Abstract
Retinitis pigmentosa (RP) shows progressive loss of photoreceptors involved with heterogeneous genetic background. Here, by exome sequencing and linkage analysis on a Chinese family with autosomal dominant RP, we identified a putative pathogenic variant, p.Gly97Arg, in the gene SPP2, of which expression was detected in multiple tissues including retina. The p.Gly97Arg was absent in 800 ethnically matched chromosomes and 1400 in-house exome dataset, and was located in the first of the two highly conserved disulfide bonded loop of secreted phosphoprotein 2 (Spp-24) encoded by SPP2. Overexpression of p.Gly97Arg and another signal peptide mutation, p.Gly29Asp, caused cellular retention of both endogenous wild type and exogenous mutants in vitro, and primarily affected rod photoreceptors in zebrafish mimicking cardinal feature of RP. Taken together, our data indicate that the two mutations of SPP2 have dominant negative effects and cellular accumulation of Spp-24 might be particularly toxic to photoreceptors and/or retinal pigment epithelium. SPP2 has a new role in retinal degeneration.
Retinitis pigmentosa (RP, MIM 268000) is the most common form of inherited retinal dystrophies (IRDs) and affect over one million individuals globally1. During the disease course, rod photoreceptors and/or retinal pigment epithelium (RPE) cells are primarily affected by most mutations leading to night blindness and gradual constriction of visual fields (VFs). Degeneration of cone photoreceptors is generally secondary to rods degeneration2,3, and therefore may ultimately cause the loss of central vision.
RP exhibits great genetic heterogeneity involving 64 disease-causing genes, of which 24 genes are implicated in autosomal dominant RP (adRP) (www.RetNet.org). Proteins encoded by those genes are implicated in multiple functions, including phototransduction, visual cycle, homeostasis of photoreceptors, metabolism and phagocytosis of RPE cells, and some fundamental cellular activities such as pre-mRNA splicing4. Some proteins are retina specific, while others are widely, or even ubiquitously, expressed. However, mutations in all identified RP genes can only account for 50% to 60% of all cases, leaving the heritability in nearly 40% of RP patients unknown5,6,7,8.
The SPP2 gene (MIM 602637; NG_008668.1) spans approximately 27 kb at chromosome 2q37.1, contains 8 exons, and encodes the secreted phosphoprotein 24 (Spp-24; NP_008875.1), which is considered as a member of the cystatin superfamily9. Spp-24 consists of 211 amino acids including a signal peptide (the first 29 residues), a cystatin-like domain, in which two highly conserved disulfide bonds reside (Cys92 - Cys103, and Cys116 - Cys134), and a variable C-terminal region9. While the human SPP2 is a new and relatively poor characterized gene, the cystatin-like domain of the mature bovine spp-24, after the cleavage of the signal peptide, was proposed to form a tertiary structure similar to cystatins functioning as an inhibitor of the thiol proteases such as cathepsins10,11.
Herein, using whole exome sequencing (WES), we identified a heterozygous missense variant in the gene SPP2, p.Gly97Arg, in a four-generation Chinese family with RP. The p.Gly97Arg is located within the first disulfide bonded loop in the cystatin-like domain of Spp-24. To determine the pathogenicity of SPP2 p.Gly97Arg, we characterized the phenotypes in cells and zebrafish overexpressed with wild type Spp-24 (Spp-24WT), mutant Spp-24 carrying p.Gly97Arg (Spp-24Gly97Arg) and an artificial mutation, p.Gly29Asp (Spp-24Gly29Asp), a presumable positive control mutant predicted to affect the signal peptide cleavage and protein secretion. We found that overexpression of Spp-24Gly97Arg and Spp-24Gly29Asp, but not Spp-24WT, caused cellular retention of exogenous Spp-24 in HEK 293T cells and initiated demorphogenesis of rod photoreceptors in zebrafish larvae. Altogether, we demonstrate that SPP2 is a new disease-causing gene for RP.
Results
Clinical evaluations
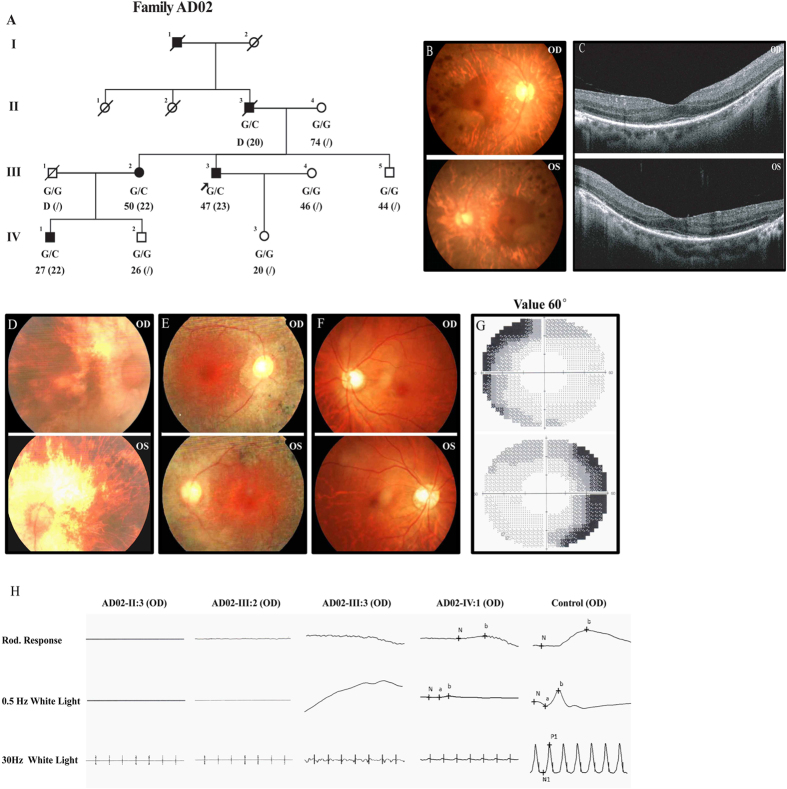
The proband of family AD02 (Fig. 1A), AD02-III:3, was first referred to ophthalmic examinations at age 23 due to poor night vision. At his last visit (age 47), his VF was constricted to less than 15 degrees in both eyes, while his central vision was relatively preserved (Table 1). Funduscopy showed bilateral RP characteristics including waxy pallor of the optic discs, retinal atrophy with macular region relatively preserved, and pigmental deposits in midperiphery (Fig. 1B). Optical coherence tomography (OCT) demonstrated moderate degeneration of the outer nuclear layer (ONL) in macular region with significant loss of inner/outer segments (IS/OS) (Fig. 1C). His rod photoreceptor responses were nearly diminished as demonstrated by full-field electroretinogram (ERG) (Fig. 1H). Patients AD02-II:3 and AD02-III:2 noticed decreased night vision at 20 and 22-year-old respectively. Patients AD02-II:3 recently deceased, but at his last visit (age 77), he had developed server bilateral cataract with poor best-corrected visual acuities (BCVAs). Patient AD02-III:2 still had functional BCVAs and preserved maculae at her latest visit (age 50). Their latest clinical documentations including BCVAs, VF, fundus change and full-field ERG were detailed in Table 1 and Fig. 1D,E,H. Patient AD02-IV:1 reported decreased night vision at age 22, but his fundus showed no remarkable change with normal BCVAs at the last visit (27-year-old). However, significantly reduced ERG responses and moderately reduced peripheral VF were detected (Fig. 1F–H and Table 1).
