---|---|---|---|---|
| 20.63 | 2 | <0.0001 | Digit Symbol | 1.07 | (1.01, 1.13) | 5.42 | 0.02 |
| AES-C | 1.19 | (1.09, 1.32) | 13.02 | 0.0003 |
Initial predictors of transition from MCI to AD dementia included baseline total scores for the subject-reported Apathy Evaluation Scale (AES-S), informant-reported Apathy Evaluation Scale (AES-I), and clinician-reported Apathy Evaluation Scale (AES-C). Initial covariates included: sex, baseline age, baseline American National Adult Reading Test verbal intelligence quotient, baseline Digit Symbol score, and baseline Ray Auditory Verbal Learning Test total learning score.
AD (Alzheimer’s disease); CI (confidence interval); df (degrees of freedom); HR (hazard ratio); MCI (mild cognitive impairment).
Likelihood Ratio chi-square (χ2) test is a test of the model as a whole, whereas chi-square tests based on the Wald Test are used for individual predictors.
The hazard ratio (HR) displayed for each retained predictor is the ratio of the probabilities of transitioning to AD dementia for a one unit increase in continuous predictor or a change in categories for a categorical predictor. Displayed HR’s are inverted from the original analysis results to best illustrate the effect of increasing apathy (as denoted by a decreasing AES score) on risk of progression from MCI to AD dementia.
Figure 4.
Predicted Kaplan-Meier curves from the Cox Proportional Hazards Regression model using AES sub-scale scores illustrating progression from baseline diagnosis of MCI to AD dementia over time, as predicted by AES-C score (with lower score indicating greater apathy) and Digit Symbol score combined. Note the curve representing individuals with both AES-C scores and Digit Symbol scores one standard deviation above the mean (indicating less apathy and less cognitive impairment) show the slowest rate of progression to AD dementia, while the curve representing individuals with both AES-C scores and Digit Symbol scores one standard deviation below the mean (indicating greater apathy and greater cognitive impairment) show the fastest rate of progression to AD dementia. AD (Alzheimer’s disease); AES-C (Apathy Evaluation Scale, clinician-reported sub-scale); MCI (mild cognitive impairment); +1 SD (one standard deviation above the mean); −1 SD (one standard deviation below the mean).
DISCUSSION
In a cohort of elderly individuals with MCI (n=57) or normal cognition (n=18) comparing the three AES sub-scales, lower AES-C score, denoting greater clinician-reported apathy, best predicted progression from MCI to AD dementia. Therefore, our data suggest that clinician assessment, as opposed to informant or subject report, of apathy symptoms best predicts progression to AD dementia in patients at risk for AD. When looking at progression of apathy symptoms across CN and MCI subjects using AES sub-scale scores, we found that apathy increased over time and was associated with a baseline diagnosis of MCI as opposed to CN and with being male as opposed to female. Furthermore, these findings were more prominent in the clinician and informant-reported sub-scales of the AES as opposed to the subject-reported sub-scale.
AD is a growing epidemic in urgent need of effective therapies [43, 44]. Despite ongoing and completed AD clinical trials employing multiple classes of drugs, most disease-modifying candidates, especially amyloid-modifying therapies, have failed to show clinical benefit [45]. The lack of efficacy observed in these trials may be due to the need for earlier diagnosis and intervention in the AD course to produce clinically-apparent benefit [46]. Better characterization of neuropsychiatric symptoms such as apathy in MCI and preclinical AD using more nuanced clinical assessments, such as the AES sub-scales presented here, as well as biomarkers, may facilitate early diagnosis [17, 22, 47, 48]. In order to accurately characterize apathy for such purposes, a standard metric for apathy must be chosen and thoroughly validated in the context of MCI and CN elderly at risk for preclinical AD, which to our knowledge has yet to be done. This work represents a first attempt at this important endeavor.