Figure 1. Detailed clinical evaluations of patients from family AD02.
(A) The pedigree of family AD02 indicates a dominant inheritance pattern of four generations. Genotypes, current and onset ages of RP (inside parentheses) are shown below the pedigree symbols. (B,D–F) Fundus photos demonstrate typical RP changes including waxy optic disks, artery attenuations, pigment deposits, and macular degeneration, in both eyes of patient AD02-III:3 (B), AD02-II:3 (D) and patient AD02-III:2 (E), while the fundus of patient AD02-IV:1 was normal (F). (C) Macular degeneration is also indicated by OCT examinations, which reveal attenuated ONL and RPE with complete loss of OS and IS. (G) Peripheral vision loss is revealed by automated visual field examination of patient AD02-IV:1. (H) Scotopic and photopic ERG responses of patients AD02-II:3, III:2, and III:3 are undetectable, while are significantly reduced for patient AD02-IV:1. ERG responses of a negative control are also presented.
Table 1. Clinical Features of Attainable Patients.
| Patient ID | Genotype | Status | Age at last visit (Age of Onset)* | Sex | Fundus Appearance |
ERG | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCVA (logMAR) | Refractive Error | O.D. |
O.S. |
VF | |||||||||||||||
| O.D. | O.S. | O.D. | O.S. | MD | OD | AA | PD | MD | OD | AA | PD | O.D. | O.S. | ||||||
| AD02-II:3 | c.289G>C | Het | D (20) | M | 0.05 | 0.1 | NA | NA | Yes | Waxy | Yes | Yes | Yes | Waxy | Yes | Yes | Diminished | <5° | <5° |
| AD02-III:2 | c.289G>C | Het | 50 (22) | F | 0.5 | 0.6 | −1.75 DS | −2.25 DS | Yes | Waxy | Yes | Yes | Yes | Waxy | Yes | Yes | Diminished | <15° | <15° |
| AD02-III:3 | c.289G>C | Het | 47 (23) | M | 0.6 | 0.5 | −1.50 DS | −2.00 DS | Yes | Waxy | Yes | Yes | Yes | Waxy | Yes | Yes | Diminished | <15° | <15° |
| AD02-IV:1 | c.289G>C | Het | 27 (22) | M | 1.0 | 1.0 | −0.50 DS | −1.00 DS/−0.50 DC*120 | NOR | NOR | NOR | NOR | NOR | NOR | NOR | NOR | Reduced | NOR | NOR |
Abbreviations: Het: heterozygous; *: year(s); D: dead; M: male; F: female; BCVA: best corrected visual acuity; logMAR: logarithm of the minimum angle of resolution; O.D.: right eye; O.S.: left eye; NA: not available; MD: macular degeneration; OD: optic disk; AA: artery attenuation; PD: pigment deposits; NOR: normal; ERG: electroretinography; VF: visual field.
Identification of putative variant in SPP2 gene
The pedigree of the family AD02 indicated a likely autosomal dominant inheritance mode because of the presence of patients in each generation with roughly 50% occurrence and the male-to-male transmission (Fig. 1). To determine the RP causative mutation for this family, targeted next-generation sequencing was initially conducted on 3 individuals, including patient AD02-II:3, patient AD02-IV:1, and the unaffected AD02-III:5, to detect potential disease causative mutation in identified RP relevant genes. No potential disease causative mutation was revealed. WES was further performed on these 3 individuals. The mean depths of targeted region yielded by WES were 66.72-, 82.10-, and 74.60-fold for individuals AD02-II:3, AD02-IV:1, and AD02-III:5, respectively. The detailed WES data were summarized in Supplemental Table 1. After initial screening, a total of 37 single nucleotide variations (SNVs) were shared by the two patients (AD02-II:3 and AD02-IV:1) but not the unaffected member (AD02-III:5), and were absent in 5 SNP databases. Further co-segregation analysis of these SNVs among all family members recruited was performed by Sanger sequencing with their primers detailed in Supplemental Table 2. Among them, four SNVs were found to be false positive, five did not fit with the autosomal dominant inheritance mode, and another 27 SNVs did not co-segregate with the disease phenotype in the family. Only one heterozygous SNV, c.G289C in the exon 3 of SPP2 gene (Fig. 2A–C), was completely co-segregated with RP phenotype of this family. It was further found to be absent in 800 unrelated ethnically matched alleles and the in-house exome sequencing database derived from 1400 Chinese unrelated to RP. The variant c.G289C would result in an amino acid substitution at position 97 of Spp-24 (p.Gly97Arg), a switch from a neutral amino acid, glycine, to an alkaline amino acid, arginine. We next directly sequenced all 7 coding exons of SPP2 gene in patients AD02-II:3 and AD02-IV:1. No other pathogenic variant in SPP2 was detected.
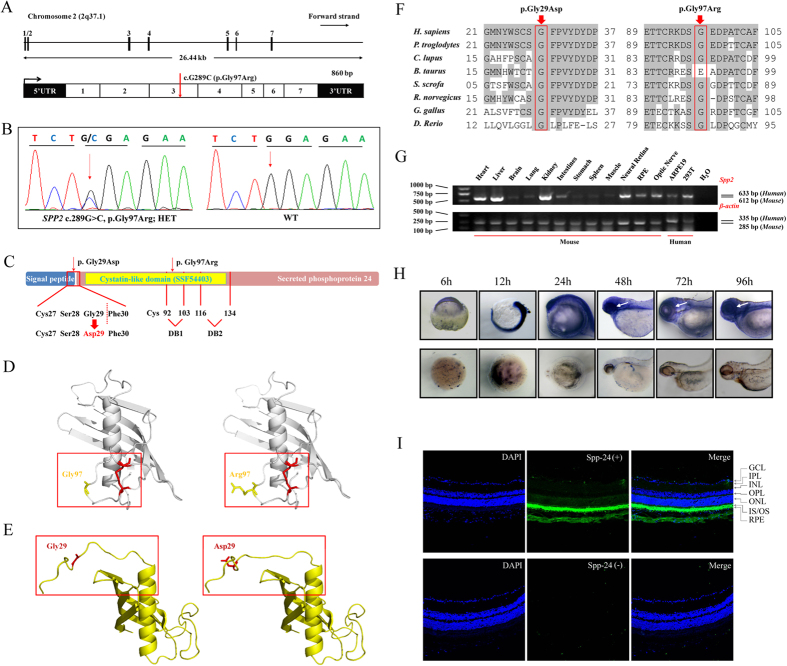
Figure 2. Genetic analyses of the SPP2 variants, and expression profiling of Spp-24.
(A) SPP2 gene spanning 26.44 kb on chromosome 2q37.1 contains 7 exons (upper panel). The identified heterozygous variant, c.G289C (p.Gly97Arg), was located within exon 3 (below panel). (B) Sequencing chromatogram of patient AD02-III:3 shows the c.289G>C substitution. WT sequence is also shown. (C) Schematic representation of the relative linear location of the two SPP2 mutations in context of Spp-24 protein structure. The p.Gly97Arg is located in the cystatin-like domain, and the artificial mutation, p.Gly29Asp, is located at the last residue of signal peptide. The red dotted line denotes the cleavage site of the signal peptide. The two internal disulfide bonds are indicated by DB1 and DB2 respectively. (D–E) Structural modeling of Spp-24. The mutated residue 97 is located within the first disulfide bond loop of Spp-24 (D, cys92-103 disulfide bond is denoted by red color). The other substitution p.Gly29Asp would lead to the distortion of the signal peptide cleavage site in the mutant when compared with the WT monomer (E). (F) Conservation analyses of the mutated residues 29 and 97 of Spp-24 in multiple species, including human (H. sapiens), chimpanzees (P. troglodytes), dogs (C. lupus), cows (B. taurus), pigs (S. scrofa), rats (R. norvegicus), chickens (G. gallus), and zebrafish (D. rerio). Conserved residues are shaded. (G) Expression of SPP2 in multiple murine tissues and human cell lines, namely ARPE19 and HEK 293T (upper panel). Expression of β-actin serves as internal control (lower panel). (H) Zebrafish whole mount in situ hybridization reveals the ubiquitously expression of the spp2 transcript throughout the development of zebrafish. A relatively higher expression in the eye wall after 48 hours post fertilization is indicated by white arrows. (I) Immunostaining for spp-24 (green) on murine retinal frozen section. Abundant reactivity of spp-24 was detected in the inner/outer segments (IS/OS) and retinal pigment epithelium (RPE) layers, and moderate staining was also found in sclera, choroidal vessels and ganglion cells (GC) (upper panel). Sections incubated with secondary antibody alone serve as negative control (lower panel). Scale bar: 100 μm.
Comparative and structural analyses of SPP2 variants
To predict the potential pathogenic impact of p.Gly97Arg on protein level, we carried out comparative and structural analyses. Multiple orthologous sequence alignment (MSA) revealed that p.Gly97Arg was conserved among all species tested except for cow (Fig. 2F). Similarly, p.Gly97Arg was predicted to be possibly damaging by 3 out of the 4 programs (Table 2).
Table 2. Characteristics of the mutations.
| Variation |
Exon | Bioinformatics Analysis |
Frequency in Controls | |||||
|---|---|---|---|---|---|---|---|---|
| Nucleotide | Amino Acid | Status | SIFT | PolyPhen | CONDEL | PROVEN | ||
| c.289G>C | p.Gly97Arg | Het | E1 | Damaging | Benign | Deleterious | Deleterious | 0/800 |
| c.85G>A | p.Gly29Asp | - | E3 | Damaging | PD | Deleterious | Deleterious | 0/800 |
Abbreviations: Het: heterozygous; E: exon; PD: probably damaging.