To our knowledge, this is the first study to longitudinally compare the reporter-dependent apathy constructs, AES-C, AES-I and AES-S, in CN elderly and MCI, and the first study to assess the utility of these measures as predictors of progression from MCI to AD dementia. However, many previous studies have compared subject (self) and informant reporting of cognitive and functional symptoms across CN elderly, MCI, and AD dementia. In the context of AD dementia, multiple studies have demonstrated that patients with AD dementia may report significantly different symptoms compared to informants, usually under-reporting their symptoms [49–52], and do not accurately reflect objective measures of cognitive performance [51, 53]. In the context of MCI, studies have shown that individuals with MCI under-report cognitive and functional decline compared to informants as well [54–56], and that informant report of cognitive deficits in the context of MCI better predicts transition to AD dementia than self-report [51]; however one study showed no significant difference between subject and informant report of daily functioning in individuals with MCI [57]. In elderly individuals without cognitive deficits on formal neuropsychological testing, Caselli and colleagues demonstrated that both subject and informant-reported cognitive concerns predict incidence of MCI, and also found subject-reported cognitive concerns to precede informant-reported decline in individuals who developed MCI [58]. Amariglio and colleagues reported similar findings with subject and informant-reported cognitive and functional concerns using the CDR and objective cognitive performance as the longitudinal outcomes [59]. These findings are supported by others, who have demonstrated an association in CN individuals between subject-reported cognitive concerns and incidence of MCI and AD dementia [60, 61], although this has not been consistent across all studies [62]. Further strengthening the importance of self and informant-reported cognitive concerns for AD are studies of CN elderly demonstrating associations of such cognitive concerns with underlying brain atrophy similar to that of MCI [63, 64] and elevated cortical amyloid burden [65]. Taken in aggregate, these prior studies of self and informant-reported cognitive and functional symptoms appear to parallel the findings of the current study of apathy, wherein CN individuals reported greater severity of apathy over time compared to informant or clinician report of apathy, while individuals with MCI at baseline reported lower apathy severity compared to informants and clinicians.
Prior cross-sectional and longitudinal studies have shown that apathy is greater in those with greater AD severity, ranging from MCI to severe dementia, and that apathy worsens as AD progresses over time [3, 4, 14, 19, 66]. In the current study we too showed that apathy measured in various fashions worsens over time in individuals at risk for AD due to MCI or old age. Moreover, in our supplementary analyses, we explored symptom clusters related to apathy and their progression over time and found that nearly all clinician and informant-reported apathy-related symptom clusters worsened over time. In contrast, subjects only reported worsening over time for one of the three symptom clusters.
Another striking and consistent finding in our study was the association of being male, as opposed to female, with worsening of apathy over time as measured by multiple constructs. These findings are in line with those of Brodaty and colleagues, where in a study of 76 healthy, community-dwelling elderly using the AES-I, there was a significantly greater increase in apathy over time in men than women [67], as well as a study by Geda et al of 1,587 CN elderly demonstrating greater apathy in men compared to women [16]. Finally, a study of 425 demented and non-demented elderly in acute geriatric wards also showed a greater prevalence of apathy in men compared to women [68]. The mechanism underlying this association between apathy and aging men is unclear [67]. Of note, differences in apathy between sexes have not been previously observed in the specific contexts of AD dementia [4] or MCI [5].
In our supplementary analyses, we applied factor analysis to the AES to identify symptom clusters driving AES scores in MCI and CN elderly. Prior studies have applied factor analysis to the AES in the context of other populations and identified similar symptom clusters, including lack of interest, lack of insight or concern, and general apathy [32, 69]. These results correspond in part to our own findings: while a general apathy factor was not identified in our current analysis, interest and lack of insight or concern correspond to two of our three factors (“interest and motivation” and “awareness”). We also identified a “task completion” factor. Of the three factors we identified, the interest and motivation factor had the strongest signal in the various analyses. Of note, two of our factors (“interest and motivation” and “task completion”) correspond to the proposed diagnostic criteria for apathy, which include the core feature of decreased motivation, as well as the dimension of reduced goal-directed activity; the third criterion of functional impairment was not clearly captured in our subjects since they were CN elderly or had MCI and therefore lacked significant functional impairment [70].
The current study has several notable limitations. First, our study was limited in statistical power due to small sample size, and therefore significant effects may have gone undetected by our analyses. Due to small sample size, we were also limited in the number of covariates that could be included in the multivariate models. However, our findings in the mixed effects models were statistically significant after adjusting for baseline diagnosis, sex, the interactions with baseline diagnosis and sex with time, baseline age, and premorbid IQ, and our findings in the Cox proportional hazards regression model was significant after adjusting for sex, baseline age, premorbid IQ, processing speed, and memory. Second, many analyses were performed in the current study. In considering the possibility that chance effects due to multiple testing were responsible for our findings, it might be noted that although the 0.05 level was used as a cutoff in all of the backward elimination analyses for mixed effects and Cox models, almost all significant effects of interest were significant at much more stringent levels and would have survived even a conservative Bonferroni correction. Also, the consistency of the significant findings across the different mixed effects models further suggests that the results were unlikely to be chance effects. Third, our study did not include data on other potentially important biological predictors of AD such as Apolipoprotein E status, amyloid and tau burden, regional atrophy, and hypometabolism. However, prior studies from our group, some employing the AES, have already explored these associations in the early AD spectrum [22, 47, 71]; for the current investigation, we chose not to repeat those analyses so that we could instead focus on the clinical aspects of apathy and the AES in MCI and CN elderly. Finally, none of the CN subjects in our cohort progressed to MCI, and future studies with larger samples of CN elderly followed over longer periods of time will be needed if the value of AES score as a predictor of progression from CN to MCI is to be determined.