We next modeled the crystal structure of wild type and mutant Spp-24 protein with p.Gly97Arg using a template of human cystatin F [Protein Data Base (PDB) ID: 2CH9]12, a member of type II cystatins12 that shows the highest structural similarity to Spp-24 according to the analysis from SWISS MODEL. The predicted structure of Spp-24 was covered from residues number 22 to 136 and shared 28% homology to the template protein in sequence. Variant p.Gly97Arg was located in the cystatin-like domain within the first of the two disulfide bonded loops of Spp-24 (Fig. 2D,E), which are key motifs shared by all Spp-24 proteins and type II cystatins9,12. Of note, the p.Gly97Arg represented a substitution from a relatively hydrophobic and conformational flexible residue to a highly hydrophilic and conformational rigid residue.
Expression of the SPP2 gene
We first analyzed the expression of SPP2 gene in a panel of murine tissues, and human HEK 293T and ARPE19 cells using reverse transcriptase polymerase chain reaction (RT-PCR) analysis. Expression of Spp2 was clearly detected in murine liver, kidney, heart, RPE and neural retina (Fig. 2G), and was also detectable in several other tissues and the two tested human cell lines, suggesting a wide expression of this gene. We also determined the expression of Spp2 in zebrafish at different stages by whole mount in situ hybridization, and similarly, the spp2 transcript was ubiquitously expressed during development of zebrafish and was significantly expressed in the eye wall after 48 hours post fertilization (Fig. 2H).
To further localize the spp-24 protein in ocular tissues, we conducted immunostaining for spp-24 in murine eye sections. Reactivity of the Spp-24 protein was most abundant in IS/OS and RPE, and was also detectable in the ONL, ganglion cells (GC), choroidal vessels and sclera (Fig. 2I). In the negative control sections incubated with second antibody only, no non-specific staining was found (Fig. 2I).
SPP2 variants cause Spp-24 cellular retention in the endoplasmic reticulum
We hypothesized that the p.Gly97Arg may affect the cellular localization and secretion of Spp-24. To this end, we transiently expressed the C-terminally Flag-tagged fusion protein of Spp-24WT, Spp-24Gly97Arg, and Spp-24Gly29Asp in HEK 293T cells respectively. The plasmid Spp-24Gly29Asp carries an artificial mutation, p.Gly29Asp, which serves as a potential positive control for the secretory defect of Spp-24. The p.Gly29Asp affects an absolutely conserved site according to MSA analysis (Fig. 2C), and is predicted to be deleterious by all online prediction programs applied (Table 2). More importantly, the p.Gly29Asp is located at the last residue of the signal peptide. As indicated by SignalP program, the Y score peaks at the residue Phe30 of Spp-24WT, and at the residue Ser28 of the mutant Spp-24Gly29Asp. In addition, modeling of Spp-24 revealed that the p.Gly29Asp altered the orientation of the corresponding residue (Fig. 2E). Thus, p.Gly29Asp highly affects the conformation and the cleavage of the signal peptide leading to secretory defect of Spp-24.
The subcellular localizations of the exogenous Spp-24 were first determined by immunofluorescent staining of Flag (Fig. 3A). As expected, for cells transfected with Spp-24WT, the exogenous Spp-24WT is found mainly localized to the cell membrane, while the endogenous Spp24 protein shows a uniform distribution in the transfected cells (Fig. 3A). However, for cells overexpressing mutant Spp-24Gly29Asp or Spp-24Gly97Arg, the exogenous Flag-tagged mutant Spp-24 showed extensive co-localization with the endogenous Spp-24 protein in the cytoplasm and perinucleus region, indication a potential interaction between the mutant and the wild type Spp-24 proteins (Fig. 3A). The membranous Spp-24Gly97Arg and Spp-24Gly29Asp was much less distinct when compared to Spp-24WT as indicated by the staining of Flag (Fig. 3A). In addition, the exogenous mutant Spp-24 protein also showed colocalization with the endoplasmic reticulum (ER), indicating retention of the Spp-24 proteins in ER (Fig. 3A).
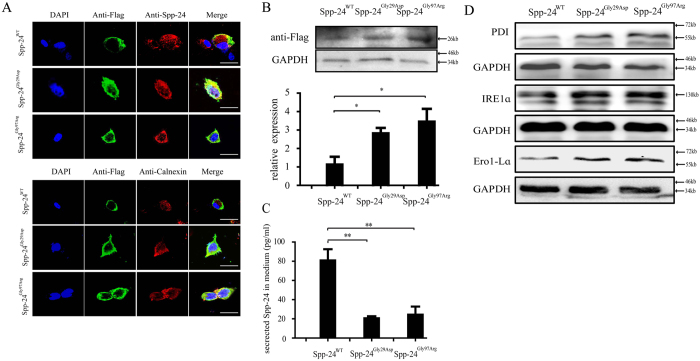
Figure 3. Analyses of the cellular processing of wild type and mutant Spp-24 in transfected HEK 293T cells.
(A) Immunofluorescence staining of HEK 293T cells transiently transfected with Flag-tagged wild type secreted phosphoprotein 2 (Spp-24WT), mutant Spp-24 carrying p.Gly29Asp (Spp-24Gly29Asp) and p.Gly97Arg (Spp-24Gly97Arg), respectively. Cell nuclei are presented using DAPI. Images are also shown as anti-Flag (detecting exogenous Flag-tagged Spp-24), anti-Spp-24 (detecting both endogenous and exogenous Spp-24), anti-Calnexin (endoplasmic reticulum), and merged formats. Scale bar: 20 μm. (B) Immunoblot of Flag on cellular protein extracts from HEK 293T cells transfected with Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serves as internal control. The intracellular amount of exogenous Spp-24 as indicated by the density of bands for Flag was quantified and normalized to internal control (indicated by bar graph at bottom, Spp-24WT VS Spp-24Gly29Asp, P = 0.0463; Spp-24WT VS Spp-24Gly97Arg, P = 0.0313). Error bars represent standard deviation (STDV) from biological triplicates. (C) Enzyme-linked immuno sorbent assay (ELISA) is performed to determine the amount of Spp-24 in the culture medium of the cells transfected with Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg, respectively (Spp-24Gly29Asp VS Spp-24WT, P = 0.0024; Spp-24Gly97Arg VS Spp-24WT, P = 0.003). Error bars represent STDV from biological triplicates. *P < 0.05, **P < 0.01. (D) Immunoblots of PDI, IRE1α, and Ero1-Lα on cellular protein extracts from HEK 293T cells transfected with Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg. The GAPDH serves as internal control.
We further assessed the cellular protein amount of exogenous Spp-24 in HEK 293T cells by immunoblot of Spp-24. Consistently, we found that the cellular Spp-24Gly29Asp and Spp-24Gly97Arg were increased when compared with Spp-24WT (Fig. 3B). As Spp-24 is a secreted protein, we next applied ELISA to determine the secreted Spp-24 in the culture medium of the cells transfected with each of the three different Spp-24 plasmids. Consistent to the immunostaining result, the secreted Spp-24 was found significantly less in the cells transfected with Spp-24Gly29Asp or Spp-24Gly97Arg when compared with cells overexpressing Spp-24WT (Fig. 3C). Immunoblotting also indicated that expressions of markers relevant to ER-stress, including protein disulfide isomerase (PDI), IRE1α, and ER protein endoplasmic oxidoreductin-1-Lα (Ero1-Lα), were elevated in cells overexpressing Spp-24Gly29Asp or Spp-24Gly97Arg when compared with cells overexpressing Spp-24WT. Taken together, our cellular studies thus indicated that the RP-associated variant, p.Gly97Arg, and the artificial variant, p.Gly29Asp, have similar pathogenic effect causing cellular retention of both endogenous and exogenous Spp-24 in ER, which further induced ER stress. Overexpression of the two mutants in zebrafish could result in retinal phenotypes via dominant-negative effect leading to loss-of-function of the protein.
Overexpressing mutant Spp-24 initiates defects in rod photoreceptors and increases systemic deformity in zebrafish
To further investigate the pathogenicity of the two Spp-24 mutations in vivo, we used the zebrafish model. Embryos were divided into four groups and injected with 100 ng (1 nl) of distinct human mRNA including SPP2 antisense mRNA (Spp-24Anti, n = 64) as negative control, wild type SPP2 mRNA (Spp-24WT, n = 72), and two mutant SPP2 mRNAs (Spp-24Gly29Asp, n = 68; Spp-24Gly97Arg, n = 70). Quantitative PCR (Q-PCR) showed similar levels of expressions of exogenous SPP2 at 2 days post fertilization (dpf) among the three groups injected with Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg (Fig. 4A). However, the expression of endogenous spp2 was significantly elevated by the injection of Spp-24Gly29Asp (3.45 ± 1.49 fold) and Spp-24Gly97Arg (2.45 ± 0.82 fold), when compared with Spp-24WT-injected larvae and non-injected group (default as 1 fold). Presumably there was a compensatory response of endogenous spp2 to the over-expressed mutant human SPP2 (Fig. 4A). Because we found that SPP2 was widely expressed, we next compared the systemic effects relevant to the injection of different SPP2 mRNAs. At 4 dpf, we observed dramatic systemic deformation in substantial number of larvae overexpressing Spp-24Gly29Asp (~32%) and Spp-24Gly97Arg (~47%), including brain malformation, cardiac edema, short trunks, and curved body axis. The proportions of zebrafish with deformity in the two groups were significantly increased when compared to the Spp-24WT-injected (~14%) and Spp-24Anti-injected (~7%) zebrafish (Fig. 4B,C). The overexpression of Spp-24Gly97Arg has also caused significantly increased death rate (~21%) when compared to the Spp-24WT-injected (~11%) and the Spp-24Anti-injected (~6%) groups (Fig. 4B). Injection of Spp-24WT, compared to injection of Spp-24Anti, has also slightly increased the rates of deformity and death, presumably due to toxicity of overexpressed exogenous protein as we previously found in other zebrafish models13.
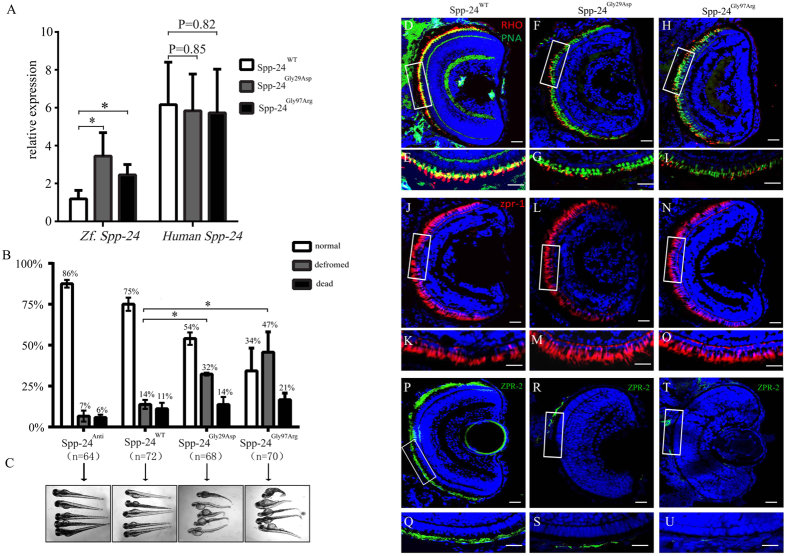
Figure 4. Deleterious effects identified in zebrafish overexpressing Spp-24 mutants.