In conclusion, our results suggest that apathy increases over time in CN elderly and individuals with MCI and tends to be more severe in men and MCI. Compared to informants and clinicians, cognitively normal subjects reported more severe apathy, while subjects with MCI, in contrast, reported less severe apathy than informants and clinicians. Finally, of the three reporter types included in this study, apathy scores reported by clinicians were most useful in predicting progression from MCI to AD dementia. This is in spite of the fact that clinicians who determined the diagnosis for subjects at each time point were blinded to apathy scores. Future studies will be required to replicate these findings in larger cohorts with greater longitudinal follow-up. Such studies should include information on AD risk factors, such as amyloid burden and Apolipoprotein E status, with intent of developing means for earlier AD diagnosis. Finally, future studies may compare other metrics for apathy to the AES, in order to determine the optimal apathy metric for use in preclinical AD.
Supplementary Material
Acknowledgements
This research was supported by R01 AG027435, K23 AG033634, K24 AG035007, the Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Awards in Alzheimer’s Disease, the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134), and the Harvard Aging Brain Study (P01 AGO36694, R01 AG037497). Authors have also received research salary support from the Harvard Medical School Scholars in Medicine Office.
The authors have received research salary support from Janssen Alzheimer Immunotherapy (GAM, REA), Wyeth/Pfizer Pharmaceuticals (GAM, REA), Eisai Inc. (GAM), Eli Lilly and Company (GAM), Avid Radiopharmaceuticals (KAJ), and Bristol-Myers-Squibb (GAM, RAS).
References
- 1.Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 4.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer's disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 5.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 6.Kang HS, Ahn IS, Kim JH, Kim DK. Neuropsychiatric symptoms in korean patients with Alzheimer's disease: exploratory factor analysis and confirmatory factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord. 2010;29:82–87. doi: 10.1159/000264629. [DOI] [PubMed] [Google Scholar]
- 7.Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis. 2009;18:11–30. doi: 10.3233/JAD-2009-1120. [DOI] [PubMed] [Google Scholar]
- 8.Lopez OL, Becker JT, Sweet RA. Non-cognitive symptoms in mild cognitive impairment subjects. Neurocase. 2005;11:65–71. doi: 10.1080/13554790490896893. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DK, Watts AS, Chapin BA, Anderson R, Burns JM. Neuropsychiatric profiles in dementia. Alzheimer Dis Assoc Disord. 2011;25:326–332. doi: 10.1097/WAD.0b013e31820d89b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aalten P, Verhey FR, Boziki M, Bullock R, Byrne EJ, Camus V, Caputo M, Collins D, De Deyn PP, Elina K, Frisoni G, Girtler N, Holmes C, Hurt C, Marriott A, Mecocci P, Nobili F, Ousset PJ, Reynish E, Salmon E, Tsolaki M, Vellas B, Robert PH. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dement Geriatr Cogn Disord. 2007;24:457–463. doi: 10.1159/000110738. [DOI] [PubMed] [Google Scholar]
- 11.Aalten P, Verhey FR, Boziki M, Brugnolo A, Bullock R, Byrne EJ, Camus V, Caputo M, Collins D, De Deyn PP, Elina K, Frisoni G, Holmes C, Hurt C, Marriott A, Mecocci P, Nobili F, Ousset PJ, Reynish E, Salmon E, Tsolaki M, Vellas B, Robert PH. Consistency of neuropsychiatric syndromes across dementias: results from the European Alzheimer Disease Consortium. Part II. Dement Geriatr Cogn Disord. 2008;25:1–8. doi: 10.1159/000111082. [DOI] [PubMed] [Google Scholar]
- 12.Geda YE, Smith GE, Knopman DS, Boeve BF, Tangalos EG, Ivnik RJ, Mrazek DA, Edland SD, Petersen RC. De novo genesis of neuropsychiatric symptoms in mild cognitive impairment (MCI) Int Psychogeriatr. 2004;16:51–60. doi: 10.1017/s1041610204000067. [DOI] [PubMed] [Google Scholar]
- 13.Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, Tekin S, Lane R, Ferris S. Behavioral symptoms in mild cognitive impairment. Neurology. 2004;62:1199–1201. doi: 10.1212/01.wnl.0000118301.92105.ee. [DOI] [PubMed] [Google Scholar]