(A) Expressions of endogenous spp2 (Zf.) and exogenous SPP2 (Human) in zebrafish at 2 days post fertilization (dpf) after injection of Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg were determined by Q-PCR and were relative to the expression of spp2 in uninjected larvae. (Spp-24Gly29Asp VS Spp-24WT, P = 0.0463; Spp-24Gly97Arg VS Spp-24WT, P = 0.0379). (B) Quantification of normal, deformed, and dead zebrafish injected with different mRNAs from 2 to 4 dpf. n: total numbers of injected zebrafish from triple experiments. (Spp-24Gly29Asp VS Spp-24WT, P = 0.0142; Spp-24Gly97Arg VS Spp-24WT, P = 0.0105). (C) Morphological changes in zebrafish injected with different mRNAs at 4 dpf. Most zebrafish injected with Spp-24Anti and Spp-24WT is relatively normal, while significant systemic deformations are revealed in groups injected with Spp-24Gly29Asp and Spp-24Gly97Arg. (D–U) Immunostaining of rhodopsin (D–I), peanut agglutinin (PNA) lectin (D–I), ZPR-1 (J–O), and ZPR-2 (P–U) on retinal frozen sections of zebrafish at 4 dpf from the four groups, including groups injected with Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg. Robust staining of rhodopsin is found in the rod IS/OS and cone OS layers in Spp-24WT -injected fish, respectively (D–E). Reactivity of rhodopsin (F–I) and ZPR-2 (Q–U) are decreased in Spp-24Gly29Asp and Spp-24Gly97Arg-injected zebrafish, while reactivities of ZPR-1 and PNA was clearly detected in the IS/OS layer of all zebrafish studied (D–O). The boxed areas in (D,F,H,J,L,N,P,R,T) were shown in higher magnification in (E,G,I,K,M,O,Q,S,U). Scale bar: 20 μm.
To further correlate the SPP2 mutations with retinal phenotypes, we investigated the integrity of retina in those zebrafish less affected by overexpression of the two mutant proteins, under the assumption that the two mutations primarily affect retinal cells. To this end, zebrafish with a normal systematic appearance were randomly selected from the three groups injected with Spp-24WT, Spp-24Gly29Asp and Spp-24Gly97Arg at 4 dpf. As expected, immunofluorescent staining of rhodopsin, a specific marker for rod photoreceptors, revealed greatly decreased expression of rhodopsin in the eye sections of the majority of zebrafish injected with Spp-24Gly29Asp (n = 14 out of 25) and Spp-24Gly97Arg (n = 13 out of 20), whereas abundant specific staining of rhodopsin was constantly observed in the inner/outer segment (IS/OS) layer of all larvae injected with Spp-24WT (n = 20) (Fig. 4D–I). We next determined the morphogenesis of cone photoreceptors in these models using an antibody against ZPR-1, a cone photoreceptor-specific marker, and peanut agglutinin (PNA) lectin, labeling the cone OS (Fig. 4D–I). PNA staining, similar to ZPR-1 staining, was also clearly detected in the IS/OS layer of all zebrafish studied (Fig. 4J–O). Similar to results observed in rhodopsin staining, immunofluorescent staining of ZPR-2, a RPE specific marker, showed significant reduction in intensity of zebrafish injected with Spp-24Gly29Asp and Spp-24Gly97Arg when compared with those overexpressing Spp-24WT (Fig. 4P–U).
Discussion
In the SPP2 genes family, bovine spp-24 was the first characterized and suggested to be involved in bone metabolism and fetutin-mineral complex14. Subsequently, human Spp-24 was defined with similar structure and was detected in kidney, liver and plasma9. In the present study, we identified a heterozygous missense mutation in SPP2, p.Gly97Arg, which was associated with adRP in a four-generation Chinese family. Moreover, we found that SPP2 was detectable in retina at both transcript and protein level, and overexpression of mutant Spp-24 caused primary defects in rod photoreceptors mimicking the cardinal feature of RP. Altogether, our data indicate a new role of Spp-24 in the pathogenesis of retinal degeneration.
The Spp-24 proteins contain four absolutely conserved cysteines to form 2 internal disulfide bonded loops, which are also key motif of type II cystatins9,12. The second disulfide bonded loop in bovine spp-24 is a highly conserved bone morphogenetic protein (BMP)-binding loop similar to that in fetuin and the BMP receptor II, and is thus defined as the transforming growth factor-β (TGF-β) receptor II homology-1 (TRH1) domain15, which was shown to bind BMP16. The substitution p.Gly97Arg is located within the first disulfide bonded loop of Spp-24, which has not been previously characterized but would likely have important biological properties given that it’s the characteristic motif of spp-24 proteins. In general, a disulfide bond plays an important role in the folding and stability of proteins, particularly for secreted proteins17. Hydrophobic residues are often condensed around disulfide bond via hydrophobic interactions to form a hydrophobic core of the protein. Because the p.Gly97Arg is a switch from a hydrophobic residue to a highly hydrophilic residue that is outside of the disulfide bonded loop (Fig. 2C), this mutation may destabilize the secondary and/or tertiary structure of Spp-24 due to the interference of water molecule on amide-amide hydrogen bond, and therefore lead to folding and secretory defects of the protein. Indeed, the similarly observed cellular retention of Spp-24Gly97Arg and the artificial signal peptide mutant, Spp-24Gly29Asp, indicates that the p.Gly97Arg has caused secretory defect of Spp-24. In addition, Spp-24 is extremely sensitive to proteolysis, and the cystatin-like domain of Spp-24 is likely conformational flexible conferring the liability to proteolysis11,18. The replacement of glycine by arginine may increase the conformational rigidity of the cystatin-like domain and subsequently decrease the susceptibility of Spp-24 to proteolysis, which may also contribute to the cytoplasmic accumulation of Spp-24Gly97Arg. Consistently, the Spp-24Gly97Arg correlated with more severe phenotypes when compared to Spp-24Gly29Asp, including more intracellular retention of the protein and subsequently leading to higher rates of systemic deformity and death in zebrafish.
In cellular study, we have found that the exogenous mutant Spp-24Gly29Asp or Spp-24Gly97Arg co-localized with the endogenous Spp-24 protein, cause cellular retention of both the exogenous and endogenous Spp-24 proteins in the ER, and further induce ER stress. A reasonable hypothesis for this phenomenon would be that in the photoreceptors of RP patients, the mutant protein generated by the mutant SPP2 allele would interact with the wild type protein produced by the wild type SPP2 allele, which further leads to cellular accumulation of both Spp-24 proteins and ER stress. We therefore, based on these findings, highly hypothesized that the two SPP2 mutations may lead to the disease in a dominant negative manner. Both insufficiency of the extracellular Spp-24 protein and cellular accumulation of the irregular Spp-24 proteins would lead to the dysfunction of rod photoreceptors or neighbor cells that support photoreceptors, e.g. RPE. In support of this proposition, the best example among cystatins is cystatin C (NP_000090.1), encoded by the CST3 gene (NG_012887.2). Cystatin C is a member of type II cystatins characteristically possessing a signal peptide and two disulfide bridges as structural characteristics19. Cystatin C presents in variable ocular tissues in different species20,21,22,23 and is most abundant in the RPE of the human eye24,25, similar to the expressions of Spp-24 as detected in this study. Interestingly, the variant B cystatin C, which carries a signal peptide mutation, was correlated with increased risk of age-related macular degeneration26. It impaired the secretory pathway of proteins in RPE cells leading to abnormal cellular retention25,27, therefore similar to the pathogenic consequences caused by Spp-24Gly29Asp and Spp-24Gly97Arg. Further, in the retina of rd1 mouse, a model for RP, increased cystatin C was observed in the RPE, ganglion cell layer and photoreceptors suggesting the potential involvement of cystatin C accumulation in the pathogenesis of retinal degeneration23. In addition to cystatin C, most proteins of the cystatin superfamily inhibit cathepsins, of which aberrant function has been implicated in RPE dysfunction28 and photoreceptor degenerations29. Abnormal cellular processing of Spp-24, as a member of the cystatin superfamily, may cause altered activities of cathepsins in retinal cells. Altogether, the aforementioned existing evidence indirectly support the hypothesis that cellular retention of Spp-24 may be a burden for RPE or photoreceptors leading to retinal degeneration.
In conclusion, SPP2 is very likely a novel RP-causing gene. According to the genetic findings and results from in vitro and in vivo studies, we have demonstrated the pathogenicity of the mutation SPP2 p.Gly97Arg. However, more functional studies are still needed to address the pathogenic mechanism by which the SPP2 mutation causes degeneration of rod photoreceptors. Screening for SPP2 mutations in RP patients with unknown genetic causes are also essential to gain better insights into the disease mechanism and genotype-phenotype correlations.
Methods
Details on the human subjects; animals; exome sequencing and bioinformatics analysis; in silico analyses; RT-PCR and real time PCR; plasmids construction; cell transfection; immunoblotting; immunofluorescence of SPP2 and cell components; whole mount in situ hybridization in zebrafish; mRNA synthesis and zerafish manipulations; and statistics are provided in SI Materials and Methods. All the human study was approved and prospectively reviewed by Nanjing Medical University ethnical review boards in accordance to the Declaration of Helsinki. Written informed consents were signed by all participants or their statutory guardian before their participation. The animal study was in accordance with the IACUC-approved protocol and approved by the institutional committee of NanJing Medical University. All procedures were conformed to the Guide for the Care and Use of laboratory animals.
Additional Information
How to cite this article: Liu, Y. et al. SPP2 Mutations Cause Autosomal Dominant Retinitis Pigmentosa. Sci. Rep. 5, 14867; doi: 10.1038/srep14867 (2015).
Supplementary Material
Acknowledgments
We thank all patients and family members for their participation in this study. We also thank Prof. Qingjiong Zhang from Zhongshan Ophthalmic Center for his help with mutation screening. We appreciate Liping Guan, Jingjing Jiang, and Jingjing Xiao from BGI-Shenzhen for technical support. We are also grateful to Genesky Biotechnologies Inc. (Shanghai, China) for their technical help with linkage analyses. This work was supported by National Key Basic Research Program of China (Grant 2013CB967500 to C.Z.); National Natural Science Foundation of China (Grants 81222009 and 81170856 to C.Z., and 81170867 to K.Z.); Thousand Youth Talents Program of China (to C.Z.); Jiangsu Outstanding Young Investigator Program (Grant BK2012046 to C.Z.); Jiangsu Province’s Key Provincial Talents Program (Grant RC201149 to C.Z.); the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (to C.Z.); Jiangsu Province’s Scientific Research Innovation Program for Postgraduates (Grant CXZZ13_0590 to X.C.); Foundation Fighting Blindness (to D. Vollrath); and a project funded by the priority academic program development of Jiangsu Higher Education Institutions (PAPD; JX10231801).
Footnotes
Author Contributions Study design: Y.L., X.C. and C.Z. Collected the samples and performed the experiments: Y.L., X.C., Q.X., X.G., P.O.T., X.Z., L.C. and W.J. Data interpretation and analysis: Y.L. and X.C. Wrote the manuscript: Y.L., X.C., D.V. and C.Z. Contributed to revision of the manuscript: K.Z., Q.Z., C.P. and C.Z. All authors have read and approved the final manuscript.