- 14.Caputo M, Monastero R, Mariani E, Santucci A, Mangialasche F, Camarda R, Senin U, Mecocci P. Neuropsychiatric symptoms in 921 elderly subjects with dementia: a comparison between vascular and neurodegenerative types. Acta Psychiatr Scand. 2008;117:455–464. doi: 10.1111/j.1600-0447.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 15.Palmer K, Lupo F, Perri R, Salamone G, Fadda L, Caltagirone C, Musicco M, Cravello L. Predicting disease progression in Alzheimer's disease: the role of neuropsychiatric syndromes on functional and cognitive decline. J Alzheimers Dis. 2011;24:35–45. doi: 10.3233/JAD-2010-101836. [DOI] [PubMed] [Google Scholar]
- 16.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Sochor O, Tangalos EG, Petersen RC, Rocca WA. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 18.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadsworth LP, Lorius N, Donovan NJ, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement Geriatr Cogn Disord. 2012;34:96–111. doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:214–221. [PubMed] [Google Scholar]
- 21.Onyike CU, Sheppard JM, Tschanz JT, Norton MC, Green RC, Steinberg M, Welsh-Bohmer KA, Breitner JC, Lyketsos CG. Epidemiology of apathy in older adults: the Cache County Study. Am J Geriatr Psychiatry. 2007;15:365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- 22.Guercio BJ, Donovan NJ, Ward A, Schultz A, Lorius N, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J Neuropsychiatry Clin Neurosci. 2015;21:e22–e27. doi: 10.1176/appi.neuropsych.13060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:222–228. [PubMed] [Google Scholar]
- 24.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 25.Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM. Structrual correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:91–97. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- 26.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- 27.Tunnard C, Whitehead D, Hurt C, Wahlund LO, Mecocci P, Tsolaki M, Vellas B, Spenger C, Kloszewska I, Soininen H, Lovestone S, Simmons A. Apathy and cortical atrophy in Alzheimer's disease. Int J Geriatr Psychiatry. 2011;26:741–748. doi: 10.1002/gps.2603. [DOI] [PubMed] [Google Scholar]
- 28.Lanctôt KL, Moosa S, Herrmann N, Leibovitch FS, Rothenburg L, Cotter A, Black SE. A SPECT study of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:65–72. doi: 10.1159/000103633. [DOI] [PubMed] [Google Scholar]
- 29.Craig AH, Cummings JL, Fairbanks L, Itti L, Miller BL, Li J, Mena I. Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neurol. 1996;53:1116–1120. doi: 10.1001/archneur.1996.00550110056012. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 31.Robert PH, Clairet S, Benoit M, Koutaich J, Bertogliati C, Tible O, Caci H, Borg M, Brocker P, Bedoucha P. The apathy inventory: assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17:1099–1105. doi: 10.1002/gps.755. [DOI] [PubMed] [Google Scholar]
- 32.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 33.Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. J Affect Disord. 1993;28:7–14. doi: 10.1016/0165-0327(93)90072-r. [DOI] [PubMed] [Google Scholar]
- 34.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. WMS-R Wechsler Memory Scale Revised Manual. New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- 39.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 40.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 41.Wechsler D. WAIS-R: Manual: Wechsler Adult Intelligence Scale--Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 42.Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- 43.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karran E, Hardy J. Antiamyloid therapy for Alzheimer's disease--are we on the right road? N Engl J Med. 2014;370:377–378. doi: 10.1056/NEJMe1313943. [DOI] [PubMed] [Google Scholar]
- 46.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002609. 111cm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donovan NJ, Wadsworth LP, Lorius NL, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA Alzheimer Disease Neuroimaging Initiative. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry. 2014;22:1168–1179. doi: 10.1016/j.jagp.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22:1642–1651. doi: 10.1016/j.jagp.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Debettignies BH, Mahurin RK, Pirozzolo FJ. Insight for impairment in independent living skills in Alzheimer's disease and multi-infarct dementia. J Clin Exp Neuropsychol. 1990;12:355–363. doi: 10.1080/01688639008400980. [DOI] [PubMed] [Google Scholar]
- 50.Koss E, Patterson MB, Ownby R, Stuckey JC, Whitehouse PJ. Memory evaluation in Alzheimer's disease: caregivers' appraisals and objective testing. Arch Neurol. 1993;50:92–97. doi: 10.1001/archneur.1993.00540010086023. [DOI] [PubMed] [Google Scholar]
- 51.Tierney MC, Szalai JP, Snow WG, Fisher RH. The prediction of Alzheimer disease: the role of patient and informant perceptions of cognitive deficits. Arch Neurol. 1996;53:423–427. doi: 10.1001/archneur.1996.00550050053023. [DOI] [PubMed] [Google Scholar]
- 52.Kuriansky JB, Gurland BJ, Fleiss JL, Cowan D. The assessment of self-care capacity in geriatric psychiatric patients by objective and subjective methods. J Clin Psychol. 1976;32:95–102. doi: 10.1002/1097-4679(197601)32:1<95::aid-jclp2270320129>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 53.Michon A, Deweer B, Pillon B, Agid Y, Dubois B. Relation of anosognosia to frontal lobe dysfunction in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994;57:805–809. doi: 10.1136/jnnp.57.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albert SM, Michaels K, Padilla M, Pelton G, Bell K, Marder K, Stern Y, Devanand DP. Functional significance of mild cognitive impairment in elderly patients without a dementia diagnosis. Am J Geriatr Psychiatry. 1999;7:213–220. doi: 10.1097/00019442-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Schinka JA, Brown LM, Proctor-Weber Z. Measuring change in everyday cognition: development and initial validation of the cognitive change checklist (3CL) Am J Geriatr Psychiatry. 2009;17:516–525. doi: 10.1097/JGP.0b013e31819e2d6c. [DOI] [PubMed] [Google Scholar]
- 56.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Stern Y, Devanand DP. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 57.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20:827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caselli RJ, Chen K, Locke DE, Lee W, Roontiva A, Bandy D, Fleisher AS, Reiman EM. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement. 2014;10:93–98. doi: 10.1016/j.jalz.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, Karantzoulis S, Aisen PS, Sperling RA for the Alzheimer's Disease Cooperative Study. Tracking early decline in cognitive function in older individuals at risk for Alzheimer’s disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument. JAMA Neurology. doi: 10.1001/jamaneurol.2014.3375. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, Luck T, Mösch E, van den Bussche H, Wagner M, Wollny A, Zimmerman T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 61.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mol ME, van Boxtel MP, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht Aging Study. Int J Geriatr Psychiatry. 2006;21:432–441. doi: 10.1002/gps.1487. [DOI] [PubMed] [Google Scholar]
- 63.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galvin JE, Roe CM, Morris JC. Evaluation of cognitive impairment in older adults: combining brief informant and performance measures. Arch Neurol. 2007;64:718–724. doi: 10.1001/archneur.64.5.718. [DOI] [PubMed] [Google Scholar]
- 65.Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, Mielke MM, Piercy K, Steinberg M, Rabins PV, Leoutsakos JM, Welsh-Bohme KA, Breitner JC, Lyketsos CG. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brodaty H, Altendorf A, Withall A, Sachdev P. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. Int Psychogeriatr. 2010;22:426–436. doi: 10.1017/S1041610209991335. [DOI] [PubMed] [Google Scholar]
- 68.Holtta EH, Laakkonen ML, Laurila JV, Strandberg TE, Tilvis RS, Pitkala KH. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. J Am Med Dir Assoc. 2012;13:541–545. doi: 10.1016/j.jamda.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Clarke DE, Reekum Rv, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci. 2007;19:57–64. doi: 10.1176/jnp.2007.19.1.57. [DOI] [PubMed] [Google Scholar]
- 70.Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, Verhey FR, Yessavage J, Clement JP, Drapier D, Bayle F, Benoit M, Boyer P, Lorca PM, Thibaut F, Gauthier S, Grossberg G, Vellas B, Byrne J. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24:98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Marshall GA, Donovan NJ, Lorius N, Gidicsin CM, Maye J, Pepin LC, Becker JA, Amariglio RE, Rentz DM, Sperling RA, Johnson KA. Apathy is associated with increased amyloid burden in mild cognitive impairment. J Neuropsychiatry Clin Neurosci. 2013;25:302–307. doi: 10.1176/appi.neuropsych.12060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.