References
- Anasagasti A., Irigoyen C., Barandika O., Lopez de Munain A. & Ruiz-Ederra J. Current mutation discovery approaches in Retinitis Pigmentosa. Vision Res 75, 117–129 (2012). [DOI] [PubMed] [Google Scholar]
- Punzo C., Kornacker K. & Cepko C. L. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12, 44–52 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P. & Cisneros E. Retinitis pigmentosa: cone photoreceptors starving to death. Nat Neurosci 12, 5–6 (2009). [DOI] [PubMed] [Google Scholar]
- Daiger S. P., Sullivan L. S. & Bowne S. J. Genes and mutations causing retinitis pigmentosa. Clin Genet 84, 132–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S. et al. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics 12, 238–249 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni J. N. et al. Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Curr Genomics 12, 276–284 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger S. P., Bowne S. J. & Sullivan L. S. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol 125, 151–158 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger S. P. et al. Mutations in known genes account for 58% of autosomal dominant retinitis pigmentosa (adRP). Adv Exp Med Biol 613, 203–209 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. S., Khorram Khorshid H. R., Kitchen J. A., Arteta D. & Dalgleish R. Characterization of the human secreted phosphoprotein 24 gene (SPP2) and comparison of the protein sequence in nine species. Matrix Biol 22, 641–651 (2004). [DOI] [PubMed] [Google Scholar]
- Hu B., Coulson L., Moyer B. & Price P. A. Isolation and molecular cloning of a novel bone phosphoprotein related in sequence to the cystatin family of thiol protease inhibitors. J Biol Chem 270, 431–436 (1995). [DOI] [PubMed] [Google Scholar]
- Brochmann E. J., Behnam K. & Murray S. S. Bone morphogenetic protein-2 activity is regulated by secreted phosphoprotein-24 kd, an extracellular pseudoreceptor, the gene for which maps to a region of the human genome important for bone quality. Metabolism 58, 644–650 (2009). [DOI] [PubMed] [Google Scholar]
- Schuttelkopf A. W., Hamilton G., Watts C. & van Aalten D. M. Structural basis of reduction-dependent activation of human cystatin F. J Biol Chem 281, 16570–16575 (2006). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. PRPF4 Mutations Cause Autosomal Dominant Retinitis Pigmentosa. Hum Mol Genet 23, 2926–2939 (2014). [DOI] [PubMed] [Google Scholar]
- Sun A. et al. Alanine-scanning mutations of the BMP-binding domain of recombinant secretory bovine spp24 affect cytokine binding. Connect Tissue Res 51, 445–451 (2010). [DOI] [PubMed] [Google Scholar]
- Demetriou M., Binkert C., Sukhu B., Tenenbaum H. C. & Dennis J. W. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem 271, 12755–12761 (1996). [DOI] [PubMed] [Google Scholar]
- Mace P. D., Cutfield J. F. & Cutfield S. M. High resolution structures of the bone morphogenetic protein type II receptor in two crystal forms: implications for ligand binding. Biochem Biophys Res Commun 351, 831–838 (2006). [DOI] [PubMed] [Google Scholar]
- Gabis L. V., Yangala R. & Lenn N. J. Time lag to diagnosis of stroke in children. Pediatrics 110, 924–928 (2002). [DOI] [PubMed] [Google Scholar]
- Behnam K. et al. BMP binding peptide: a BMP-2 enhancing factor deduced from the sequence of native bovine bone morphogenetic protein/non-collagenous protein. J Orthop Res 23, 175–180 (2005). [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Grubb A., Olafsson I. & Lundwall A. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human cysteine proteinase inhibitor cystatin C. FEBS Lett 216, 229–233 (1987). [DOI] [PubMed] [Google Scholar]
- Barka T. & van der Noen H. Expression of the cysteine proteinase inhibitor cystatin C mRNA in rat eye. Anat Rec 239, 343–348 (1994). [DOI] [PubMed] [Google Scholar]
- Colella R. Cystatin mRNA is expressed by the ciliary epithelium of the chick eye. J Histochem Cytochem 44, 77–79 (1996). [DOI] [PubMed] [Google Scholar]
- Wasselius J., Hakansson K., Johansson K., Abrahamson M. & Ehinger B. Identification and localization of retinal cystatin C. Invest Ophthalmol Vis Sci 42, 1901–1906 (2001). [PubMed] [Google Scholar]
- Ahuja S. et al. rd1 Mouse retina shows an imbalance in the activity of cysteine protease cathepsins and their endogenous inhibitor cystatin C. Invest Ophthalmol Vis Sci 49, 1089–1096 (2008). [DOI] [PubMed] [Google Scholar]
- Selim N., Branum G. D., Liu X., Whalen R. & Boyer T. D. Differential lobular induction in rat liver of glutathione S-transferase A1/A2 by phenobarbital. Am J Physiol Gastrointest Liver Physiol 278, G542–550 (2000). [DOI] [PubMed] [Google Scholar]
- Paraoan L., Hiscott P., Gosden C. & Grierson I. Cystatin C in macular and neuronal degenerations: implications for mechanism(s) of age-related macular degeneration. Vision Res 50, 737–742 (2010). [DOI] [PubMed] [Google Scholar]
- Zurdel J., Finckh U., Menzer G., Nitsch R. M. & Richard G. CST3 genotype associated with exudative age related macular degeneration. Br J Ophthalmol 86, 214–219 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraoan L. et al. Unexpected intracellular localization of the AMD-associated cystatin C variant. Traffic 5, 884–895 (2004). [DOI] [PubMed] [Google Scholar]
- Krohne T. U., Kaemmerer E., Holz F. G. & Kopitz J. Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp Eye Res 90, 261–266 (2010). [DOI] [PubMed] [Google Scholar]
- Kinser R. D. & Dolph P. J. Cathepsin proteases mediate photoreceptor cell degeneration in Drosophila. Neurobiol Dis 46, 655–662 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.